Содержание
- 2. GOVERNMENT REGULATIONS AND RECOMMENDATION AND STANDARDS a. Maintain general quality of food supply, ensuring food provided
- 3. NUTRITIONAL LABELING Regulations pertaining to the nutritional labeling of foods Mandatory for all food products to
- 4. AUTHENTICITY The price of food or food product may vary based on the quality and the
- 5. FOOD INSPECTION AND GRADING/CLASSIFICATION Routinely analyses the properties of food products to ensure they meet laws
- 6. Quality control Food industry is highly competitive. Thus, product must be of higher quality, less expensive
- 7. Monitoring food properties during processing Monitoring will help to detect problems, change in properties and thus
- 8. Food properties analysed a. Composition Composition determines safety, nutrition, physico-chemical properties, quality attributes and sensory characteristics.
- 9. c. Physico-chemical properties Rheological (shape of the food changes or flow), Optical (interaction with electromagnetic radiation
- 10. Sensory evaluation Quality and desirability of food and food products depends on the acceptability by the
- 11. Choosing an Analytical Technique The choice depends on : Types of food to be analysed The
- 12. Criteria in selecting a technique Precision – reproducible Reproducibility – even in different laboartories Accuracy –
- 13. Analysis of carbohydrates Carbohydrates may be present as isolated molecules but can also be associated to
- 14. Analysis methods The amount of sample depends on the nature of food (solid or liquid), carbohydrate
- 15. Chemical methods Based on the fact that many of the substances are reducing agents which can
- 16. Enzymatic Method Rapid, highly specific and sensitive to low concentration of carbohydrates in food (enzyme assay
- 17. Analysis of starch Digestible polysaccharides, major source of energy, 2 glucose homopolysaccharides amylose (linear 500 –
- 18. Analysis of dietary Fibers Indigestible polysaccharides, major componenst include cellulose, hemicellulose, pectin, hydrocolloid and lignin. Procedures
- 19. Analysis of proteins (Polymers of amino acids) Determination of overall protein concentration Kjeldahl method (Food is
- 20. Methods using Spectrophotometry Natural ability for protein to absorb light (or scatter) in the UV-visible region
- 21. Methods based on Solubility Characteristics of proteins Salting out [(NH4)2SO4] Isoelectric precipitation (Proteins aggregate and precipitate
- 22. Analysis of lipid Important properties of concern : Total lipid concentration Type of lipid Physicochemical properties
- 23. Determination of Total Lipid Concentration Solvent Extraction Drying sample (water affect efficiency of extraction) Particle size
- 24. Determination of Lipid composition Lipid extremely diverse group of compounds, important to determine the type of
- 25. Rheology Deformation and flow of matter Creaminess, juiciness, smoothness, brittleness, tenderness, hardness etc Flow properties –
- 27. Скачать презентацию
Слайд 2GOVERNMENT REGULATIONS AND RECOMMENDATION AND STANDARDS
a. Maintain general quality of food supply,
GOVERNMENT REGULATIONS AND RECOMMENDATION AND STANDARDS
a. Maintain general quality of food supply,

b. Government Department (Food and Drug Administration, (FDA), US Department of Agriculture (USDA), National Marine Fisheries Service (NMFS), Environmental Protection Agency (EPA)
Responsible for regulating particular sectors of the food industry and publish document on the standards concerning the composition, quality, inspection and labeling
Mandatory standards (standards of identity, standards of quality and standards of fill-of-containers)
Voluntary Standards (standards of grade) – food is graded based on quality, as superior product are costly priced.
Слайд 3NUTRITIONAL LABELING
Regulations pertaining to the nutritional labeling of foods
Mandatory
NUTRITIONAL LABELING
Regulations pertaining to the nutritional labeling of foods
Mandatory

Important for consumer – informed choice for their diet
Total calorie value of food, total fat, saturated fatty acids, cholesterol, sodium, carbohydrate, dietary fiber, sugars, protein, vitamins, calcium and iron – information provided on food labels
Approved health claims – link between food components and certain diseases. (Eg. Calcium and osteoporosis, sodium and high blood pressure, soluble fiber or cholesterol and heart diseases.
Consumers can plan nutritional and balanced or healthy diet; over consumption of certain food may cause health problem or otherwise good food will be benficial to health.
Слайд 4AUTHENTICITY
The price of food or food product may vary based on the
AUTHENTICITY
The price of food or food product may vary based on the

Claims made for certain products. How do we verify these claims?
Food manufacturers may made false claims to get higher prices
Techniques should be developed to determine the authenticity of food products
Consumers should not be victims of frauds and the competition between manufacturers mus be fair.
