Слайд 2The earth’s crust, including its oceans, is approximately 16 km thick but

consists of merely 0.04% by mass of the element phosphorus.
Слайд 3However, upon the examination of living organisms, we find that phosphorus is

present in many of the molecules that play an essential role in living processes and that this element constitutes 2–4% of the dry weight of living cells.
Слайд 4To illustrate just how crucial phosphorus is to our lives, consider the
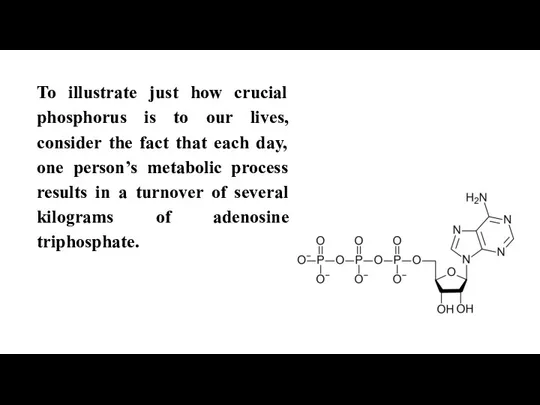
fact that each day, one person’s metabolic process results in a turnover of several kilograms of adenosine triphosphate.
Слайд 5Some of the most important roles of biophosphorus compounds – cellular energy
storage and transfer, cell signaling, molecular media for genetic information storage and transmission, formation of lipid bilayers in membranes – are exemplified by the well-known structures, not to mention calcium phosphate, the key mineral constituent of bone.




 Человек – представитель животного мира
Человек – представитель животного мира Кошки. Виды кошек
Кошки. Виды кошек Транскрипція та реплікація
Транскрипція та реплікація Свертывание крови
Свертывание крови Kletochnaya_teoria
Kletochnaya_teoria Отделы головного мозга
Отделы головного мозга Микроорганизмы. Водоросли. Простейшие. Приложение 3
Микроорганизмы. Водоросли. Простейшие. Приложение 3 Презентация на тему Гигиена органа слуха
Презентация на тему Гигиена органа слуха  Метаболические пути
Метаболические пути Класс млекопитающие. Заключительный урок (1)
Класс млекопитающие. Заключительный урок (1) Первичный метаболизм растений
Первичный метаболизм растений Динамический стереотип
Динамический стереотип Многообразие водорослей
Многообразие водорослей Физиология микроорганизмов
Физиология микроорганизмов Комплекс Гольджи. Строение и функции
Комплекс Гольджи. Строение и функции Растения - флора
Растения - флора Устройство микроскопа
Устройство микроскопа Презентация на тему "Генетический тест" - презентации по Биологии
Презентация на тему "Генетический тест" - презентации по Биологии Значение и мероприятия селекционно-племенной работы в животноводстве. Структура организаций
Значение и мероприятия селекционно-племенной работы в животноводстве. Структура организаций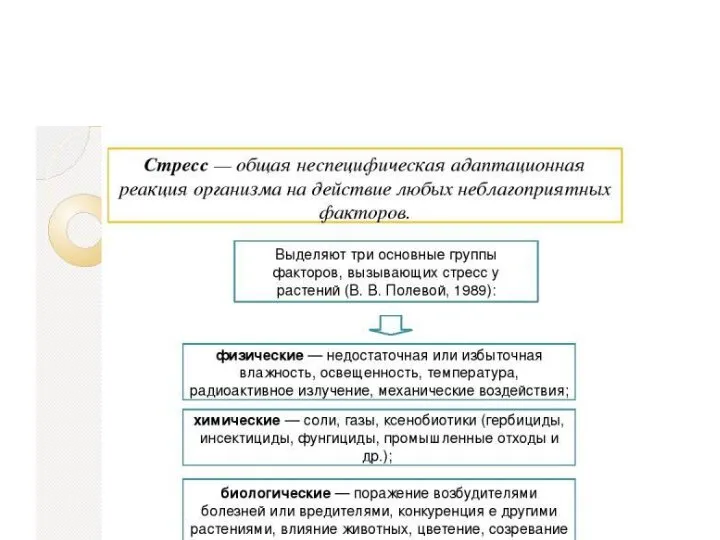 Устойчивость растений
Устойчивость растений Мир животных. Класс млекопитающие. 8 класс
Мир животных. Класс млекопитающие. 8 класс Amoebozoa. Царство Conosea. Тип Archamoeba
Amoebozoa. Царство Conosea. Тип Archamoeba урок 7 Факторы эволюции
урок 7 Факторы эволюции Размножение Плаунов
Размножение Плаунов Признаки живых организмов
Признаки живых организмов Limfrites sistēma
Limfrites sistēma Презентация на тему: Как видит человек
Презентация на тему: Как видит человек Сенсорные возможности животных
Сенсорные возможности животных