Содержание
- 2. Chemistry has always existed. The formation of the Earth and the development of life involved many
- 3. Sheltering Medicine Clothing Protection
- 4. After producing fire, ancient men started to construct tools to make their lives easier. They made:
- 5. An Alchemy Recap The People, Places and Discoveries
- 6. What is Alchemy? ( key words: philosophy – goals- cure- diseases- prolonging – infinitely) ( A
- 7. The Goals of Alchemy Philosopher’s Stone: a stone to make everything gold.( A tool that would
- 8. Alchemists used trial and error method. Alchemists didn’t use experimentation method. Alchemy is only a mystical
- 9. Although alchemy is not considered a science, alchemists were the first chemists. Their subscription in the
- 10. 3. They discovered some elements such as mercury, lead and antimony. 4. They discovered gun powder,
- 11. How It All Began A very brief timeline Greek Philosophy Egyptian Science Chinese Alchemy Arabic Alchemy
- 12. Empedocles (around 450 BC) A Greek philosopher. He defined elements as the basic building blocks from
- 13. Democritus (460-370 BC) A Greek philosopher Theory of Matter – all matter is made up of
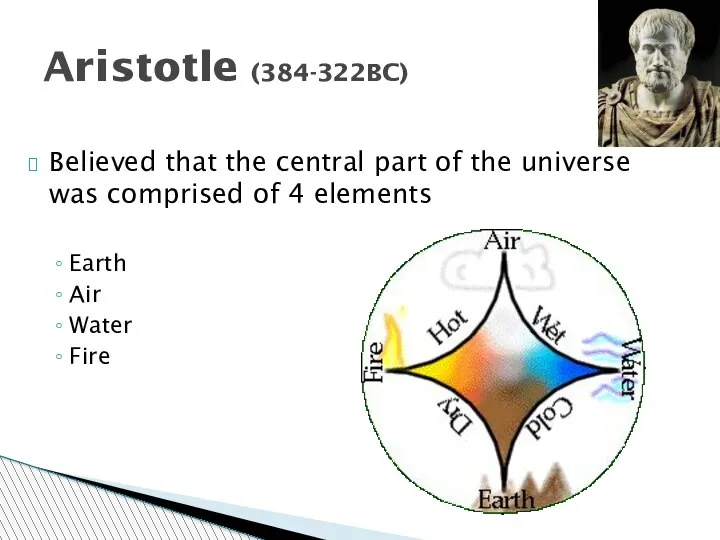
- 14. Aristotle (384-322BC) Believed that the central part of the universe was comprised of 4 elements Earth
- 15. Aristotle According to Aristotle, matter was composed of four elements: earth, fire, air and water. He
- 16. Aristotle Aristotle’s theory ruled for 2000 years because: It was comprehensive It was based on common
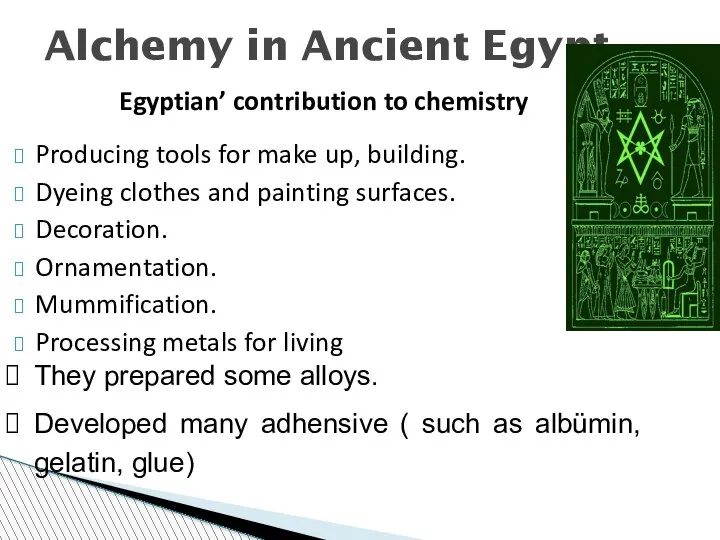
- 17. Alchemy in Ancient Egypt Egyptian’ contribution to chemistry Producing tools for make up, building. Dyeing clothes
- 18. In the Hellenistic (primary Greek or Roman) cultures, there are also some practices for alchemy and
- 19. Chinese Alchemy Its main focus was medicine Black Powder (greatest contribution – achieved) used in fireworks
- 20. Arabic Alchemy Arabic alchemy was dominated by Jabir Ibn Hayyan (Geber) and Al-Razi
- 21. Jabir Ibn Hayyan & Al- Razi Born in 721, was either Arab or Persian. He was
- 22. Al – Razi wrote two books outlining his views of matter, equipment, tools and chemical operations
- 23. Ibn Sina: He was concentrated on medicine. He developed many healing methods with different drugs. In
- 24. EL - BIRUNI
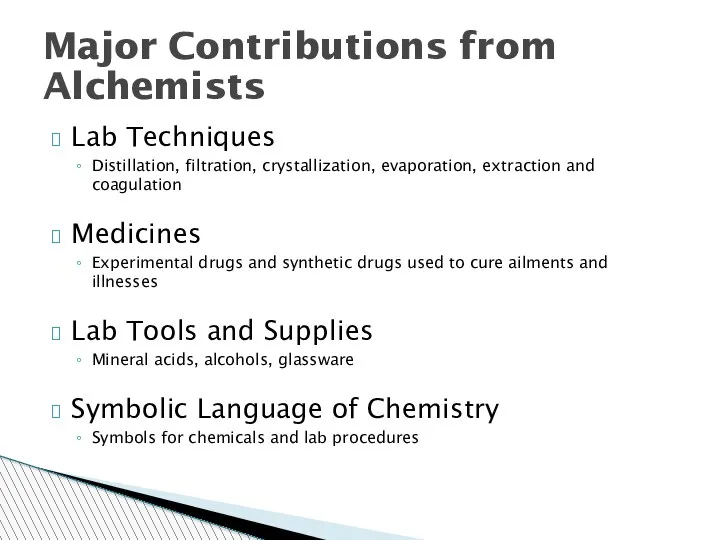
- 25. Major Contributions from Alchemists Lab Techniques Distillation, filtration, crystallization, evaporation, extraction and coagulation Medicines Experimental drugs
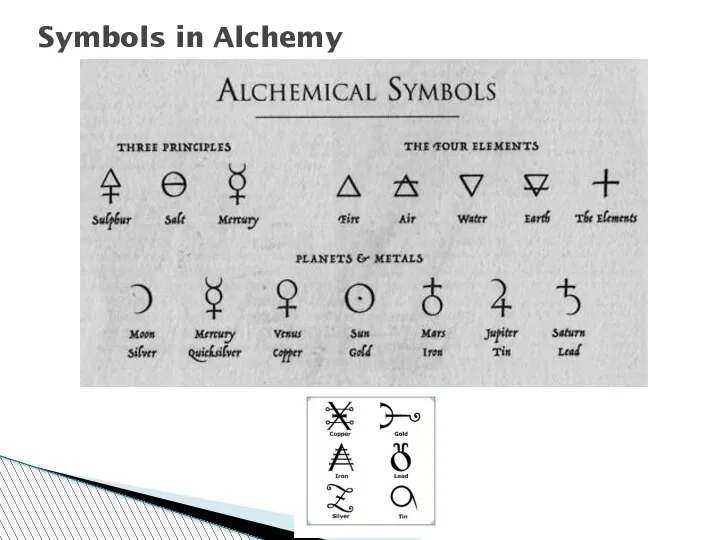
- 26. Symbols in Alchemy
- 27. “Stop making gold,” he taught “instead find medicines.” “discover new medicines rather than making gold” Paracelsus
- 28. Robert Boyle1627 - 1691) Robert Boyle redefined an element as “a substance that could not be
- 29. He believed that mass was conserved through chemical reactions The Law of Conservation of Mass Discovered
- 30. What is chemistry?