Слайд 5FOOD INSPECTION AND GRADING/CLASSIFICATION
Routinely analyses the properties of food products to ensure
FOOD INSPECTION AND GRADING/CLASSIFICATION
Routinely analyses the properties of food products to ensure

Food safety – safe to the standpoint of manufacturers and consumers, unsafe food is an economical disastrous if it contains harmful microorganisms, toxic chemicals, foreign substances/matters.
Manufacturers must ensure food are safe before it reaches the consumers
Good manufacturing practices, analytical techniques to detect harmful substances
Food production facilities is free for harmful effect on food and operate effectively.
Слайд 6Quality control
Food industry is highly competitive. Thus, product must be of
Quality control
Food industry is highly competitive. Thus, product must be of

Final food products must meet all the standards; from raw materials, processes (heat, mix cool etc), packaging, storage, transported and sold to consumers
Final food products – consistent properties (appearance, texture, flavour, color and stability); should not vary from one batch to another
However, the raw materials can cause variation in the properties of food and the manufacturers should control the variations
Role of different food ingredients and processing procedures
Monitor properties of food during the production steps and appropriate steps can be taken if variation occurs
Characterization of raw materials - measure the properties of the raw materials to ensure it meets certain standards of quality. Quality of raw materials determines the quality of finished food products
Слайд 7Monitoring food properties during processing
Monitoring will help to detect problems, change in
Monitoring food properties during processing
Monitoring will help to detect problems, change in

Improve quality of food products, reduce amount of materials and save time – samples will be removed from the process and analysed in a quality assurance laboratory (time consuming)
Analytical methods which are rapid without having to remove a sample from the process
An on-line technique is preferable, non-destructive and can be done using automation
Characterization of Final Products
Properties must meet appropriate legal and labeling requirements, safe and high quality, desirable properties at the time it is consumed
HACCP
Слайд 8Food properties analysed
a. Composition
Composition determines safety, nutrition, physico-chemical properties,
Food properties analysed
a. Composition
Composition determines safety, nutrition, physico-chemical properties,
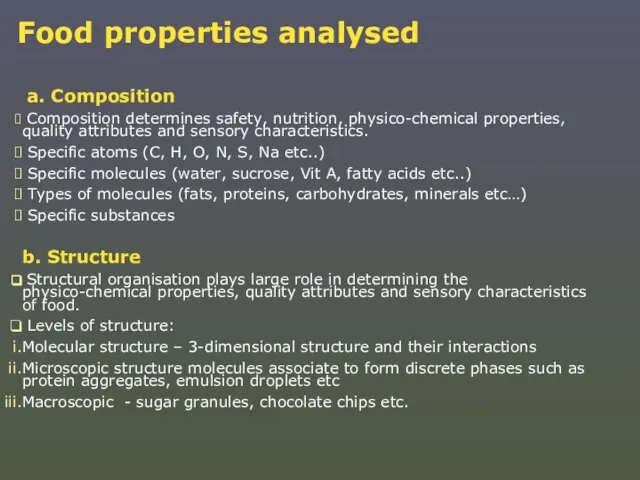
Specific atoms (C, H, O, N, S, Na etc..)
Specific molecules (water, sucrose, Vit A, fatty acids etc..)
Types of molecules (fats, proteins, carbohydrates, minerals etc…)
Specific substances
b. Structure
Structural organisation plays large role in determining the physico-chemical properties, quality attributes and sensory characteristics of food.
Levels of structure:
Molecular structure – 3-dimensional structure and their interactions
Microscopic structure molecules associate to form discrete phases such as protein aggregates, emulsion droplets etc
Macroscopic - sugar granules, chocolate chips etc.
Слайд 9c. Physico-chemical properties
Rheological (shape of the food changes or flow),
c. Physico-chemical properties
Rheological (shape of the food changes or flow),

Stablity (ability to resist biological, chemical or physical changes in its properties over time. Biological refers to change in microorganism present in food, chemical refers to change in types of molecules such as fat rancidity or non-enzymatic browning, physical changes movement of molecules)
Flavour (molecules interact with receptors in the mouth (taste) or smell (nose). The perceived flavour depends on the type and concentration of falvour constituents
Affect the quality, sensory attributes and behaviour during production, storage and consumption.
Food must be prepared so that they have the required properties over a wide range of environmental conditions, in relation to their condition during processing, storage and consumption (variation in temperature or mechanical stress).
