Содержание
- 2. Physical-chemical and medical-biological properties of Netropsin (NT) and Proflavine (PF)
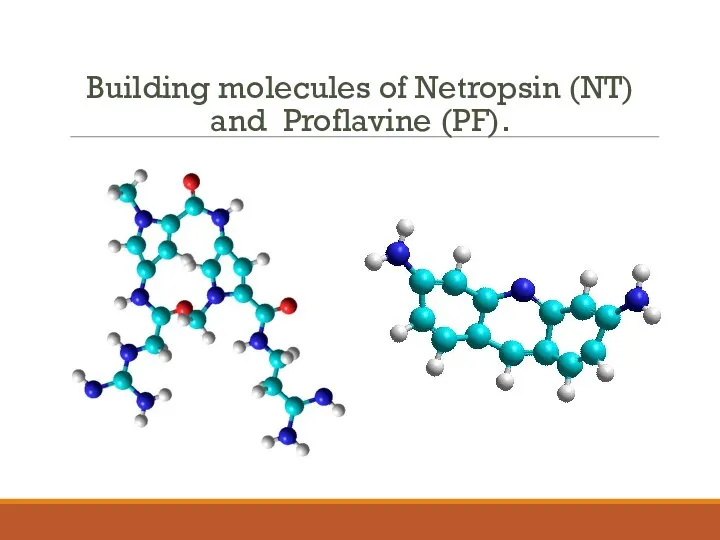
- 3. Building molecules of Netropsin (NT) and Proflavine (PF).
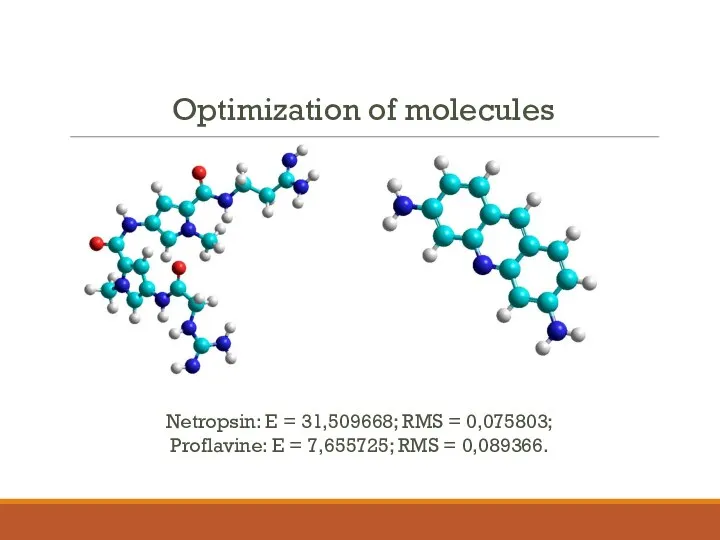
- 4. Optimization of molecules Netropsin: E = 31,509668; RMS = 0,075803; Proflavine: E = 7,655725; RMS =
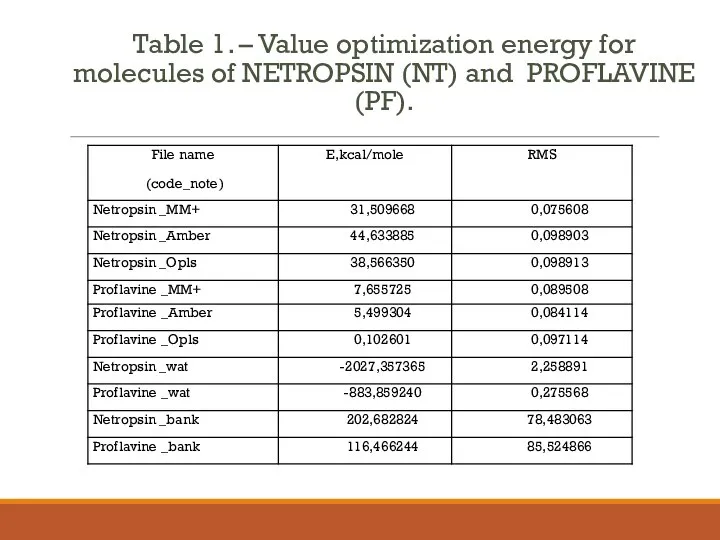
- 5. Table 1. – Value optimization energy for molecules of NETROPSIN (NT) and PROFLAVINE (PF).