- 31. 2.1 The Fundamental Disciplines of Chemistry Analytical chemistry is a branch of chemistry which performs analysis,
- 32. Polymer chemistry is a discipline that deals with long chemical chains. These long chemical chains are
- 33. https://quizlet.com/6483467/six-major-branches-of-chemistry-flash-cards/ Six major branches of Chemistry
- 34. 2.2 Application Areas of Chemistry Disciplines( chemistry at work) Chemistry in Fertilizer Processing A fertilizer is
- 35. Chemistry in Processing of Hardwood Wood is composed of cellulose and once raw wood is obtained,
- 36. Chemistry in Textile-Dyeing Process Textile chemistry: Textile chemistry is a highly specialized field that applies the
- 37. OTHER USES OF CHEMISTRY Chemistry is also used for detecting the doping materials in the body
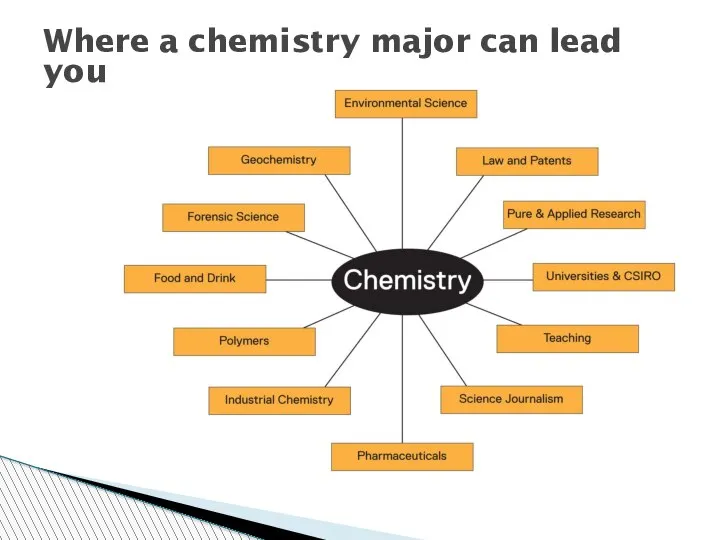
- 38. Where a chemistry major can lead you
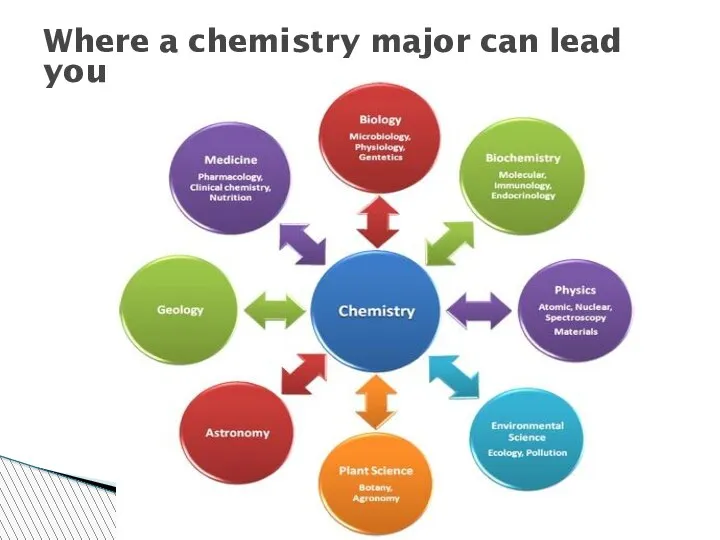
- 39. Where a chemistry major can lead you