Слайд 10Sensory evaluation
Quality and desirability of food and food products depends on the
Sensory evaluation
Quality and desirability of food and food products depends on the

Evaluation of sensory properties is important before the product is launched in the market
Factors affecting the sensory evaluation include nutritional knowledge and education, climate, age, health, social, culture and religion
Thus in food testing, untrained consumer will be used to test the improved or new products . However, trained consumers will be used to sense specific food products
Disadvantages of sensory evaluation test
Time consuming and expensive, test are not objective
Not for foods that may contain poison or toxin
Cannot provide information related to safety, composition or nutritional value of food
Standardized procedures may be used, however which can related to sensory characteristics, such as tenderness, palatability, chewiness etc.)
Слайд 11Choosing an Analytical Technique
The choice depends on :
Types of food to be
Choosing an Analytical Technique
The choice depends on :
Types of food to be

The reasons for carrying out the analysis
Sources;
Books (Introduction to Food Analysis, S.S. Nielsen 1998, Aspen Publisher)
Official Methods of Analysis Eg. Association of the Official Analytical Chemists (AOAC) and American Oil Chemists Society (AOCS)
Journals
Equipment and reagent suppliers
Internet
Слайд 12Criteria in selecting a technique
Precision – reproducible
Reproducibility – even in different laboartories
Accuracy
Criteria in selecting a technique
Precision – reproducible
Reproducibility – even in different laboartories
Accuracy

Simplicity of operation – even by unskilled researchers
Cost
Speed
Sensivity – lowest concentration
Safe – non harzardous reagents used in the tests
Destructive/non-destructive approach
On line/off line (during food processing
Approval or recognition by international bodies
Nature of food analysed
Слайд 13Analysis of carbohydrates
Carbohydrates may be present as isolated molecules but can also
Analysis of carbohydrates
Carbohydrates may be present as isolated molecules but can also
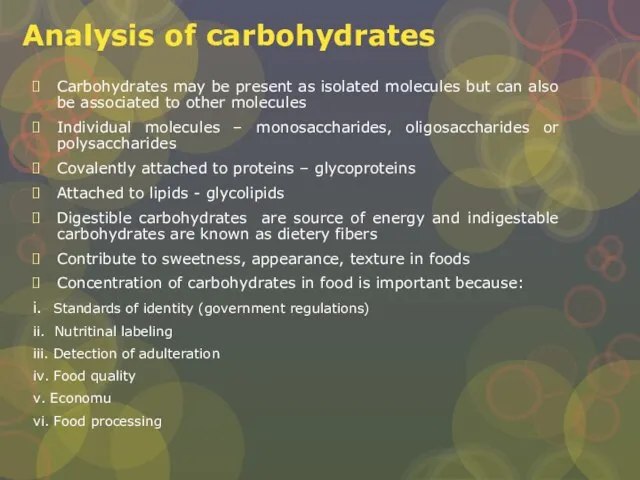
Individual molecules – monosaccharides, oligosaccharides or polysaccharides
Covalently attached to proteins – glycoproteins
Attached to lipids - glycolipids
Digestible carbohydrates are source of energy and indigestable carbohydrates are known as dietery fibers
Contribute to sweetness, appearance, texture in foods
Concentration of carbohydrates in food is important because:
i. Standards of identity (government regulations)
ii. Nutritinal labeling
iii. Detection of adulteration
iv. Food quality
v. Economu
vi. Food processing
Слайд 14Analysis methods
The amount of sample depends on the nature of food (solid
Analysis methods
The amount of sample depends on the nature of food (solid
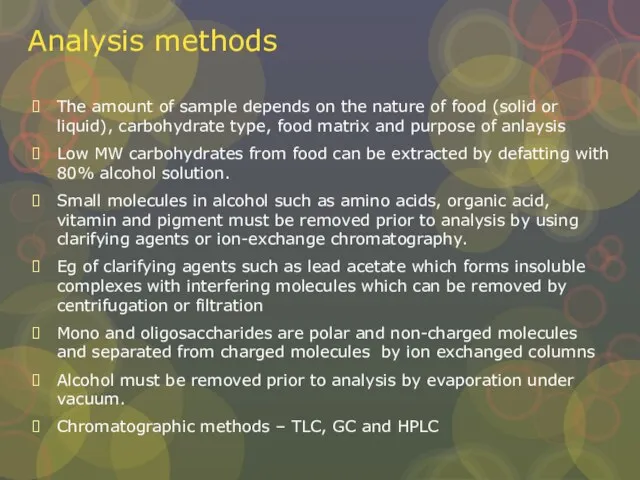
Low MW carbohydrates from food can be extracted by defatting with 80% alcohol solution.
Small molecules in alcohol such as amino acids, organic acid, vitamin and pigment must be removed prior to analysis by using clarifying agents or ion-exchange chromatography.
