Содержание
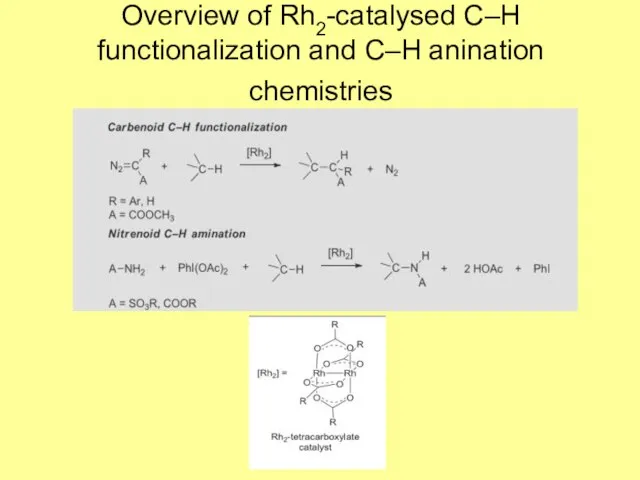
- 2. Overview of Rh2-catalysed C–H functionalization and C–H anination chemistries
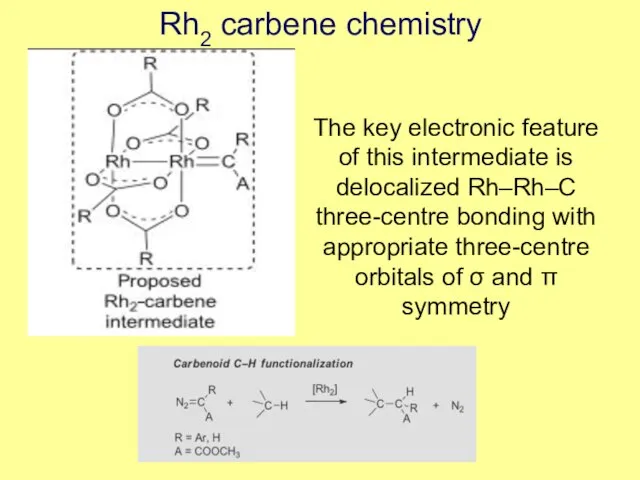
- 3. Rh2 carbene chemistry The key electronic feature of this intermediate is delocalized Rh–Rh–C three-centre bonding with
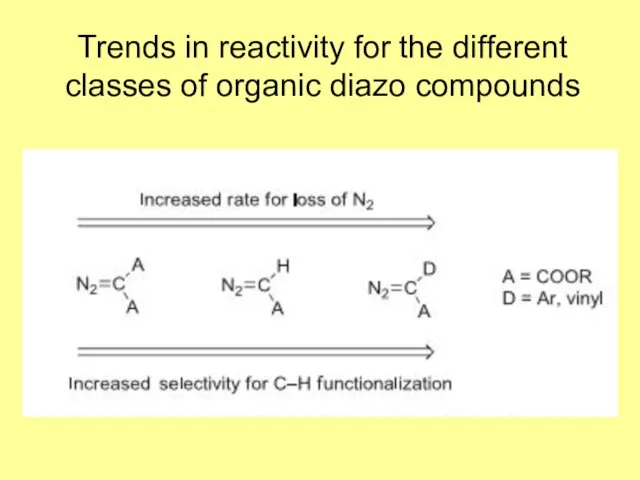
- 4. Trends in reactivity for the different classes of organic diazo compounds
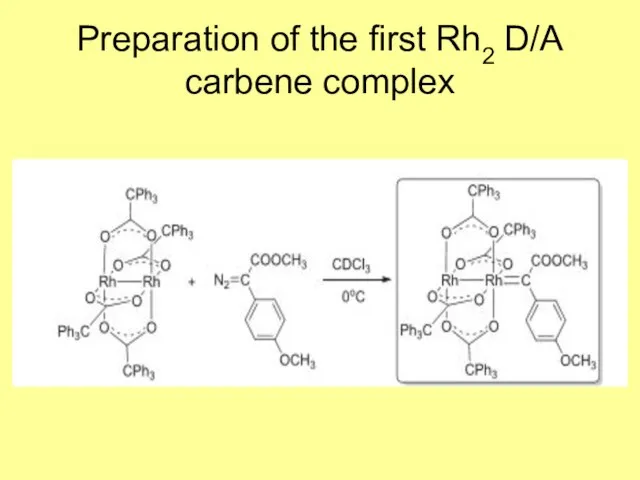
- 5. Preparation of the first Rh2 D/A carbene complex
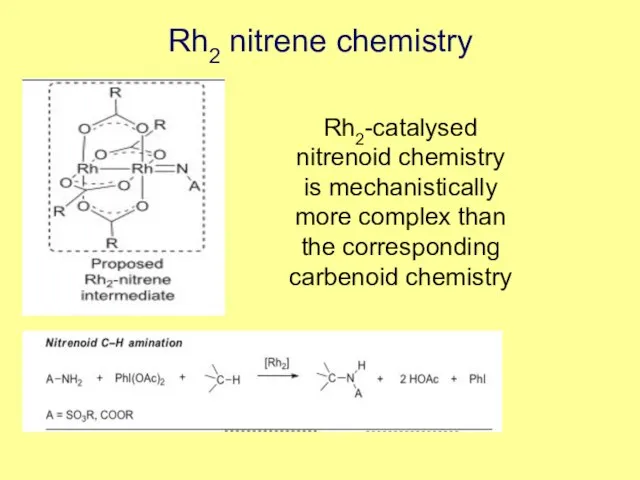
- 6. Rh2 nitrene chemistry Rh2-catalysed nitrenoid chemistry is mechanistically more complex than the corresponding carbenoid chemistry
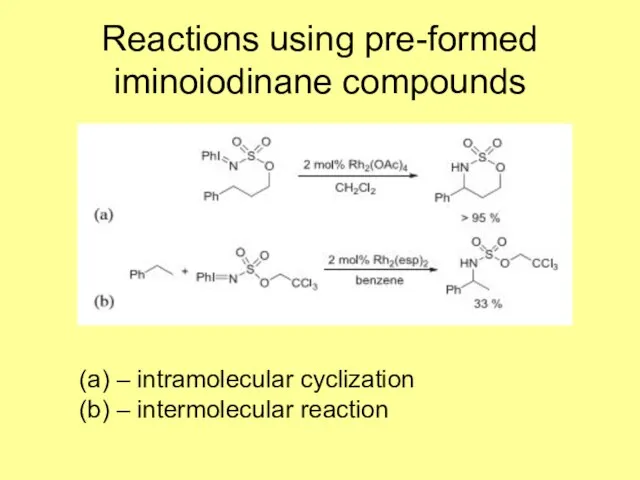
- 7. Reactions using pre-formed iminoiodinane compounds (a) – intramolecular cyclization (b) – intermolecular reaction
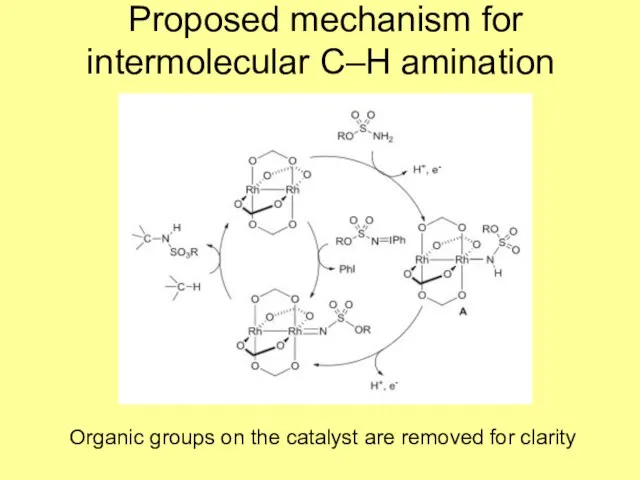
- 8. Proposed mechanism for intermolecular C–H amination Organic groups on the catalyst are removed for clarity
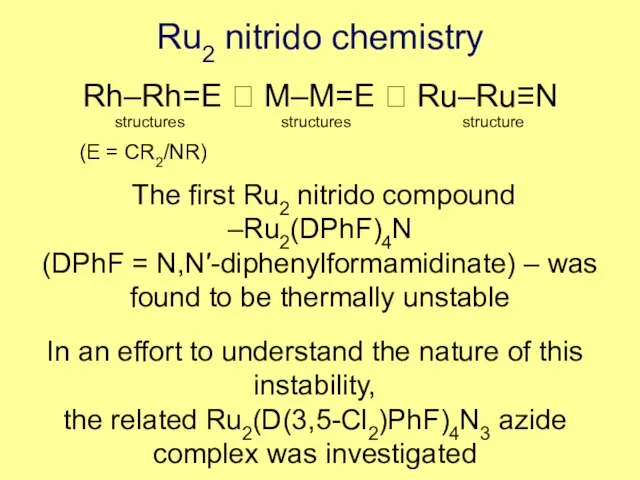
- 9. Ru2 nitrido chemistry Rh–Rh=E ? M–M=E ? Ru–Ru≡N structures structures structure (E = CR2/NR) The first
- 10. Crystal structure of Ru2[(D(3,5-Cl2)PhF)3(D(3,5-Cl2-2-NH)PhF)]
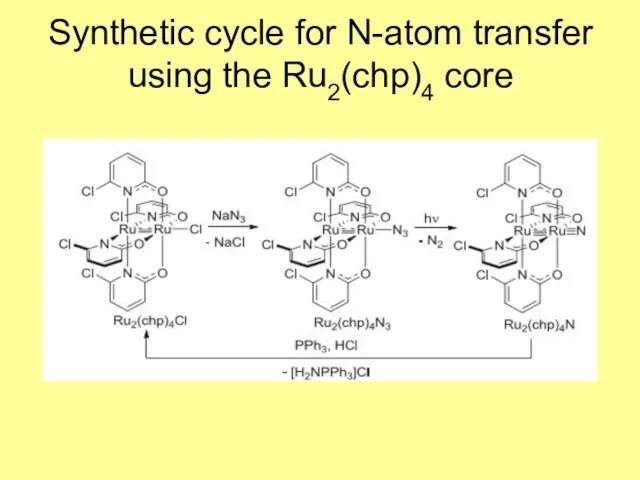
- 11. Synthetic cycle for N-atom transfer using the Ru2(chp)4 core
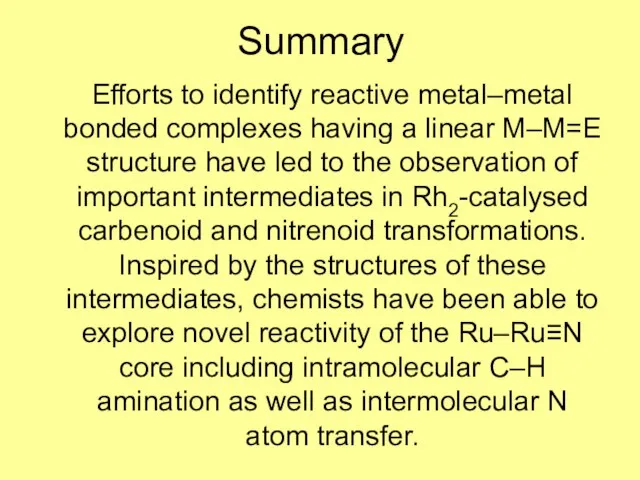
- 12. Summary Efforts to identify reactive metal–metal bonded complexes having a linear M–M=E structure have led to
- 14. Скачать презентацию