- 40. CHEMISTRY RELATED OCCUPATIONS Chemical Engineering: Chemical engineering is all about turning raw materials into useful, everyday
- 41. Chemist: A chemist is a scientist who researches and experiments with the properties of chemical substances.
- 42. Metallurgical Engineering: Metallurgical engineering involves the study, innovation, design, implementation, and improvement of processes that transform
- 43. Pharmacology: Pharmacology is the science of drug action on biological systems. It involves chemical properties, biological
- 44. Chemistry Teacher: A chemistry teacher teaches high school students about chemicals. chemistry teachers facilitate student learning
- 45. Do we use any symbols in our life? Why do we need symbols? Why do we
- 46. ELEMENTS AND COMPOUNDS The Historical Development of the Symbolic Language of Chemistry The modern symbols used
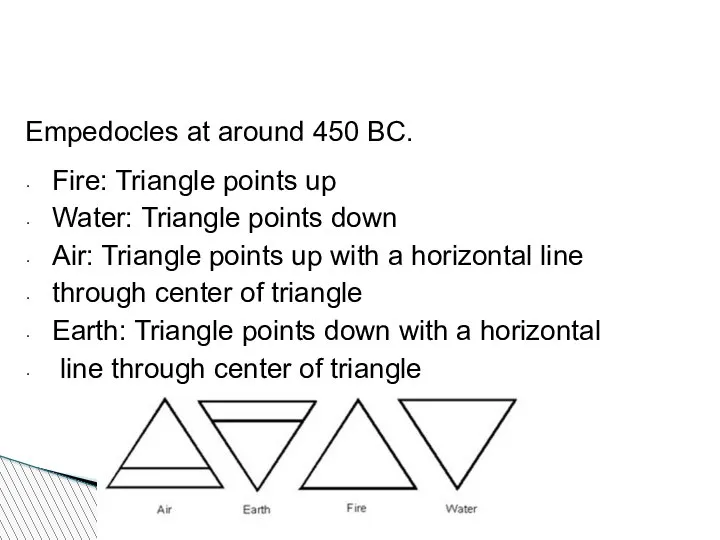