Eg of clarifying agents such as lead acetate which forms insoluble complexes with interfering molecules which can be removed by centrifugation or filtration
Mono and oligosaccharides are polar and non-charged molecules and separated from charged molecules by ion exchanged columns
Alcohol must be removed prior to analysis by evaporation under vacuum.
Chromatographic methods – TLC, GC and HPLC
Слайд 15Chemical methods
Based on the fact that many of the substances are reducing
Chemical methods
Based on the fact that many of the substances are reducing
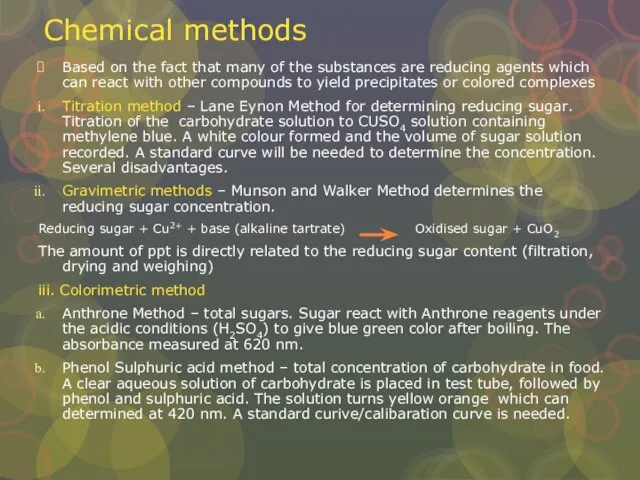
Titration method – Lane Eynon Method for determining reducing sugar. Titration of the carbohydrate solution to CUSO4 solution containing methylene blue. A white colour formed and the volume of sugar solution recorded. A standard curve will be needed to determine the concentration. Several disadvantages.
Gravimetric methods – Munson and Walker Method determines the reducing sugar concentration.
Reducing sugar + Cu2+ + base (alkaline tartrate) Oxidised sugar + CuO2
The amount of ppt is directly related to the reducing sugar content (filtration, drying and weighing)
iii. Colorimetric method
Anthrone Method – total sugars. Sugar react with Anthrone reagents under the acidic conditions (H2SO4) to give blue green color after boiling. The absorbance measured at 620 nm.
Phenol Sulphuric acid method – total concentration of carbohydrate in food. A clear aqueous solution of carbohydrate is placed in test tube, followed by phenol and sulphuric acid. The solution turns yellow orange which can determined at 420 nm. A standard curive/calibaration curve is needed.
Слайд 16Enzymatic Method
Rapid, highly specific and sensitive to low concentration of carbohydrates in
Enzymatic Method
Rapid, highly specific and sensitive to low concentration of carbohydrates in
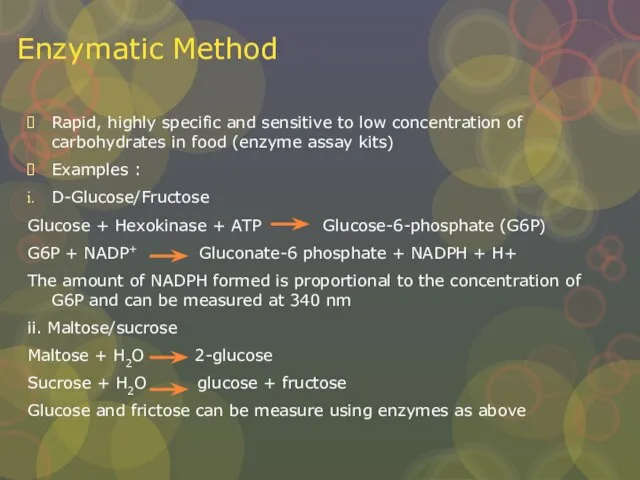
Examples :
D-Glucose/Fructose
Glucose + Hexokinase + ATP Glucose-6-phosphate (G6P)
G6P + NADP+ Gluconate-6 phosphate + NADPH + H+
The amount of NADPH formed is proportional to the concentration of G6P and can be measured at 340 nm
ii. Maltose/sucrose
Maltose + H2O 2-glucose
Sucrose + H2O glucose + fructose
Glucose and frictose can be measure using enzymes as above
Слайд 17Analysis of starch
Digestible polysaccharides, major source of energy, 2 glucose homopolysaccharides amylose
Analysis of starch
Digestible polysaccharides, major source of energy, 2 glucose homopolysaccharides amylose

Amylose and amylopectin can be separated by adding chemicals such as alcohols which precipitate amylose but not amylopectin.