![Crystal structure of Ru2[(D(3,5-Cl2)PhF)3(D(3,5-Cl2-2-NH)PhF)]](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/377408/slide-9.jpg)
 New Year Merry Christmas
New Year Merry Christmas Компания DominiSoft www.dominisoft.ru Компания “БУКА” www.buka.ru www.bukasoft.ru
Компания DominiSoft www.dominisoft.ru Компания “БУКА” www.buka.ru www.bukasoft.ru ЗДОРОВЬЕСБЕРЕГАЮЩИЕ ТЕХНОЛОГИИ, КАК ВЗАИМОДЕЙСТВИЕ УЧЕНИКА И УЧИТЕЛЯ.
ЗДОРОВЬЕСБЕРЕГАЮЩИЕ ТЕХНОЛОГИИ, КАК ВЗАИМОДЕЙСТВИЕ УЧЕНИКА И УЧИТЕЛЯ. Презентация на тему природа коми края
Презентация на тему природа коми края Синдром эмоционального выгорания
Синдром эмоционального выгорания Система образования и проблемы её структуры
Система образования и проблемы её структуры Волейбол. 5-7 классы
Волейбол. 5-7 классы Война — жесточе нету слова,Война — печальней нету слова. Война — святее нету слова В тоске и славе этих лет.И на устах у нас иного
Война — жесточе нету слова,Война — печальней нету слова. Война — святее нету слова В тоске и славе этих лет.И на устах у нас иного Евгений Львович Шварц (1896-1958)
Евгений Львович Шварц (1896-1958) Водоёмы нашей местности
Водоёмы нашей местности Фармакоэпидемиологические и фармакоэкономические аспекты нежелательных реакций лекарственных препаратов – неявная проблема и
Фармакоэпидемиологические и фармакоэкономические аспекты нежелательных реакций лекарственных препаратов – неявная проблема и  Зиянкестердің экономикалық шекті. Зиянын табиғи жауларының тиімділігінің деңгейін анықтау. (Лекция 15)