- 47. Empedocles at around 450 BC. Fire: Triangle points up Water: Triangle points down Air: Triangle points
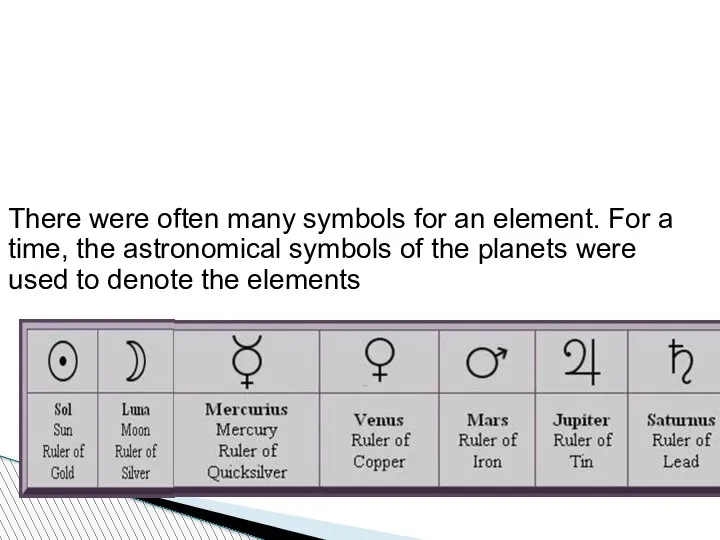
- 48. There were often many symbols for an element. For a time, the astronomical symbols of the
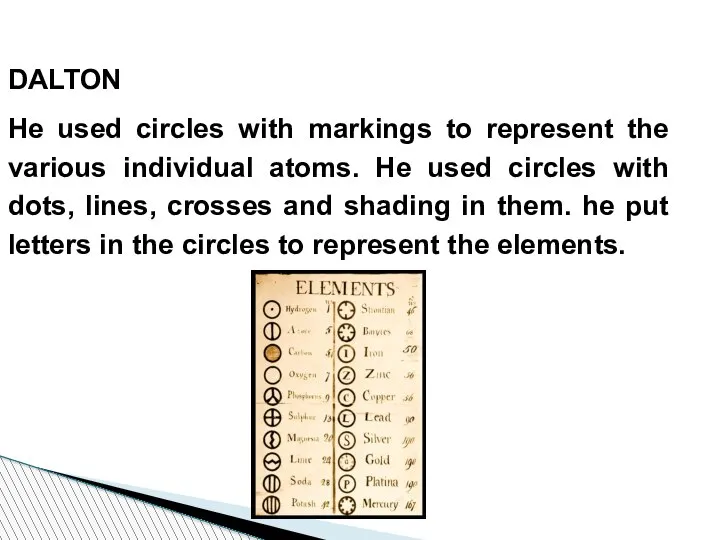
- 49. DALTON He used circles with markings to represent the various individual atoms. He used circles with
- 50. About ten years later, in Sweden, Berzelius suggested just using letters to represent atoms of each
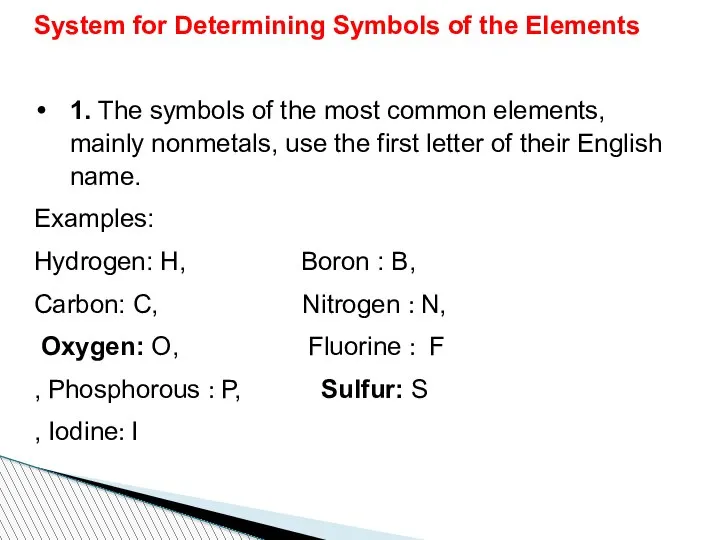
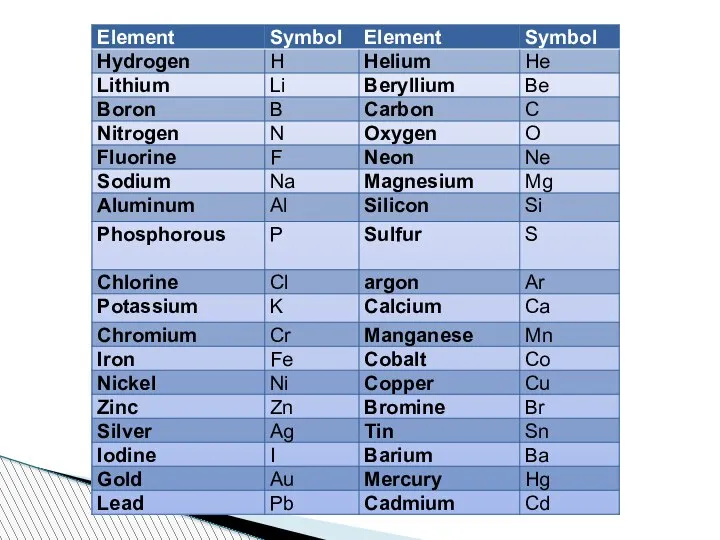
- 51. System for Determining Symbols of the Elements 1. The symbols of the most common elements, mainly
- 52. 2. If the name of the element has the same initial letter as another element, then
- 53. 3. If the first two letters of the element name are the same as another element,
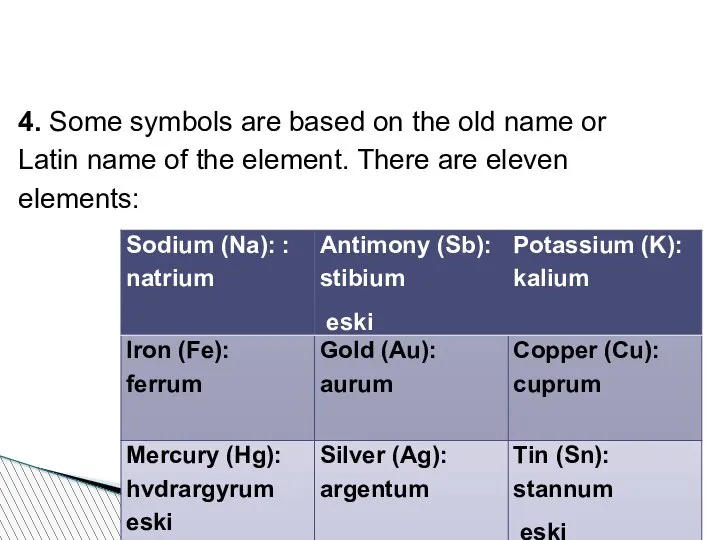
- 54. 4. Some symbols are based on the old name or Latin name of the element. There
