Содержание
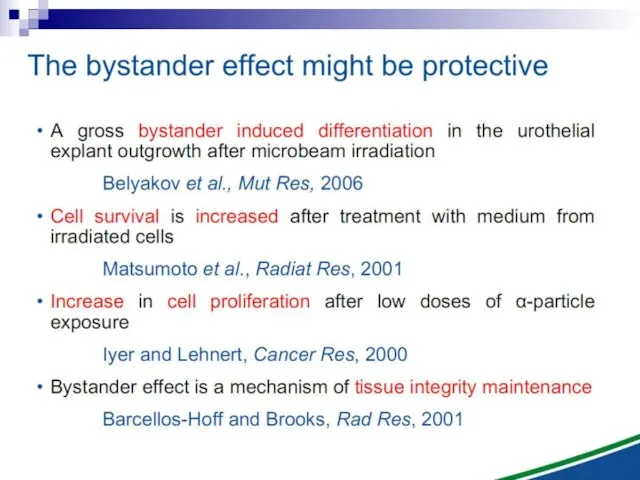
- 2. The bystander effect refers to the induction of biological effects in cells that are not directly
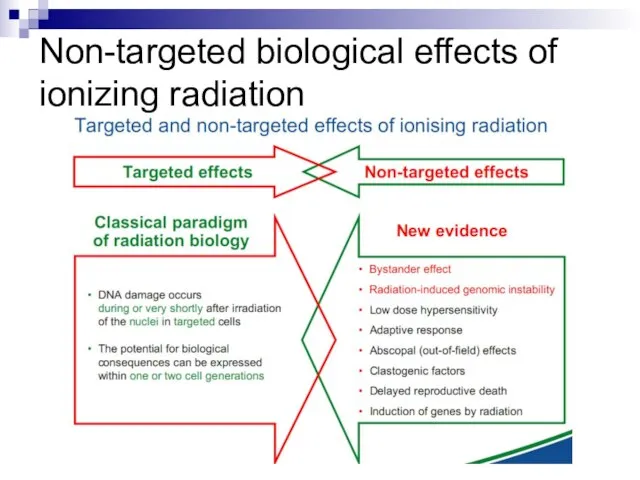
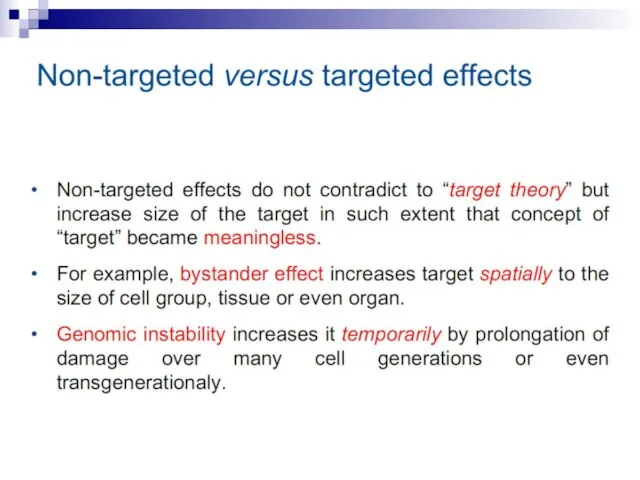
- 3. Non-targeted biological effects of ionizing radiation
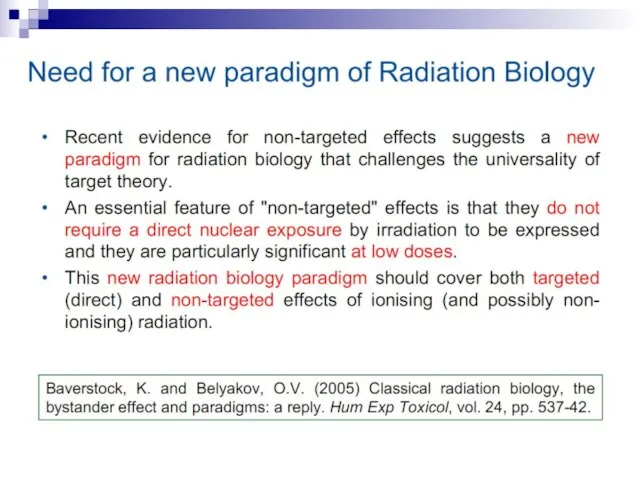
- 5. Non-targeted effects of ionizing radiation as a new paradigm of radiation biology Ward, J. (1999) New
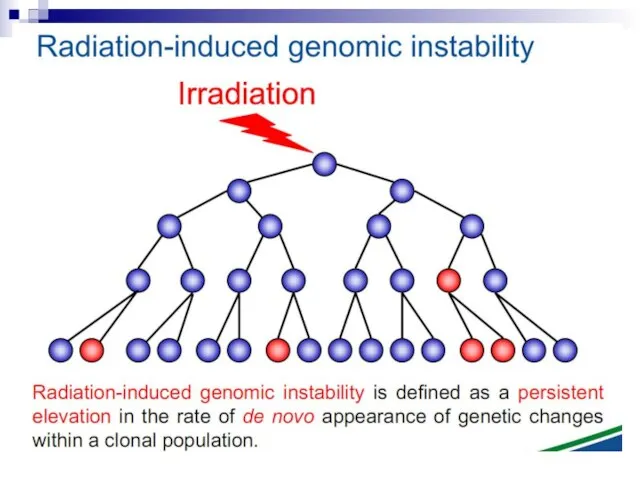