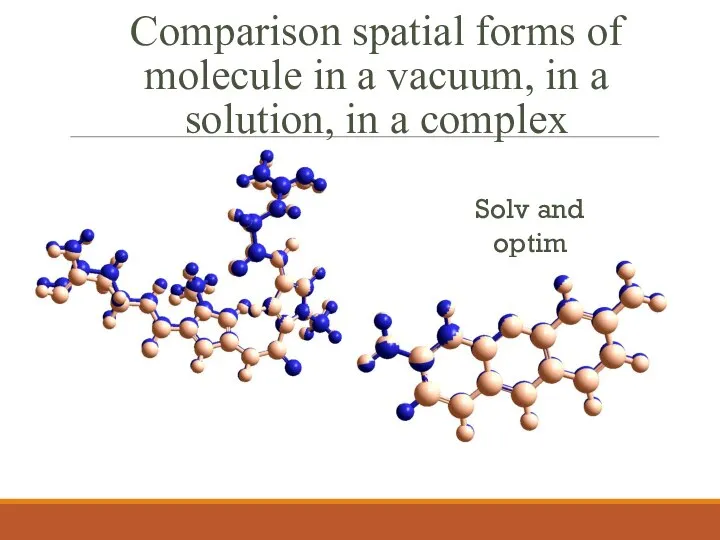
- 6. Comparison spatial forms of molecule in a vacuum, in a solution, in a complex Solv and
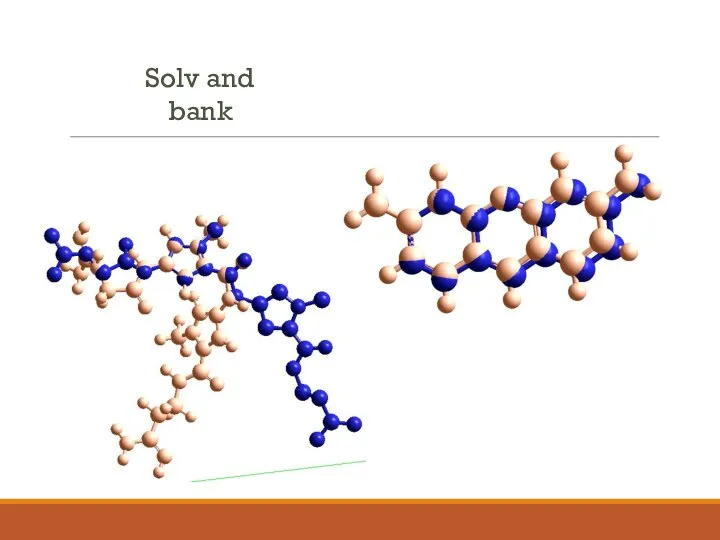
- 7. Solv and bank
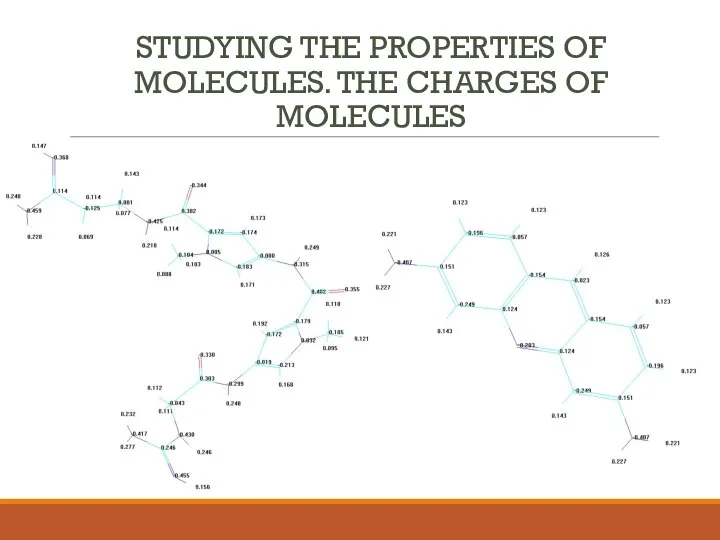
- 8. STUDYING THE PROPERTIES OF MOLECULES. THE CHARGES OF MOLECULES
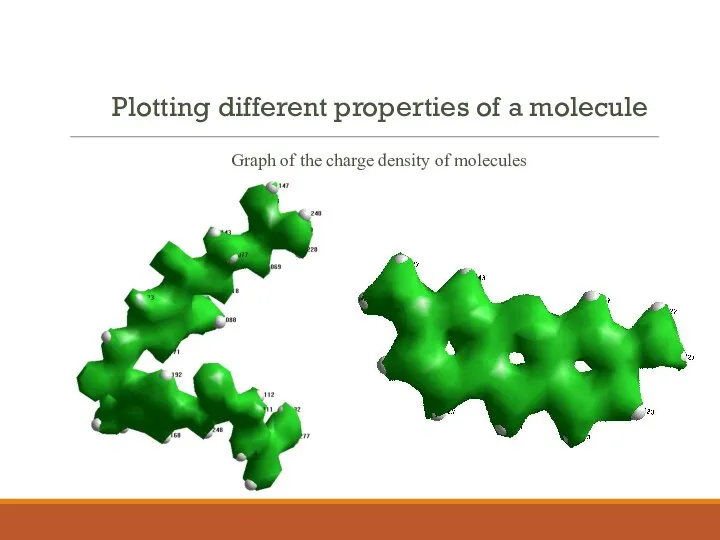
- 9. Plotting different properties of a molecule Graph of the charge density of molecules
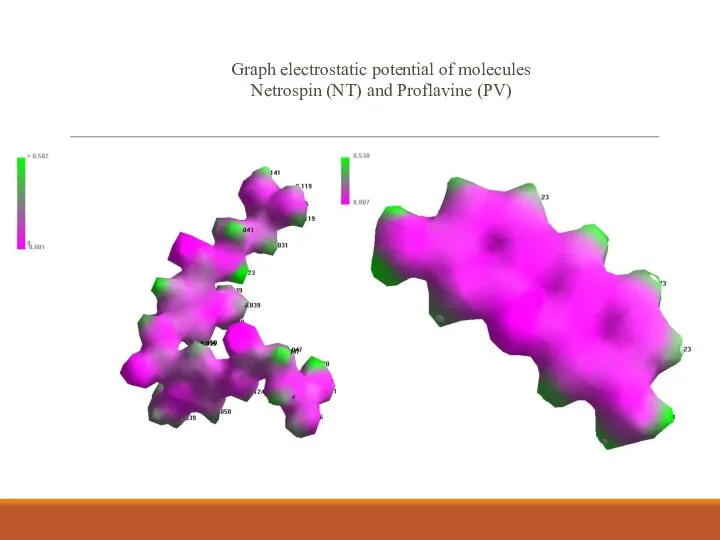
- 10. Graph electrostatic potential of molecules Netrospin (NT) and Proflavine (PV)
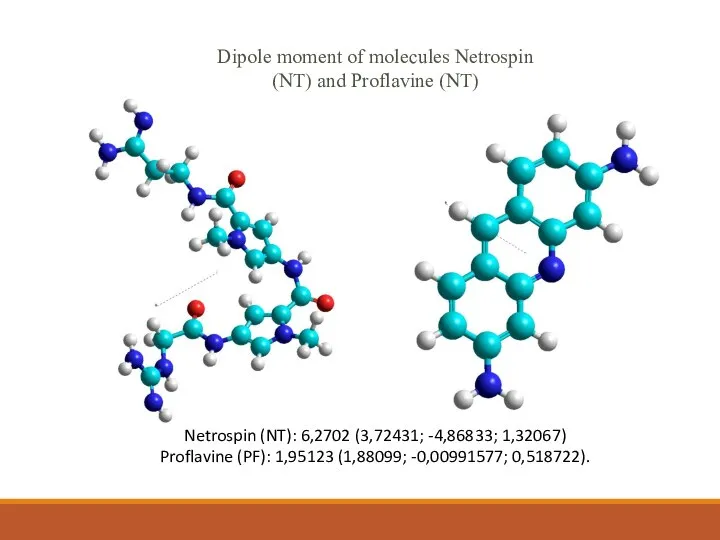
- 11. Netrospin (NT): 6,2702 (3,72431; -4,86833; 1,32067) Proflavine (PF): 1,95123 (1,88099; -0,00991577; 0,518722). Dipole moment of molecules