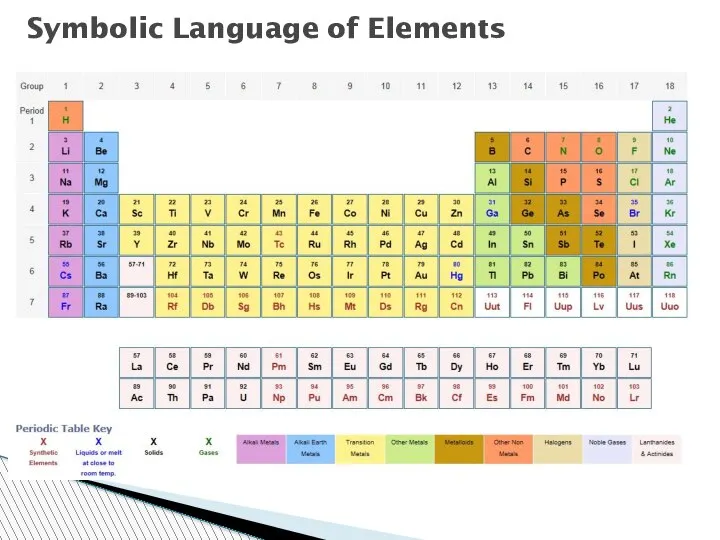
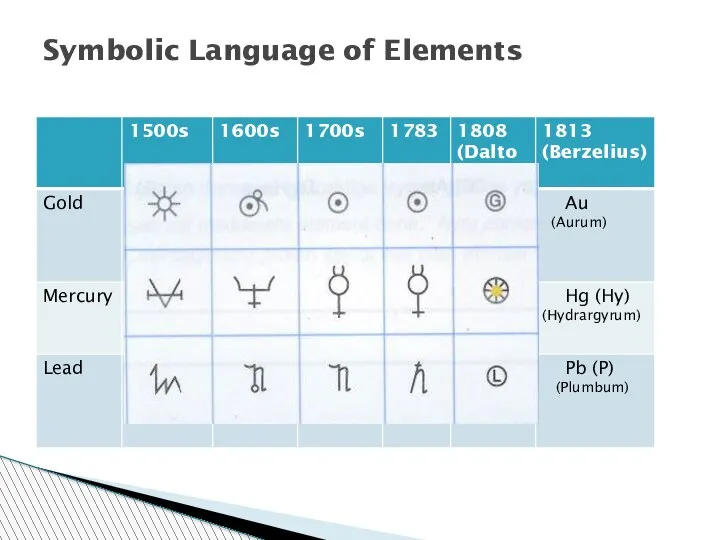
- 55. Symbolic Language of Elements
- 56. 2 Elements and Symbols of Elements All substances are made up of matter and the fundamental
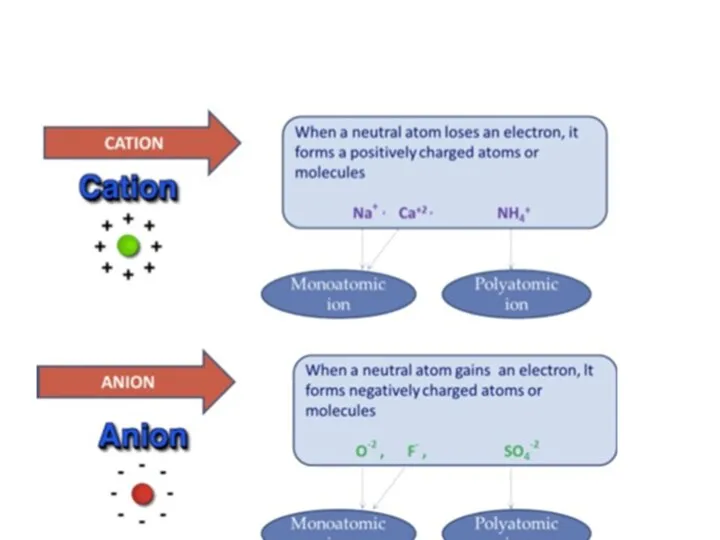
- 57. An element is a substance made up of atoms of one kind. An element: consists of
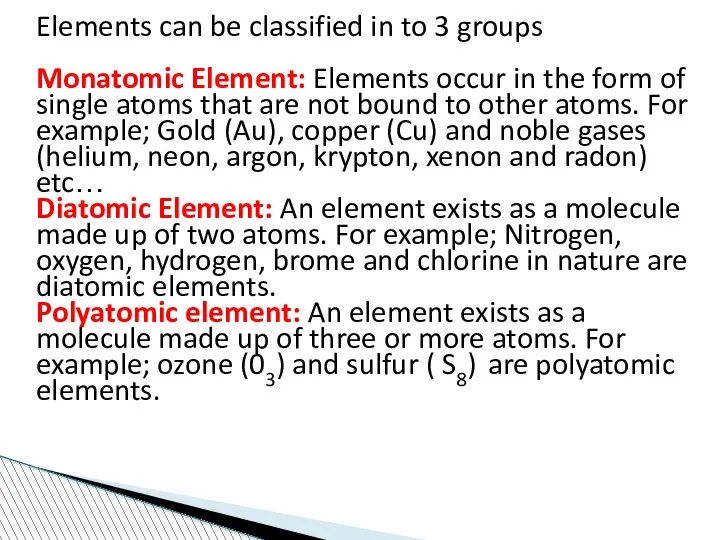
- 58. Elements can be classified in to 3 groups Monatomic Element: Elements occur in the form of
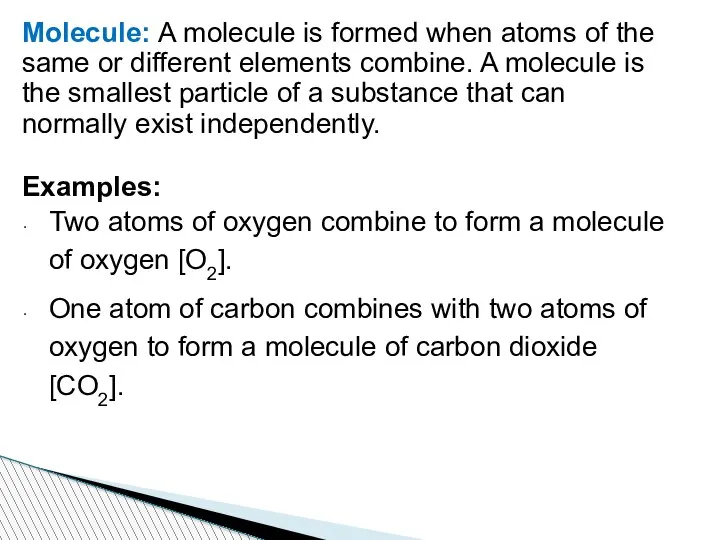
- 60. Molecule: A molecule is formed when atoms of the same or different elements combine. A molecule
- 61. A compound is a pure substance formed when two or more chemical elements are chemically bonded
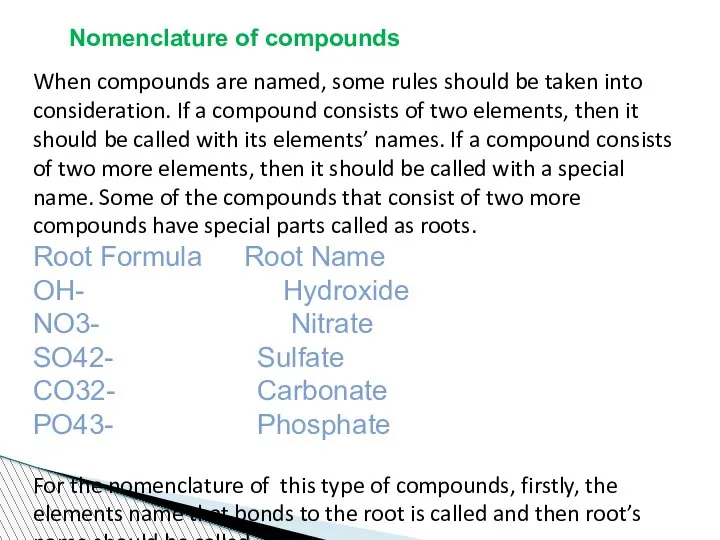
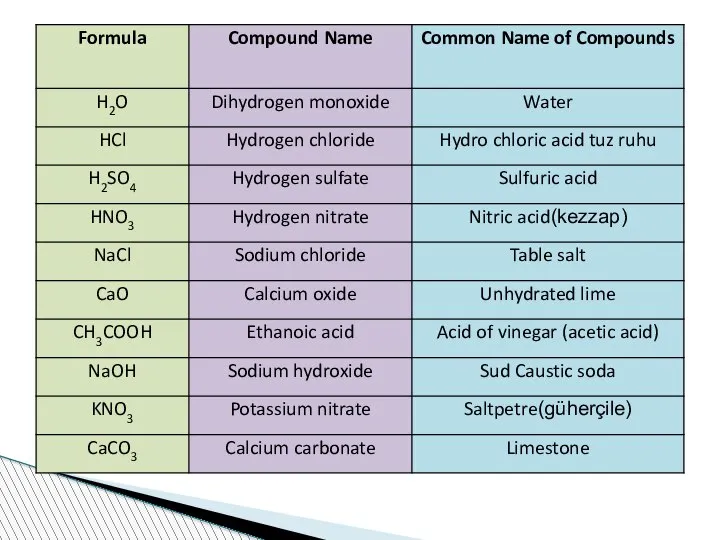
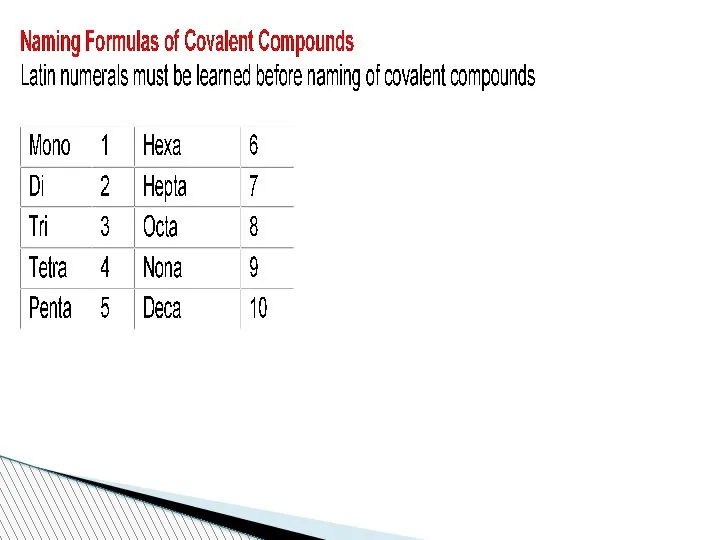
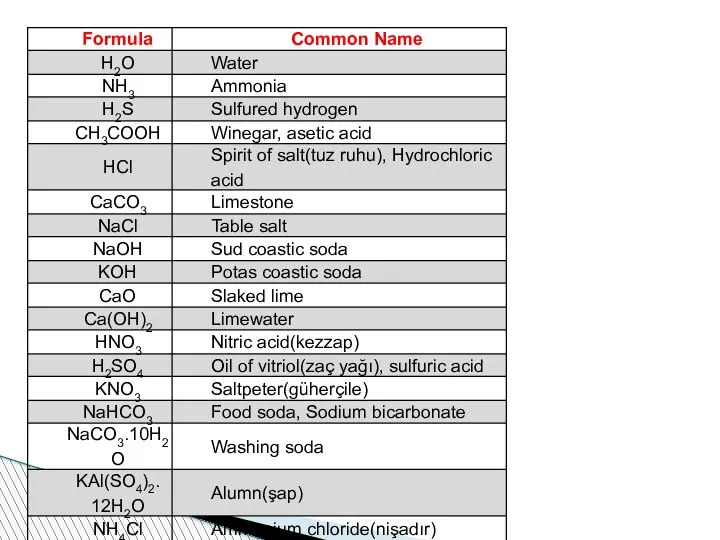