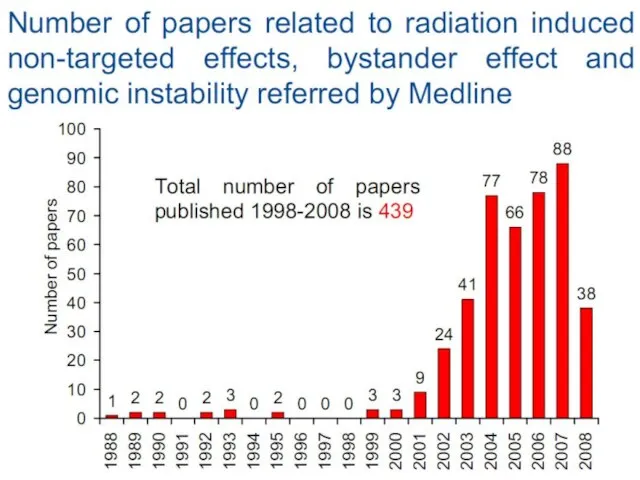
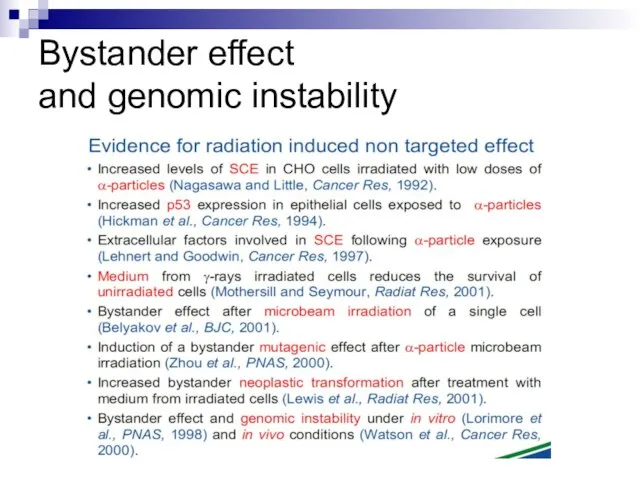
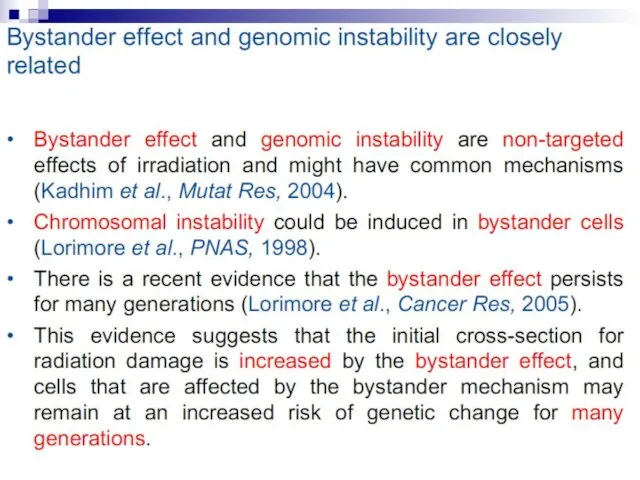
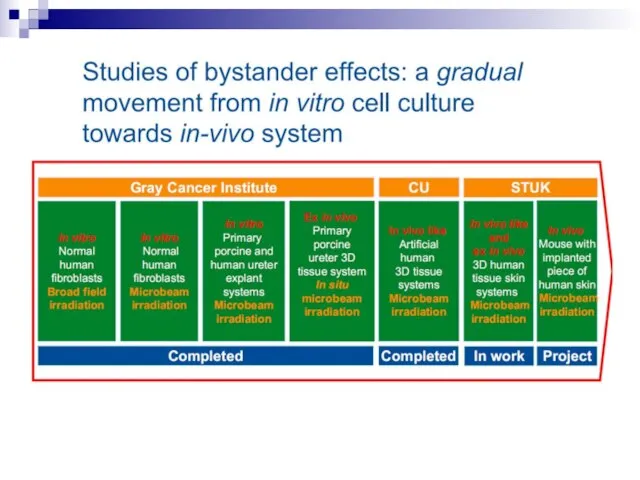
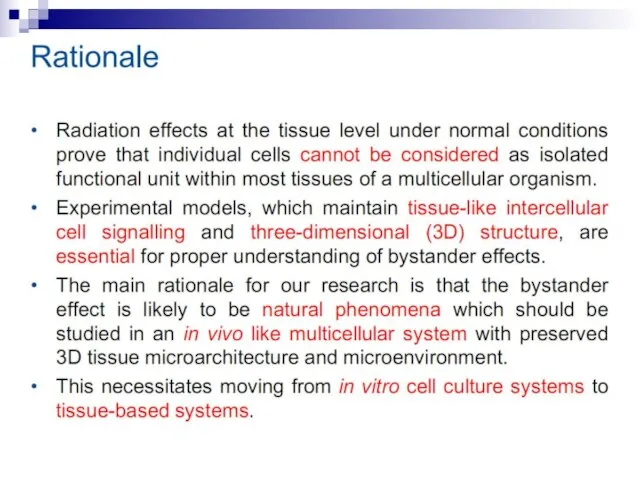
- 12. Bystander effect and genomic instability
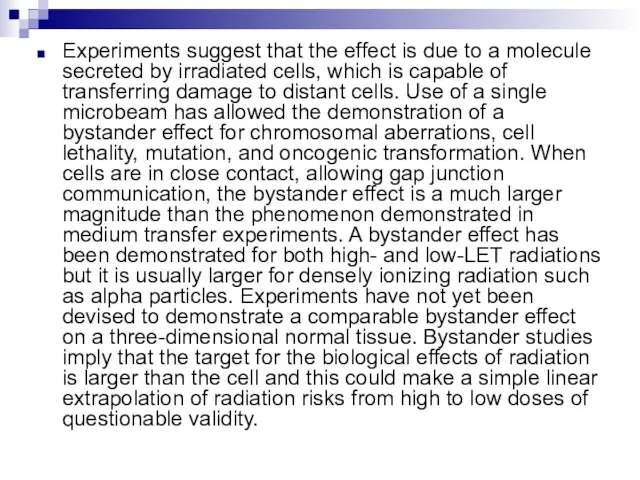
- 73. Experiments suggest that the effect is due to a molecule secreted by irradiated cells, which is
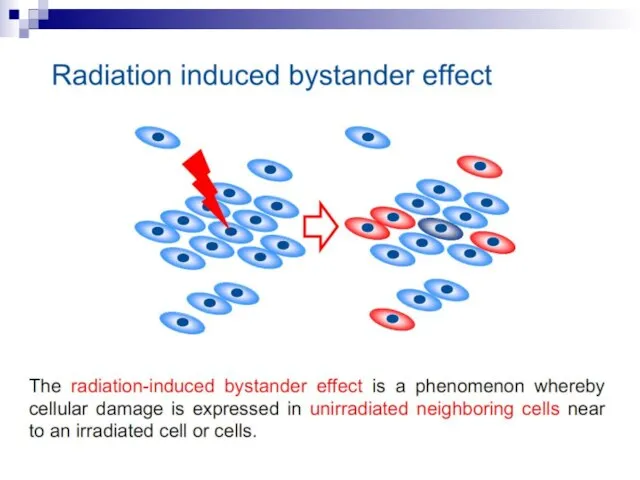
- 74. The radiation-induced bystander effect is defined as “the induction of biological effects in cells that are