Starch granules separated from other major component by drying, grinding, steeping in water, filtration and centrifugation
Food samples are dissolve in 80% ethanol which will dissolve away mono and oligosaccharides but not the starch which can be filtered or centrifuged.
Starch can be dispersed in water after heating at >65oC where it form gelatin like
Analysis methods for starch
Specific enzymes breakdowns starch to glucose
Iodine added to form insoluble starch-iodine complex which can determined gravimetrically or titrimetrically based on the amount of iodine required to precipitate the starch
Слайд 18Analysis of dietary Fibers
Indigestible polysaccharides, major componenst include cellulose, hemicellulose, pectin, hydrocolloid
Analysis of dietary Fibers
Indigestible polysaccharides, major componenst include cellulose, hemicellulose, pectin, hydrocolloid
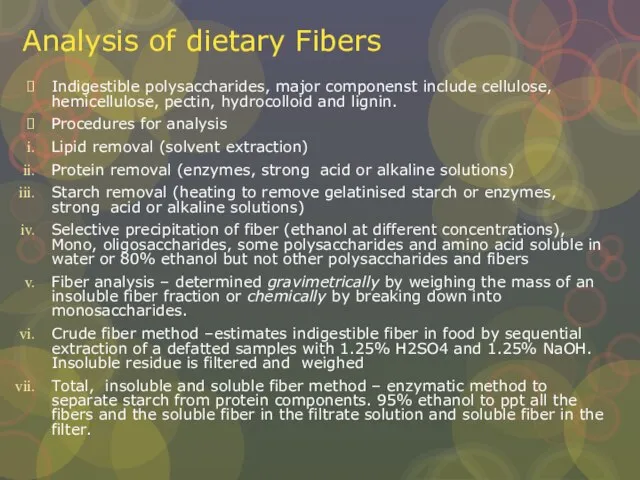
Procedures for analysis
Lipid removal (solvent extraction)
Protein removal (enzymes, strong acid or alkaline solutions)
Starch removal (heating to remove gelatinised starch or enzymes, strong acid or alkaline solutions)
Selective precipitation of fiber (ethanol at different concentrations), Mono, oligosaccharides, some polysaccharides and amino acid soluble in water or 80% ethanol but not other polysaccharides and fibers
Fiber analysis – determined gravimetrically by weighing the mass of an insoluble fiber fraction or chemically by breaking down into monosaccharides.
Crude fiber method –estimates indigestible fiber in food by sequential extraction of a defatted samples with 1.25% H2SO4 and 1.25% NaOH. Insoluble residue is filtered and weighed
Total, insoluble and soluble fiber method – enzymatic method to separate starch from protein components. 95% ethanol to ppt all the fibers and the soluble fiber in the filtrate solution and soluble fiber in the filter.
Слайд 19Analysis of proteins (Polymers of amino acids)
Determination of overall protein concentration
Kjeldahl method
Analysis of proteins (Polymers of amino acids)
Determination of overall protein concentration
Kjeldahl method
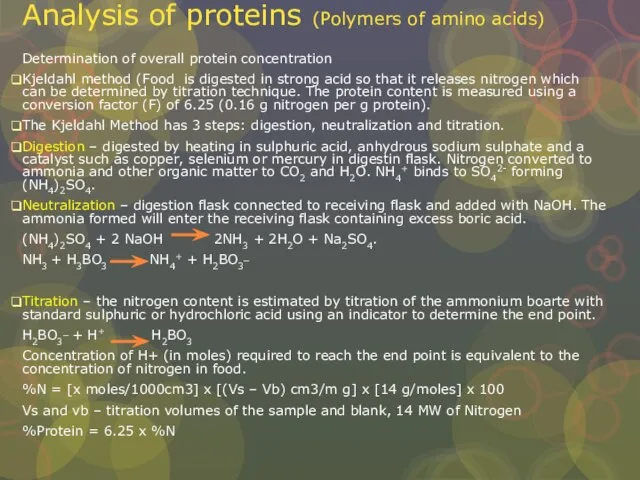
The Kjeldahl Method has 3 steps: digestion, neutralization and titration.
Digestion – digested by heating in sulphuric acid, anhydrous sodium sulphate and a catalyst such as copper, selenium or mercury in digestin flask. Nitrogen converted to ammonia and other organic matter to CO2 and H2O. NH4+ binds to SO42- forming (NH4)2SO4.
Neutralization – digestion flask connected to receiving flask and added with NaOH. The ammonia formed will enter the receiving flask containing excess boric acid.
(NH4)2SO4 + 2 NaOH 2NH3 + 2H2O + Na2SO4.