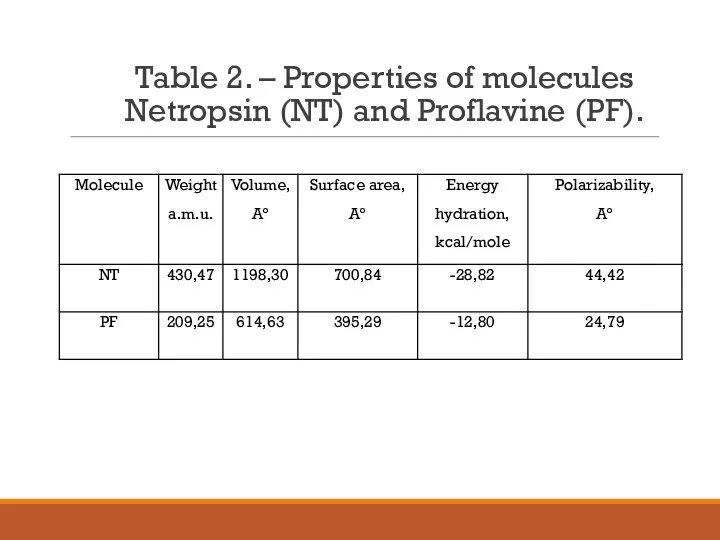
- 12. Table 2. – Properties of molecules Netropsin (NT) and Proflavine (PF).
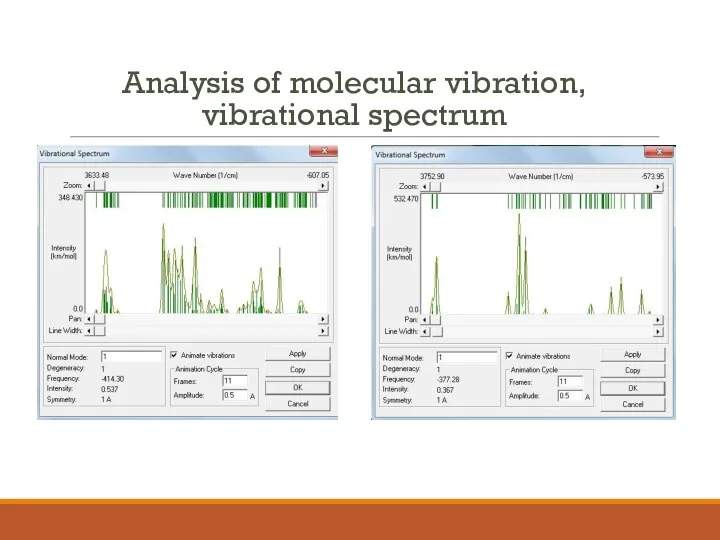
- 13. Analysis of molecular vibration, vibrational spectrum
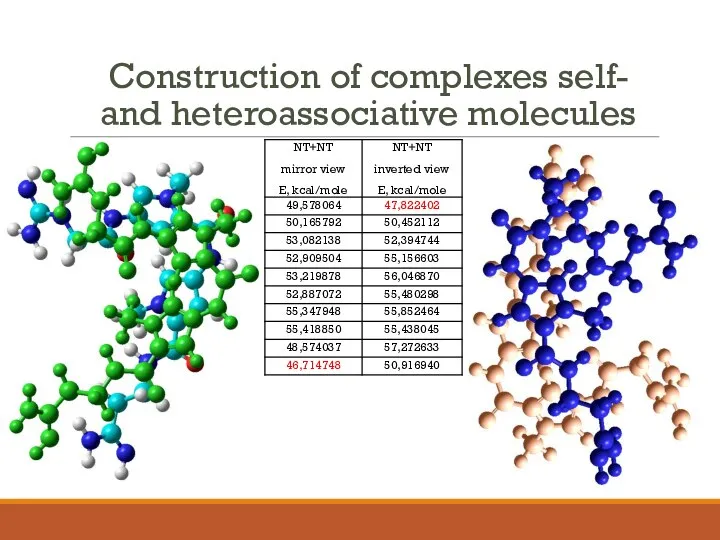
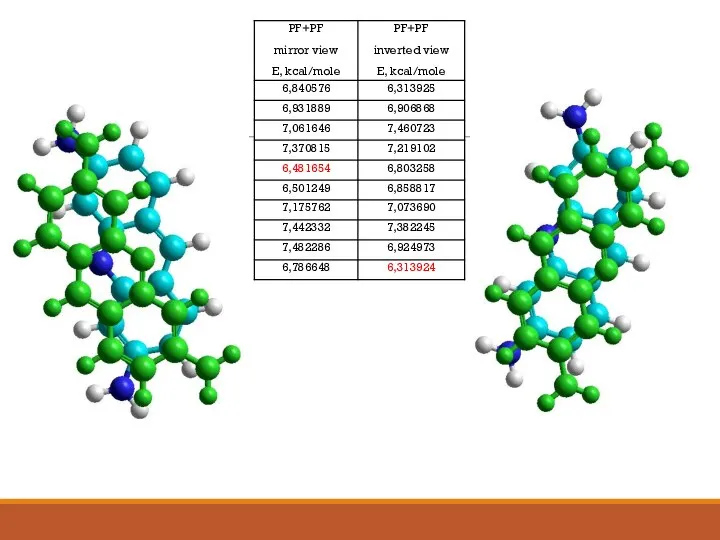
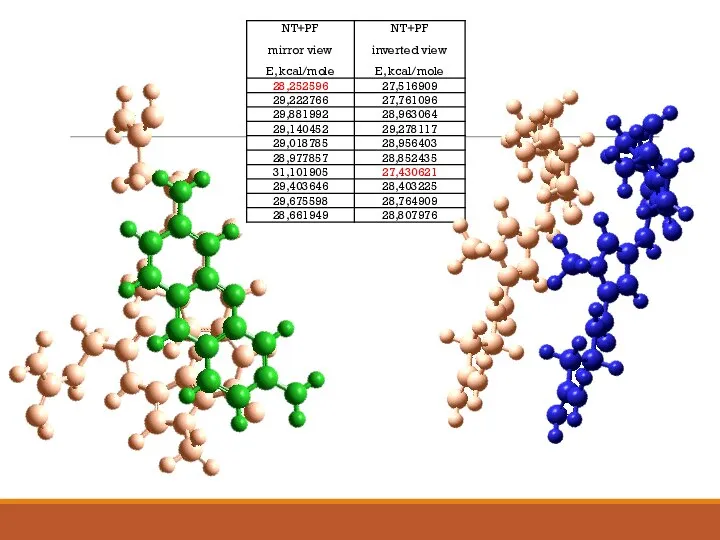
- 14. Construction of complexes self- and heteroassociative molecules
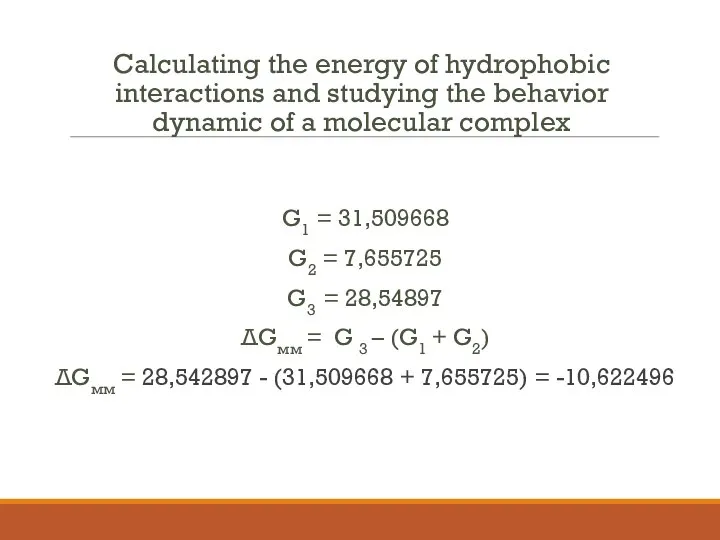
- 17. Calculating the energy of hydrophobic interactions and studying the behavior dynamic of a molecular complex G1
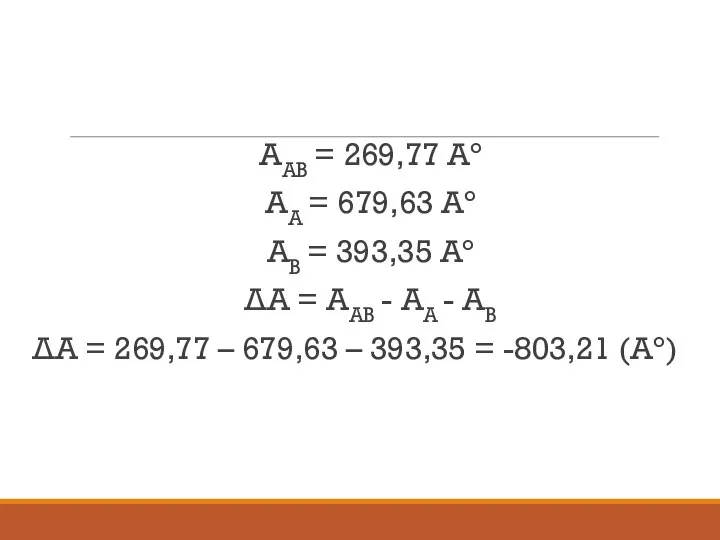
- 18. АAB = 269,77 Aо АA = 679,63 Aо АВ = 393,35 Aо ΔА = АAB -
- 19. ΔGВДВ = γВДВ * ΔA ΔGВДВ = (-38.8) * ( -803,21) = 31164,548 (кал/моль*Ао), ΔGгф =