- 75. GENERATIONS OF students in radiation biology have been taught that heritable biological effects require direct damage
- 76. In the radiation field, it has come to be loosely defined as the induction of biological
- 77. The plethora of data now available concerning the bystander effect fall into two quite separate categories,
- 78. Medium transfer experiments Experiments involving the transfer of medium from irradiated to unirradiated cells have demonstrated
- 79. This bystander effect suggested that irradiated cells secreted a molecule into the culture medium that was
- 80. Some limited progress has been made in the search for the mechanisms involved in this bystander
- 81. The majority of bystander experiments involving medium transfer have utilized low-LET x or gamma rays, in
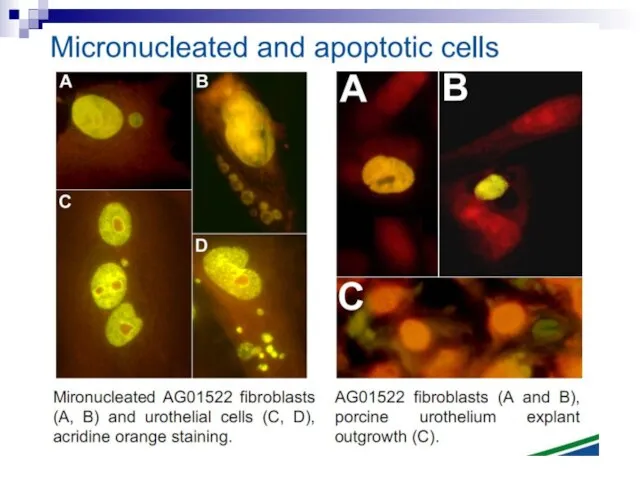
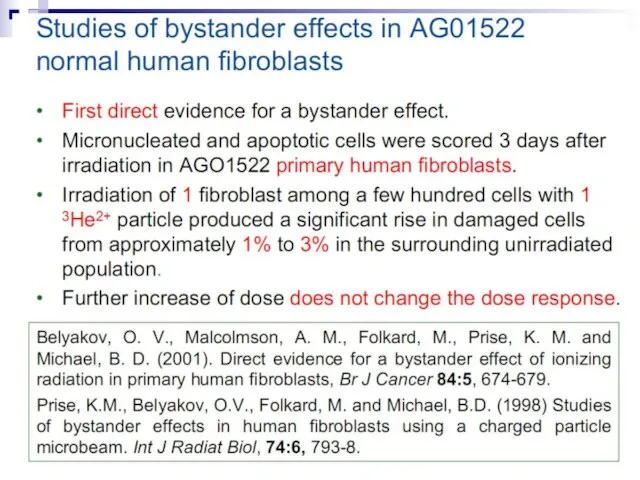
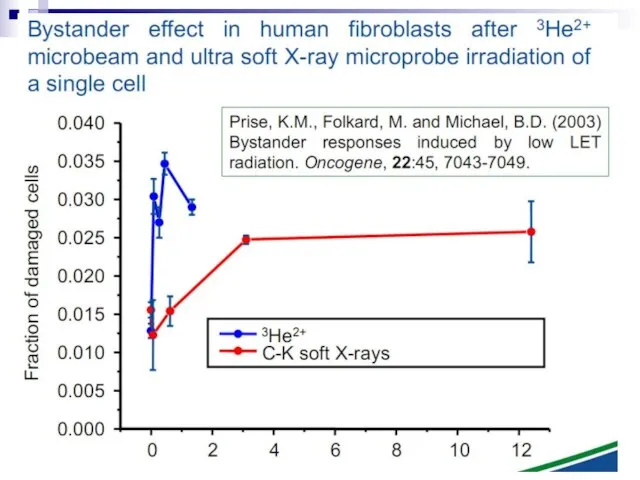
