Слайд 2You will:
CHAPTER 12.2
ALKENES

Слайд 3CHAPTER 1.1
RADIOACTIVITY
Kew terms
Operate – әрекет ету/ действовать;
Repulsion - серпу / отталкивание;
Emission - шығу / выбросы;
Dosimetrist - дозиметр / дозиметр;
Geiger counter - Гейгер санағышы / счетчик Гейгера;
Prone to-бейім / склонный к;
Decay - ыдырау / распад;
Annihilation - жойылу / уничтожение.

Слайд 4CHAPTER 1.1
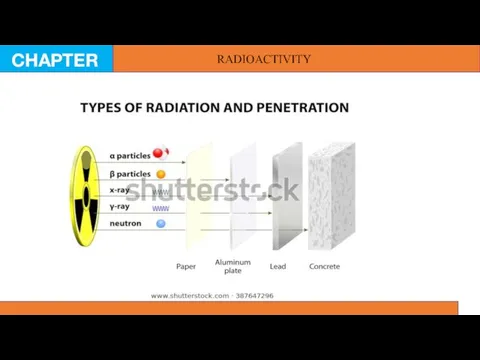
Radioactivity means a spontaneous emission of radioactive particles by an unstable

nucleus. Atoms which are prone to decay are classified as radioactive. Conversely, an isotope is considered stable if it does not spontaneously transform into another element by radioactive emission. In the late 19th century Ernest Rutherford was able to identify three common radioactive emissions which were released by radioactive atoms. He was also able to show how they behave in an electric field, which allowed him to find charges of each particle. He named them as alpha, beta and gamma radiation.
Слайд 7CHAPTER 1.1
The above reaction is called a nuclear equation, or nuclear reaction.

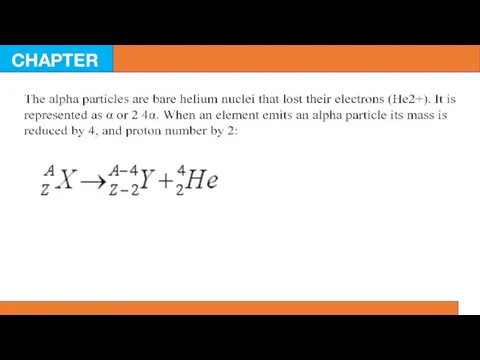
Highly energetic electrons and positrons are called beta particles or beta rays. Sometimes positrons are termed antielectrons, because they have similar mass like an electron, but they are positively charged. Beta rays are represented as βfor an electron, and β+ for a positron. If they collide with each other, they are annihilated and energy is produced in the form of gamma rays. When an electron is emitted from a nucleus, it transforms a neutron to a proton. Conversely, positron emission results in the transformation of proton to a neutron.




 Laminated group info + attendance records+students’ records
Laminated group info + attendance records+students’ records Merry Christmas and Happy New Year
Merry Christmas and Happy New Year Shopping in Ukraine
Shopping in Ukraine Sigmund Freud, or Sigismund Schlomo Freud
Sigmund Freud, or Sigismund Schlomo Freud London Museums
London Museums Body parts — части тела
Body parts — части тела Merry Christmas!
Merry Christmas! Интеллектуальный марафон. Ответы. 8 класс
Интеллектуальный марафон. Ответы. 8 класс Gastroesophageal reflux disease (GERD)
Gastroesophageal reflux disease (GERD) Complete and answer the questions
Complete and answer the questions For the creative competition in english
For the creative competition in english My teaching practice
My teaching practice Did You See Chip?
Did You See Chip? Present Continuous Tense (настоящее длительное время). Случаи употребления
Present Continuous Tense (настоящее длительное время). Случаи употребления Databases Design. Introduction to SQL
Databases Design. Introduction to SQL Ladybug home. Reading
Ladybug home. Reading Connection between regular expressions and regular languages
Connection between regular expressions and regular languages Final english test
Final english test Present Continuous (настоящее продолженное)
Present Continuous (настоящее продолженное) Present Simple или Past Simple. Тест
Present Simple или Past Simple. Тест Learn to deduce the meaning
Learn to deduce the meaning House of my dream
House of my dream Halloween jokes
Halloween jokes It, there
It, there Festive time
Festive time Laminated group info+ attendance records+students’ records. Let’s congratulate November students!
Laminated group info+ attendance records+students’ records. Let’s congratulate November students! Schwa is the name for the most common sound in English
Schwa is the name for the most common sound in English 236733 (копия)-3 (копия)
236733 (копия)-3 (копия)