- 62. Nomenclature of compounds When compounds are named, some rules should be taken into consideration. If a
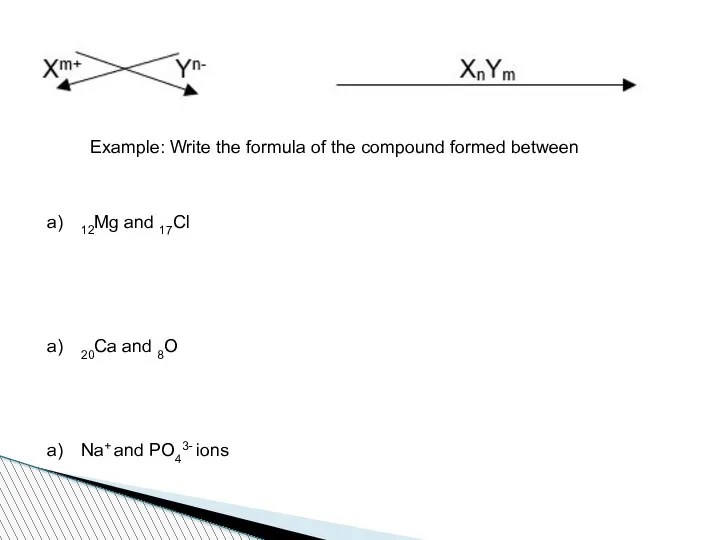
- 64. Writing Formulas of Compounds General Rules 1. Firstly, the symbol of the cation (metal) is written
- 65. Example: Write the formula of the compound formed between 12Mg and 17Cl 20Ca and 8O Na+
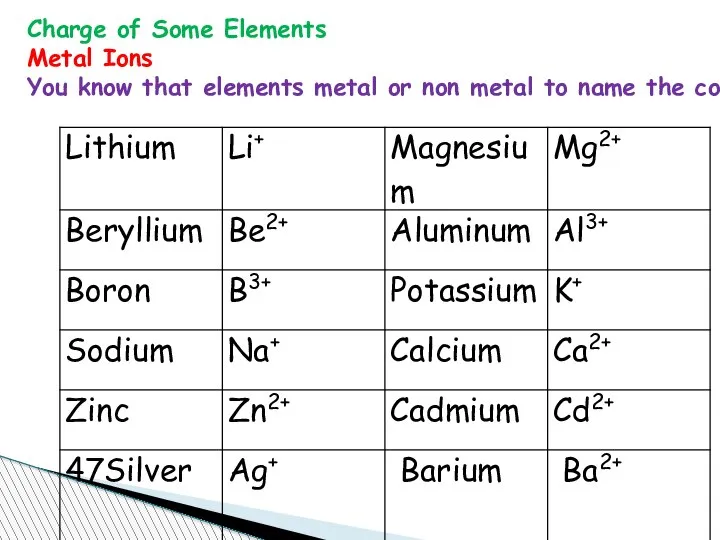
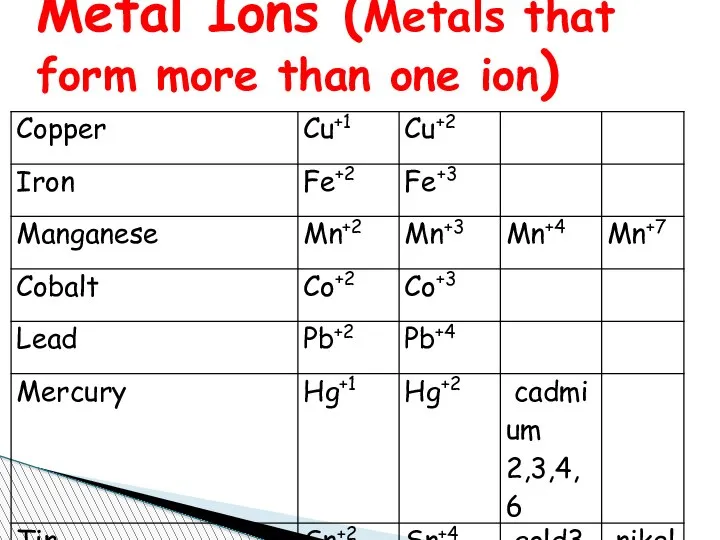
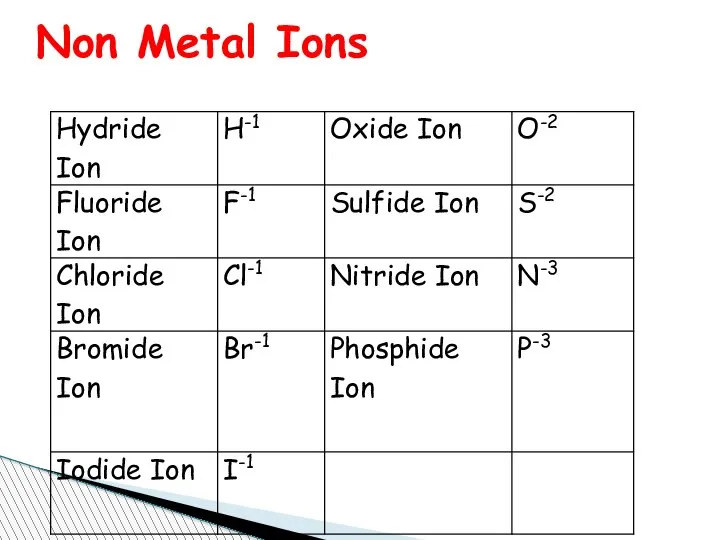
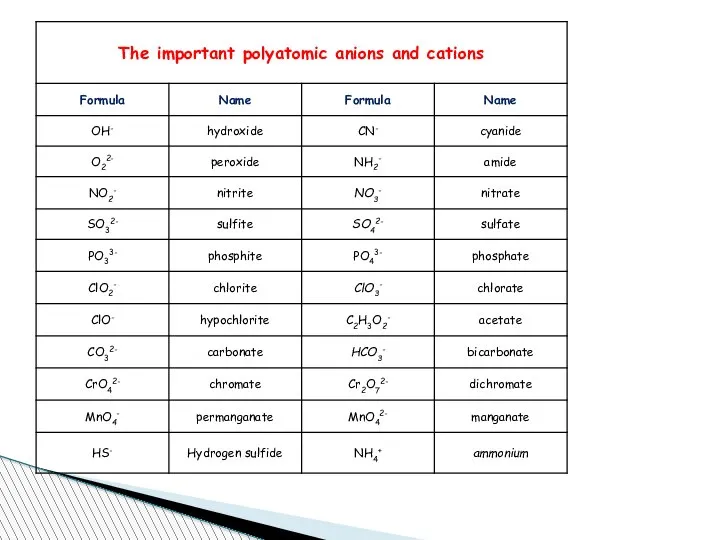
- 67. Charge of Some Elements Metal Ions You know that elements metal or non metal to name
- 68. Metal Ions (Metals that form more than one ion)
- 69. Non Metal Ions
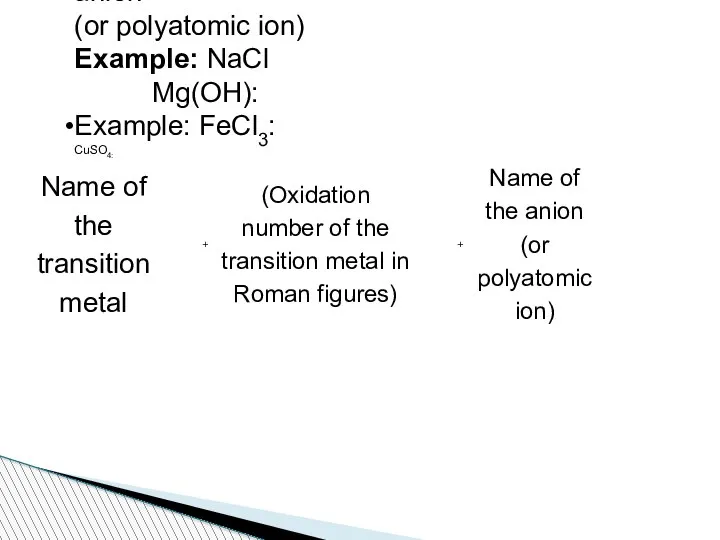
- 71. Naming Formulas of Ionic Compounds Name of the metal (cation) + Name of the anion (or
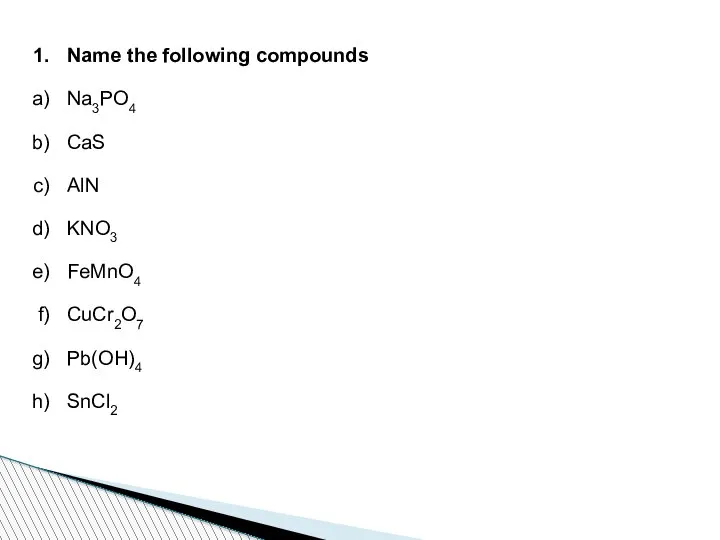
- 72. Name the following compounds Na3PO4 CaS AlN KNO3 FeMnO4 CuCr2O7 Pb(OH)4 SnCl2