NH3 + H3BO3 NH4+ + H2BO3_
Titration – the nitrogen content is estimated by titration of the ammonium boarte with standard sulphuric or hydrochloric acid using an indicator to determine the end point.
H2BO3_ + H+ H2BO3
Concentration of H+ (in moles) required to reach the end point is equivalent to the concentration of nitrogen in food.
%N = [x moles/1000cm3] x [(Vs – Vb) cm3/m g] x [14 g/moles] x 100
Vs and vb – titration volumes of the sample and blank, 14 MW of Nitrogen
%Protein = 6.25 x %N
Слайд 20Methods using Spectrophotometry
Natural ability for protein to absorb light (or scatter) in
Methods using Spectrophotometry
Natural ability for protein to absorb light (or scatter) in
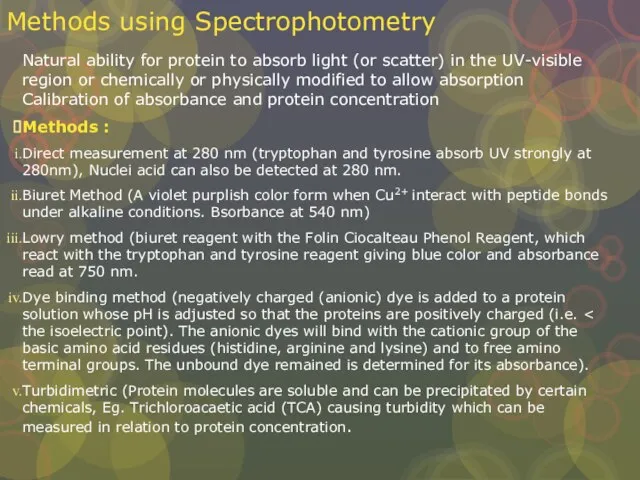
Methods :
Direct measurement at 280 nm (tryptophan and tyrosine absorb UV strongly at 280nm), Nuclei acid can also be detected at 280 nm.
Biuret Method (A violet purplish color form when Cu2+ interact with peptide bonds under alkaline conditions. Bsorbance at 540 nm)
Lowry method (biuret reagent with the Folin Ciocalteau Phenol Reagent, which react with the tryptophan and tyrosine reagent giving blue color and absorbance read at 750 nm.
Dye binding method (negatively charged (anionic) dye is added to a protein solution whose pH is adjusted so that the proteins are positively charged (i.e. < the isoelectric point). The anionic dyes will bind with the cationic group of the basic amino acid residues (histidine, arginine and lysine) and to free amino terminal groups. The unbound dye remained is determined for its absorbance).
Turbidimetric (Protein molecules are soluble and can be precipitated by certain chemicals, Eg. Trichloroacaetic acid (TCA) causing turbidity which can be measured in relation to protein concentration.
Слайд 21Methods based on Solubility Characteristics of proteins
Salting out [(NH4)2SO4]
Isoelectric precipitation (Proteins aggregate
Methods based on Solubility Characteristics of proteins
Salting out [(NH4)2SO4]
Isoelectric precipitation (Proteins aggregate
![Methods based on Solubility Characteristics of proteins Salting out [(NH4)2SO4] Isoelectric precipitation](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/375517/slide-20.jpg)
Solvent fractionation (precipitation using water soluble oragnic solvent, like ethanol or acetone)
Methods for protein separation based on adsorption characteristics
Ion exchange chromatography
Affinity chromatography
Methods for protein separation based on size differences
Dialysis
Ultrafiltration
Size exclusion chromatograph or gel filtration
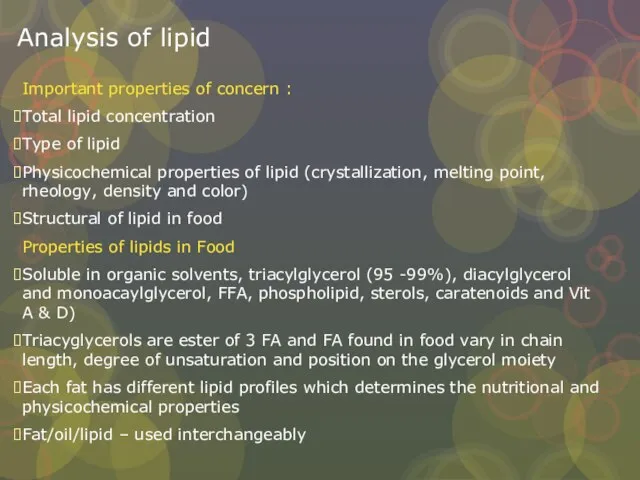
Слайд 22Analysis of lipid
Important properties of concern :
Total lipid concentration
Type of lipid
Physicochemical properties
Analysis of lipid
Important properties of concern :
Total lipid concentration
Type of lipid
Physicochemical properties
Structural of lipid in food
Properties of lipids in Food