- 82. Micronuclei in normal human fibroblasts Perhaps the most direct and most dramatic demonstration of the bystander
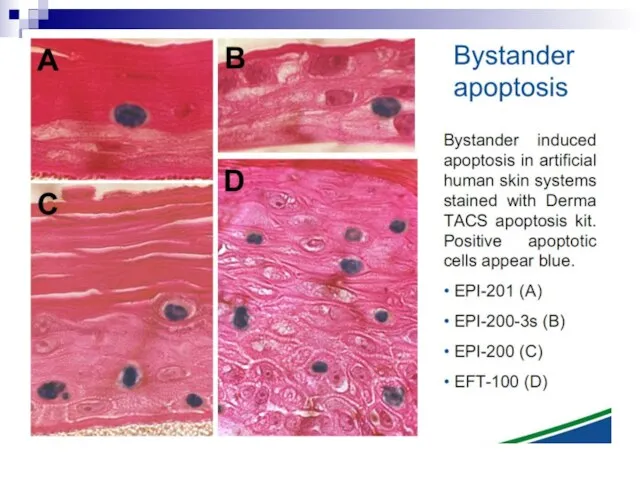
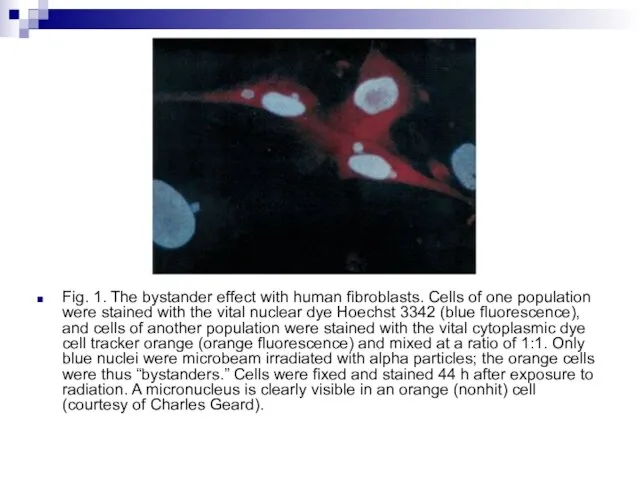
- 83. Fig. 1. The bystander effect with human fibroblasts. Cells of one population were stained with the
- 84. Cell lethality Lines of hygromycin- and neomycin-resistant V79 cells were produced. Before exposure the hygromycinresistant cells
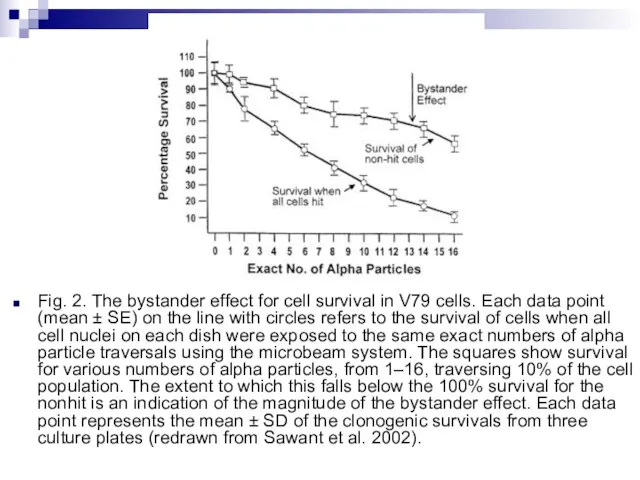
- 85. Fig. 2. The bystander effect for cell survival in V79 cells. Each data point (mean ±