- 73. Write the formula of the following ionic compounds Sodium nitrate Copper (II) hydroxide Calcium phosphate Ammonium
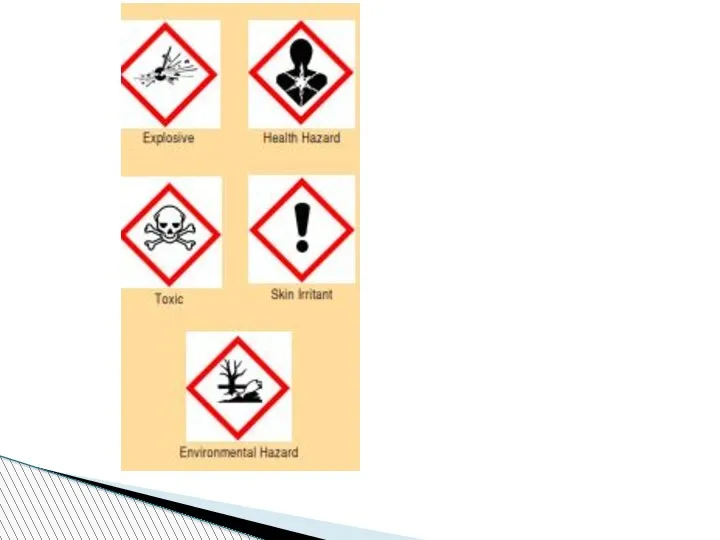
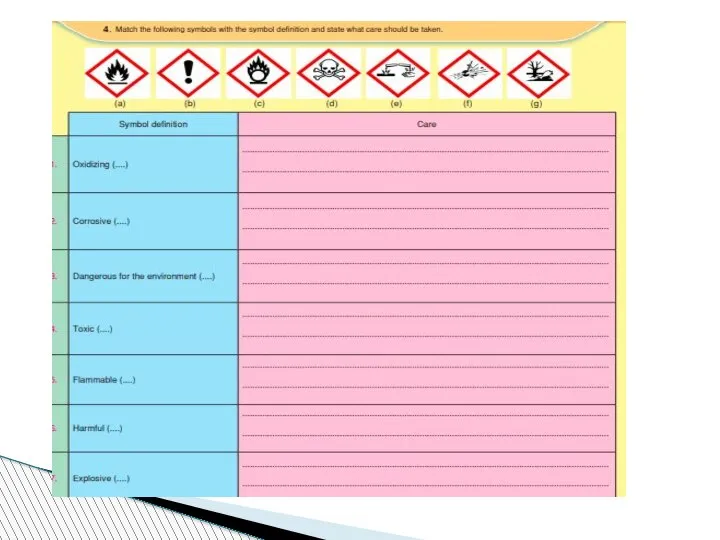
- 74. SAFETY IN THE CHEMISTRY LABORATORY 1. Always wear goggles, gloves apron for safety. 2. Never reach
- 75. 6. Never smell a chemical directly from the container. Wave your hand over the opening of
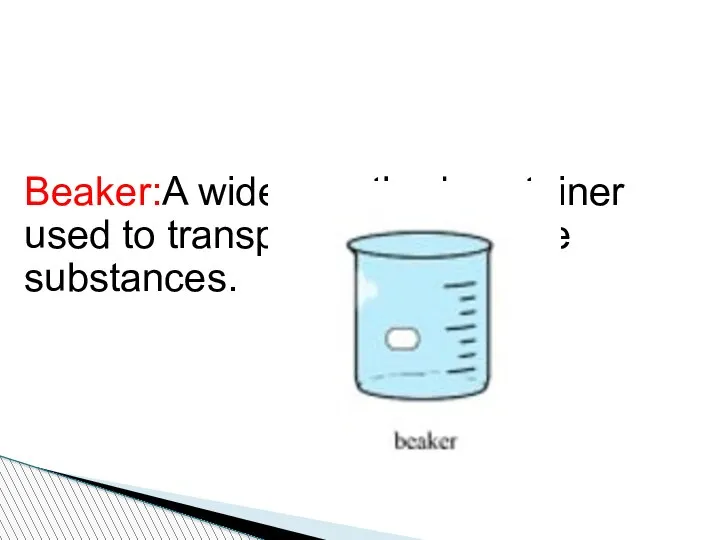
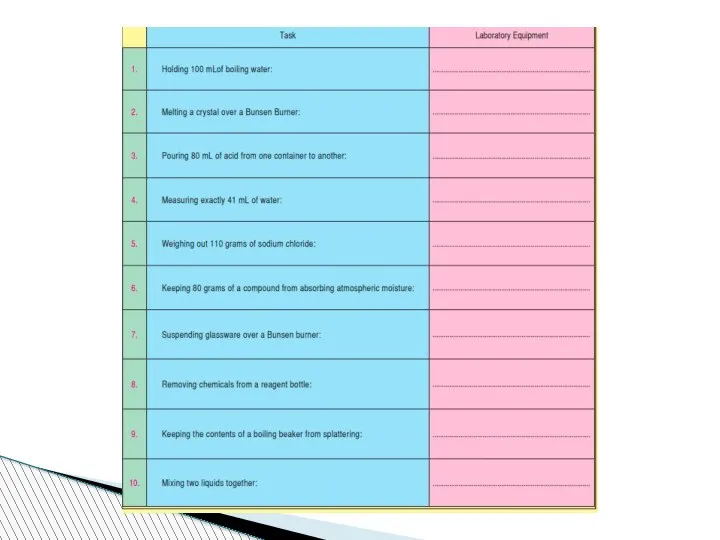
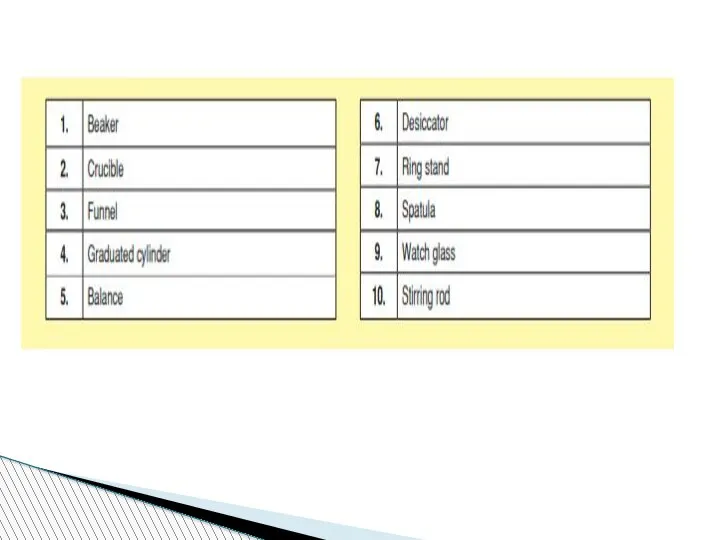
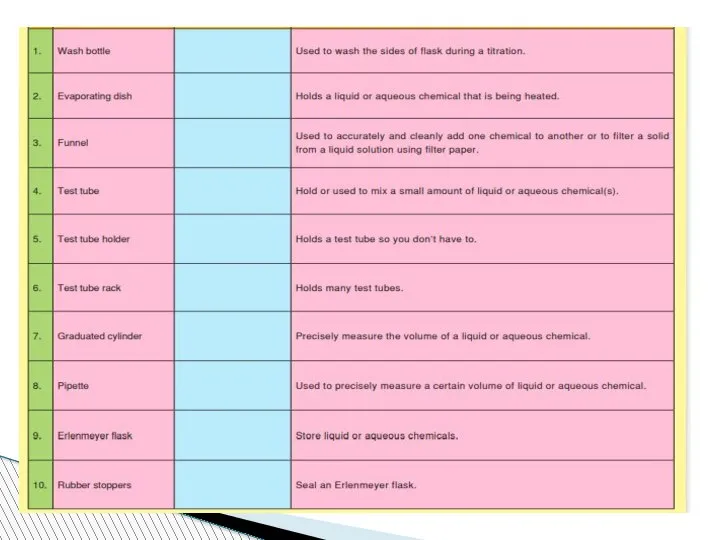
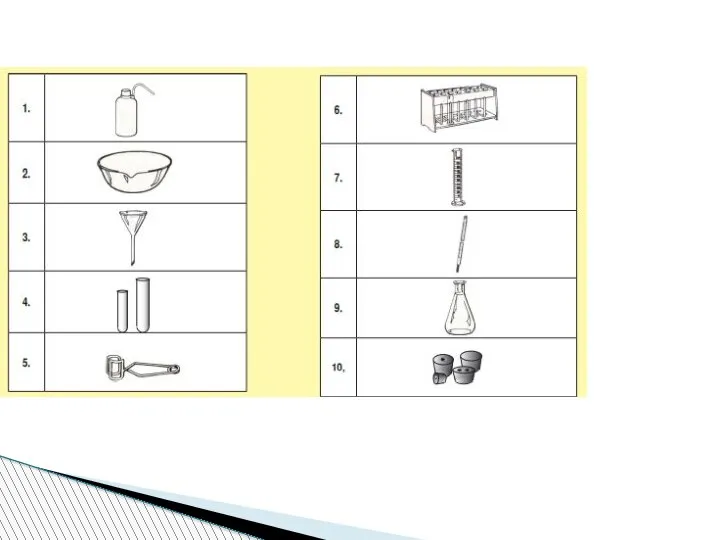
- 78. Beaker:A wide-mouthed container used to transport, heat or store substances.
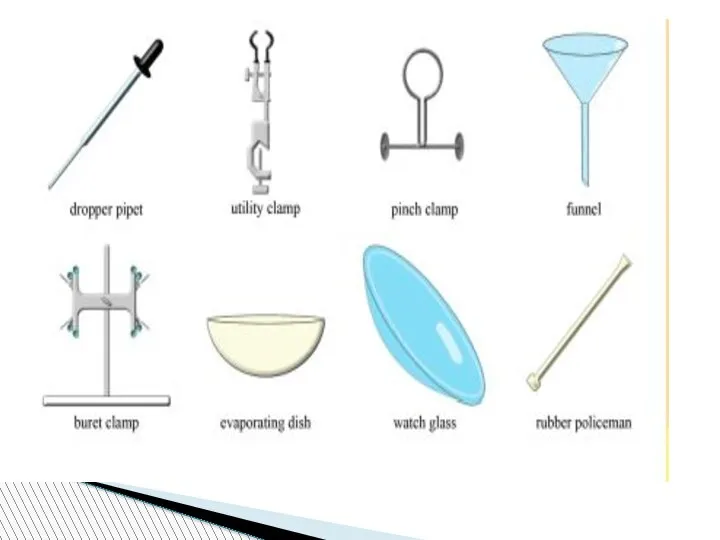
- 79. Erlenmeyer Flask : A narrow-mouthed container used to transport, heat or store substances, often used when
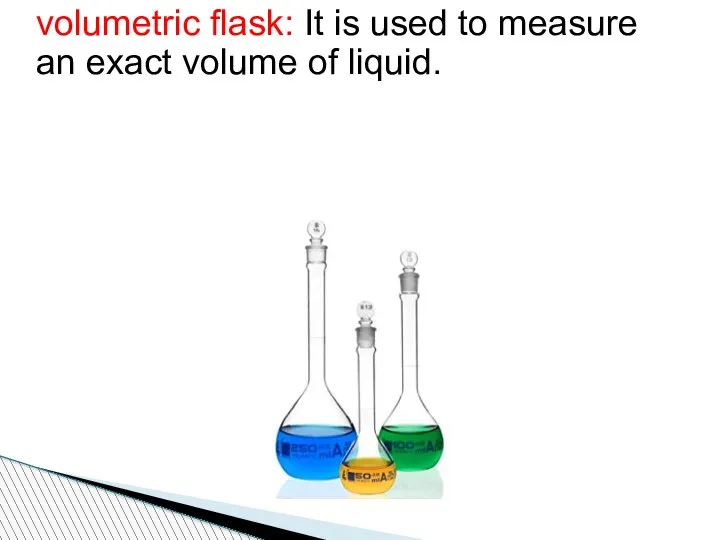
- 80. volumetric flask: It is used to measure an exact volume of liquid.