Soluble in organic solvents, triacylglycerol (95 -99%), diacylglycerol and monoacaylglycerol, FFA, phospholipid, sterols, caratenoids and Vit A & D)
Triacyglycerols are ester of 3 FA and FA found in food vary in chain length, degree of unsaturation and position on the glycerol moiety
Each fat has different lipid profiles which determines the nutritional and physicochemical properties
Fat/oil/lipid – used interchangeably
Слайд 23Determination of Total Lipid Concentration
Solvent Extraction
Drying sample (water affect efficiency of extraction)
Particle
Determination of Total Lipid Concentration
Solvent Extraction
Drying sample (water affect efficiency of extraction)
Particle
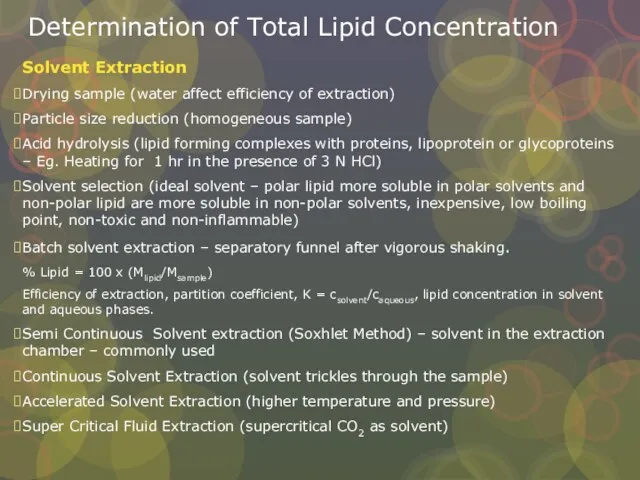
Acid hydrolysis (lipid forming complexes with proteins, lipoprotein or glycoproteins – Eg. Heating for 1 hr in the presence of 3 N HCl)
Solvent selection (ideal solvent – polar lipid more soluble in polar solvents and non-polar lipid are more soluble in non-polar solvents, inexpensive, low boiling point, non-toxic and non-inflammable)
Batch solvent extraction – separatory funnel after vigorous shaking.
% Lipid = 100 x (Mlipid/Msample)
Efficiency of extraction, partition coefficient, K = csolvent/caqueous, lipid concentration in solvent and aqueous phases.
Semi Continuous Solvent extraction (Soxhlet Method) – solvent in the extraction chamber – commonly used
Continuous Solvent Extraction (solvent trickles through the sample)
Accelerated Solvent Extraction (higher temperature and pressure)
Super Critical Fluid Extraction (supercritical CO2 as solvent)
Слайд 24Determination of Lipid composition
Lipid extremely diverse group of compounds, important to determine
Determination of Lipid composition
Lipid extremely diverse group of compounds, important to determine
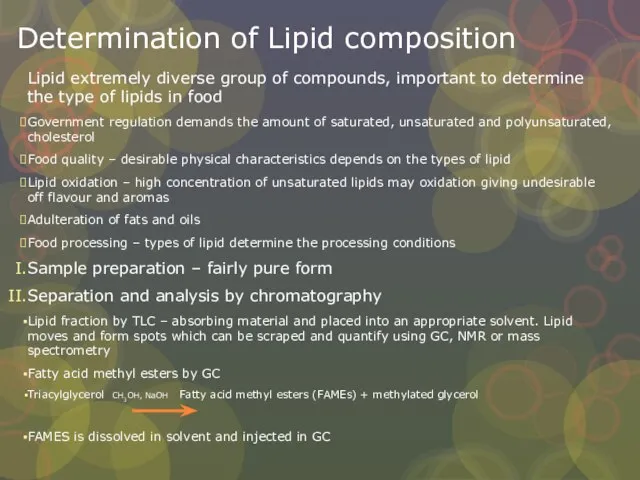
Government regulation demands the amount of saturated, unsaturated and polyunsaturated, cholesterol
Food quality – desirable physical characteristics depends on the types of lipid
Lipid oxidation – high concentration of unsaturated lipids may oxidation giving undesirable off flavour and aromas
Adulteration of fats and oils
Food processing – types of lipid determine the processing conditions
Sample preparation – fairly pure form
Separation and analysis by chromatography
Lipid fraction by TLC – absorbing material and placed into an appropriate solvent. Lipid moves and form spots which can be scraped and quantify using GC, NMR or mass spectrometry
Fatty acid methyl esters by GC
Triacylglycerol CH3OH, NaOH Fatty acid methyl esters (FAMEs) + methylated glycerol
FAMES is dissolved in solvent and injected in GC
Слайд 25Rheology
Deformation and flow of matter
Creaminess, juiciness, smoothness, brittleness, tenderness, hardness etc
Flow
Rheology
Deformation and flow of matter
Creaminess, juiciness, smoothness, brittleness, tenderness, hardness etc
Flow
