processes of their deactivation which are result of interaction of the excited molecules with system components. An exit of a luminescence is very sensitive to various intramolecular and intermolecular interactions which cause his reduction and lead to develop processes of quenching of a luminescence. Are among the most active quenchers of a luminescence:
- heavy anions and cations of I− , Br− , Cs+ , Cu2+ (at the same time S1 → by T1 transition is facilitated);
- paramagnetic ions and molecules O2, Mn2+ , nitroxyl radicals;
- solvent molecules. Usually polar solvents, such as water possess the greatest extinguishing action;
- acceptors of electronic energy of excitement.
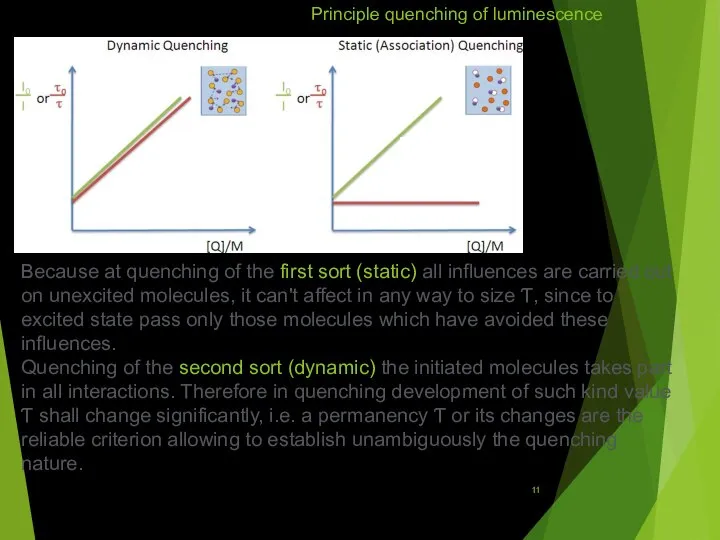
Principle quenching of luminescence








 Hidden Word (Wild animals)
Hidden Word (Wild animals) Speaking about how people changed
Speaking about how people changed Курс по изучению английских слов
Курс по изучению английских слов Jerry's door
Jerry's door Student family
Student family РАСПОЛОЖЕНИЕ АРТИКЛЕЙ
РАСПОЛОЖЕНИЕ АРТИКЛЕЙ Article partitif
Article partitif Special question
Special question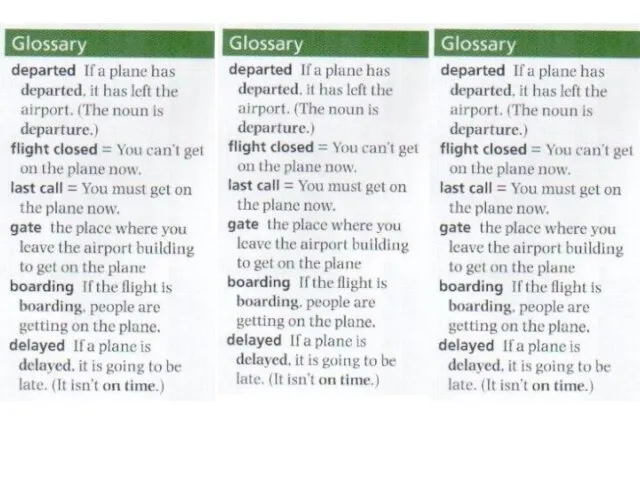 Adults. Lesson 55
Adults. Lesson 55 Welcome to the course!
Welcome to the course! Презентация на тему Cats
Презентация на тему Cats  The origin of phraseological units in the English language
The origin of phraseological units in the English language Letter Aa (Oxford Phonics World)
Letter Aa (Oxford Phonics World) Daily life
Daily life SpeakOut
SpeakOut What is the possible use of pictures at English lessons?
What is the possible use of pictures at English lessons? Open vs closed systems: the growth needs of humanity in the 21st century and beyond
Open vs closed systems: the growth needs of humanity in the 21st century and beyond English Quiz. 6 класс
English Quiz. 6 класс Лондон - столица Великобритании
Лондон - столица Великобритании Modal verbs
Modal verbs Презентация на тему New York City Attractions (Достопримечательности Нью-Йорка)
Презентация на тему New York City Attractions (Достопримечательности Нью-Йорка)  Module 8. Unit 16a
Module 8. Unit 16a We explain the way. Dialogue speech
We explain the way. Dialogue speech t = [ ʃ ] В сочетании t + суффикс типа -ion : t=[ʃ]
t = [ ʃ ] В сочетании t + суффикс типа -ion : t=[ʃ] Opposites. Quiz
Opposites. Quiz VLAN Virtual Local Area Network
VLAN Virtual Local Area Network Complex Object
Complex Object My imagine of Great Britain
My imagine of Great Britain