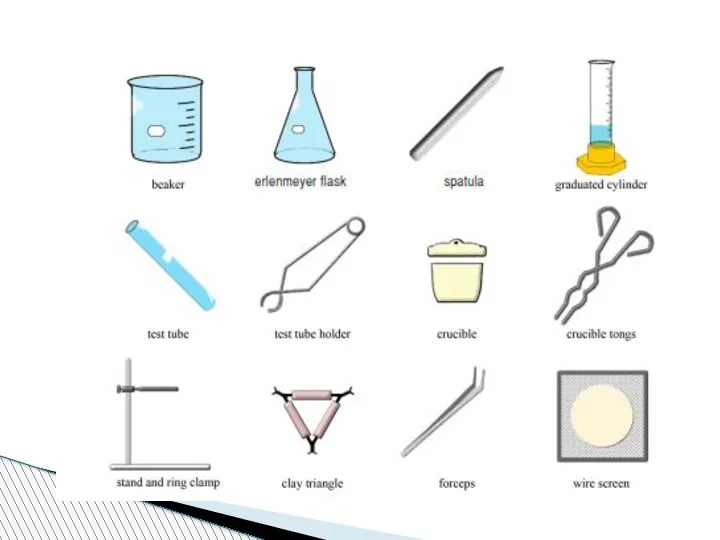
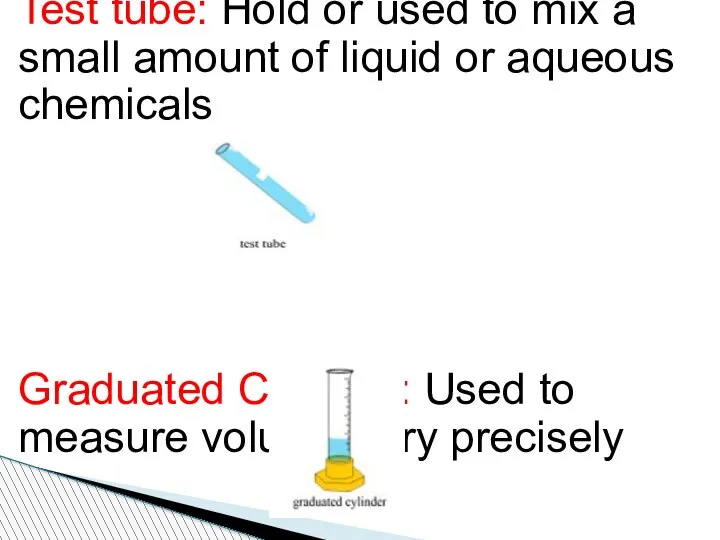
- 81. Test tube: Hold or used to mix a small amount of liquid or aqueous chemicals Graduated
- 82. Test tube holder: Holds a test tube so you don’t have to. Test tube rack: Holds
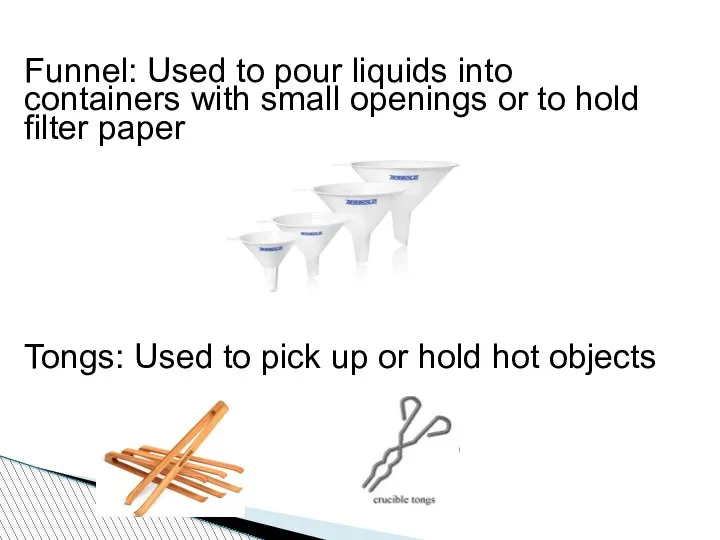
- 83. Funnel: Used to pour liquids into containers with small openings or to hold filter paper Tongs:
- 84. Triple Beam Balance: A device to measure the mass of an object or substance. .
- 85. Wacth glass:They can be used for evaporation purposes and also can function as a lid for
- 86. Spatulas and scoopulas : they are for scooping solid chemicals.
- 87. Striker: Used to light a Bunsen burner. Bunsen Burner: Used to heat objects
- 88. Ring Clamp: Attaches to a lab stand and used to hold a variety of lab equipment
- 89. Rubber stoppers: Seal an Erlenmeyer flask.
- 90. Evaporating dish: Holds a liquid or aqueous chemical that is being heated. Wash bottle: Used to
- 91. Lab Coat or Apron: Protects the scientist and the scientist’s clothes from hazardous or hot chemicals
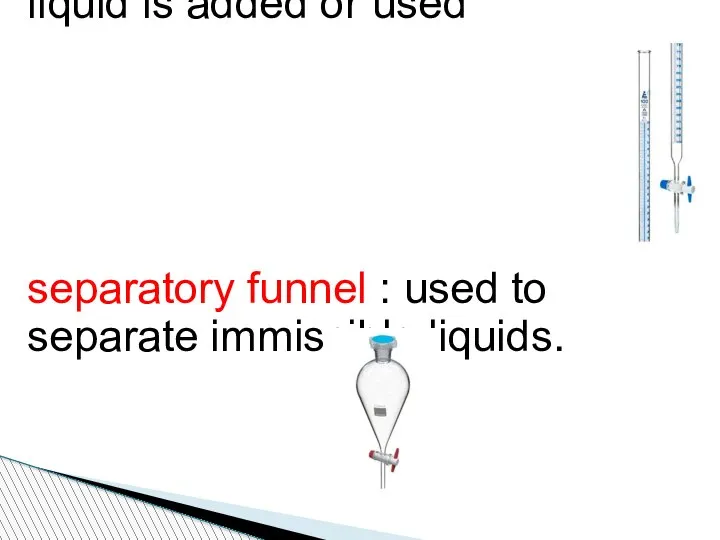
- 92. Burette: To determine how much liquid is added or used separatory funnel : used to separate
- 108. Symbolic Language of Elements
- 110. Скачать презентацию






















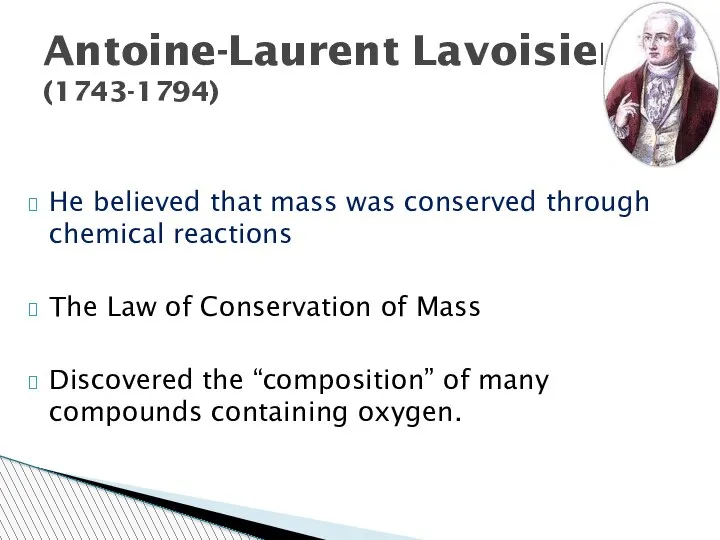




































 Too or enough
Too or enough Letters I and T
Letters I and T School things
School things Project. Endangered Species. Вымирающие животные
Project. Endangered Species. Вымирающие животные Twin towers
Twin towers The Merry Wives of Windsor
The Merry Wives of Windsor Презентация на тему Albert Einstein - Альберт Эйнштейн
Презентация на тему Albert Einstein - Альберт Эйнштейн  Презентация на тему Present Progressive
Презентация на тему Present Progressive  Likes Dislikes
Likes Dislikes Holidays in Britain
Holidays in Britain Have or has?
Have or has? Grammar “Future Tenses”
Grammar “Future Tenses” Читалка. Приложение к фонетическому курсу Честный английский
Читалка. Приложение к фонетическому курсу Честный английский Count from 1 to 5
Count from 1 to 5 Презентация на тему Westminster Abbey (Вестминстерское Аббатство)
Презентация на тему Westminster Abbey (Вестминстерское Аббатство)  Написание письма
Написание письма Grammar Check: Passive
Grammar Check: Passive Презентация на тему Noughts and crosses (Крестики – нолики)
Презентация на тему Noughts and crosses (Крестики – нолики)  Rainbow English game
Rainbow English game Interactive. English letter environment
Interactive. English letter environment Agree or disagree. Dialogs
Agree or disagree. Dialogs City/Town or Village
City/Town or Village Talented Kingdom
Talented Kingdom Презентация по английскому Жизнь на моей планете. Выполнила Садыкова Диана ученица 5 э класса.
Презентация по английскому Жизнь на моей планете. Выполнила Садыкова Диана ученица 5 э класса. Организация работы учителей иностранного языка по подготовке школьников 9-11(12) классов к ГИА
Организация работы учителей иностранного языка по подготовке школьников 9-11(12) классов к ГИА Members of the International
Members of the International Article partitif
Article partitif Презентация на тему Употребление определенного артикля the
Презентация на тему Употребление определенного артикля the