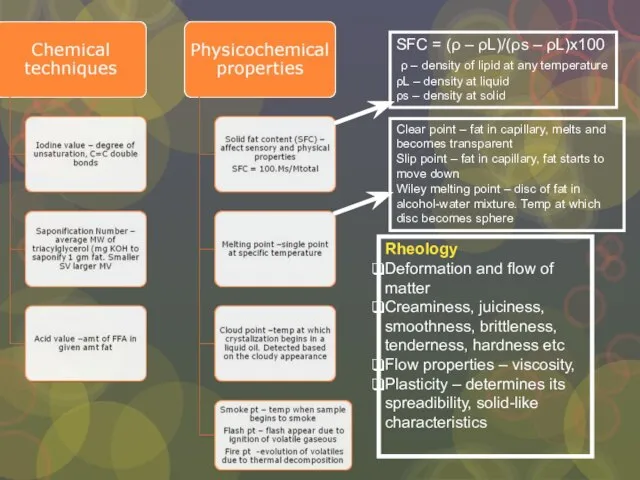
Plasticity – determines its spreadibility, solid-like characteristics
SFC = (ρ – ρL)/(ρs – ρL)x100
ρ – density of lipid at any temperature
ρL – density at liquid
ρs – density at solid
Clear point – fat in capillary, melts and becomes transparent
Slip point – fat in capillary, fat starts to move down
Wiley melting point – disc of fat in alcohol-water mixture. Temp at which disc becomes sphere
 Lluvia
Lluvia Жужжалочка. Дидактическая игра для автоматизации звука Ж в словах
Жужжалочка. Дидактическая игра для автоматизации звука Ж в словах УД ПСИХОЛОГИЯ
УД ПСИХОЛОГИЯ Храмы России
Храмы России Оптическая микроскопия
Оптическая микроскопия Менеджмент
Менеджмент Кондитерский отдел
Кондитерский отдел Органы и службы стандартизации РФ
Органы и службы стандартизации РФ Вышел зайчик погулять Художник – В. Сергеев
Вышел зайчик погулять Художник – В. Сергеев Знакомство с точкой
Знакомство с точкой Состояние и задачи управления проектами в строительстве
Состояние и задачи управления проектами в строительстве Торцевой разрез. Материаловедение
Торцевой разрез. Материаловедение чайные истории
чайные истории Шпаргалка юного покупателя
Шпаргалка юного покупателя Учебно-методический комплекс "Живая география" Живая география - учебно-методический комплекс, позволяющий использовать геоинфор
Учебно-методический комплекс "Живая география" Живая география - учебно-методический комплекс, позволяющий использовать геоинфор Классификация реакций
Классификация реакций Организация хранения документов Архивного фонда Российской Федерации и других архивных документов
Организация хранения документов Архивного фонда Российской Федерации и других архивных документов Трансляция – биосинтез белка на рибосоме
Трансляция – биосинтез белка на рибосоме Инновационный подход к жизни
Инновационный подход к жизни DaCoPAn Software Engineering Project - Система динамической визуализации событий работы протоколов при обмене данными между двумя сетевыми ЭВМ — D
DaCoPAn Software Engineering Project - Система динамической визуализации событий работы протоколов при обмене данными между двумя сетевыми ЭВМ — D Урок – размышление по рассказу К.Г. Паустовского «Телеграмма»
Урок – размышление по рассказу К.Г. Паустовского «Телеграмма» Путешествие в мир животных
Путешествие в мир животных СПАСИБО, АЗБУКА!
СПАСИБО, АЗБУКА! Rave Cosmology Today Dying, Death & Bardo . RC3.8
Rave Cosmology Today Dying, Death & Bardo . RC3.8 Основные закономерности развития информационного пространства
Основные закономерности развития информационного пространства Циклон ВЦНИИОТ с обратным конусом
Циклон ВЦНИИОТ с обратным конусом  Квантовые компьютеры
Квантовые компьютеры Разработать рекламную кампанию в стиле шоу для молодежного интернет-издания Пи-Пермь (бюджет студенческой редакции)
Разработать рекламную кампанию в стиле шоу для молодежного интернет-издания Пи-Пермь (бюджет студенческой редакции)