- 21. Скачать презентацию

 Теория и практика информационно-аналитической работы. Семинар 6 2018
Теория и практика информационно-аналитической работы. Семинар 6 2018 Косимуляция Matlab- PSIM
Косимуляция Matlab- PSIM Блок управления GDS-II - новые возможности
Блок управления GDS-II - новые возможности 314835
314835 Lektsia_1
Lektsia_1 Kotlin: ООП, классы
Kotlin: ООП, классы Единицы измерения информации
Единицы измерения информации Персональный компьютер. Компьютер как унивесальное устройство для работы с информацией
Персональный компьютер. Компьютер как унивесальное устройство для работы с информацией Информация. Объекты информации
Информация. Объекты информации Информационные системы в экономике
Информационные системы в экономике Сайт-музея военного аэродрома Арктика
Сайт-музея военного аэродрома Арктика Работа библиотеки при музее пожарной части №1 ГУ Служба пожаротушения и аварийно-спасательных работ ДЧС СКО
Работа библиотеки при музее пожарной части №1 ГУ Служба пожаротушения и аварийно-спасательных работ ДЧС СКО Кодирование информации
Кодирование информации Презентация на тему Линейный алгоритм
Презентация на тему Линейный алгоритм  Сетевые службы. Кластеры
Сетевые службы. Кластеры Презентация на тему Виды памяти, вытесняющие статическую память
Презентация на тему Виды памяти, вытесняющие статическую память  Системы управления базами данных. Информационное моделирование
Системы управления базами данных. Информационное моделирование Урок 3 (Графические примитивы)