Зиянкестердің экономикалық шекті. Зиянын табиғи жауларының тиімділігінің деңгейін анықтау. (Лекция 15) Séjour d’études linguistiques Découverte de la Provence
Séjour d’études linguistiques Découverte de la Provence Символи України. Національний український одяг
Символи України. Національний український одяг Законы развития теории судебной экспертизы
Законы развития теории судебной экспертизы Командный проект. Бизнес-задача
Командный проект. Бизнес-задача Взаимодействие с заинтересованными сторонами при подготовке интегрированных отчетов
Взаимодействие с заинтересованными сторонами при подготовке интегрированных отчетов Экономить - значит зарабатывать! Плакат для рабочих
Экономить - значит зарабатывать! Плакат для рабочих Что такое деятельность
Что такое деятельность Микеланджело
Микеланджело  Лучший центр. Белорецк. Скалодром
Лучший центр. Белорецк. Скалодром Здоровьесберегающие технологии в образовательном процессе. Подготовил: зам.директора по УВР Павлов В.В.
Здоровьесберегающие технологии в образовательном процессе. Подготовил: зам.директора по УВР Павлов В.В. Лекция 5. Проблема психологического контроля и управления спортивной деятельностью
Лекция 5. Проблема психологического контроля и управления спортивной деятельностью Опорные схемы и рисунки в помощь учащимся
Опорные схемы и рисунки в помощь учащимся Топология компьютерных сетей
Топология компьютерных сетей Процессоры фирм Intel и AMD
Процессоры фирм Intel и AMD Стили в одежде
Стили в одежде «1С:Предприятие 8. Управление водоканалом»
«1С:Предприятие 8. Управление водоканалом»