Урок 3 (Графические примитивы) Лингвистика для математиков
Лингвистика для математиков Склейка карт по фотографиям
Склейка карт по фотографиям 8 - Nested loops
8 - Nested loops Сервис интерактивных упражнений – Wordwall
Сервис интерактивных упражнений – Wordwall Технологии глобальной компьютерной сети Интернет
Технологии глобальной компьютерной сети Интернет Кодирование текстовой информации
Кодирование текстовой информации Компас
Компас Безопасность детей в сети интернет
Безопасность детей в сети интернет Представление графов. Топологическая сортировка
Представление графов. Топологическая сортировка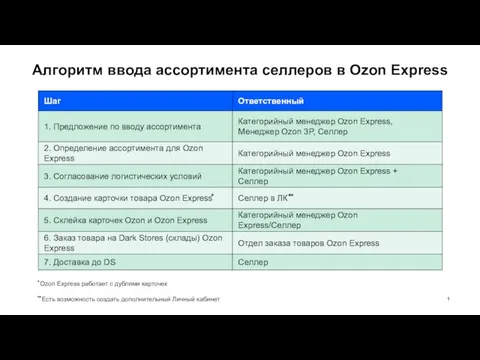 Алгоритм ввода ассортимента селлеров в Ozon Express
Алгоритм ввода ассортимента селлеров в Ozon Express