- 86. There is a considerable degree of cell killing in the nonhit cells, implying a substantial bystander
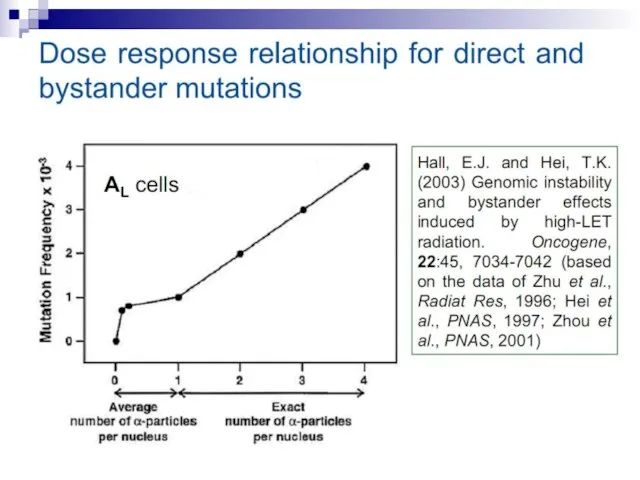
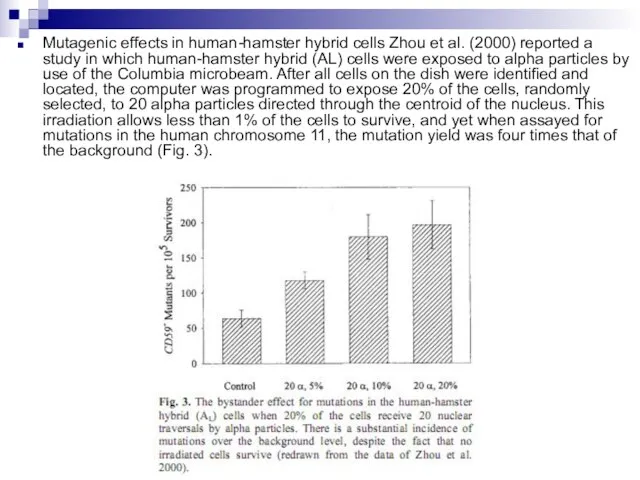
- 87. Mutagenic effects in human-hamster hybrid cells Zhou et al. (2000) reported a study in which human-hamster
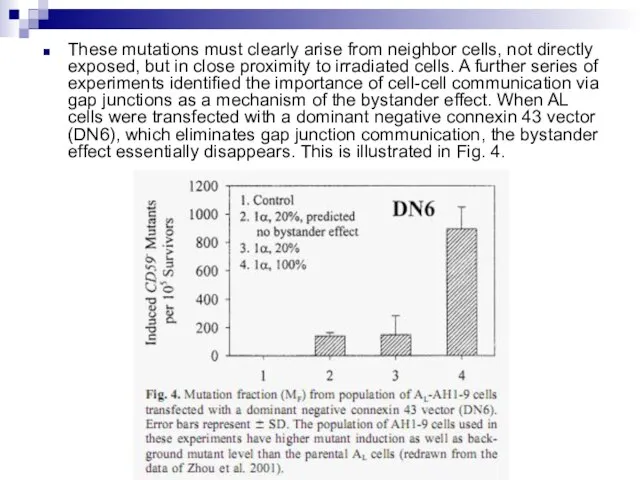
- 88. These mutations must clearly arise from neighbor cells, not directly exposed, but in close proximity to
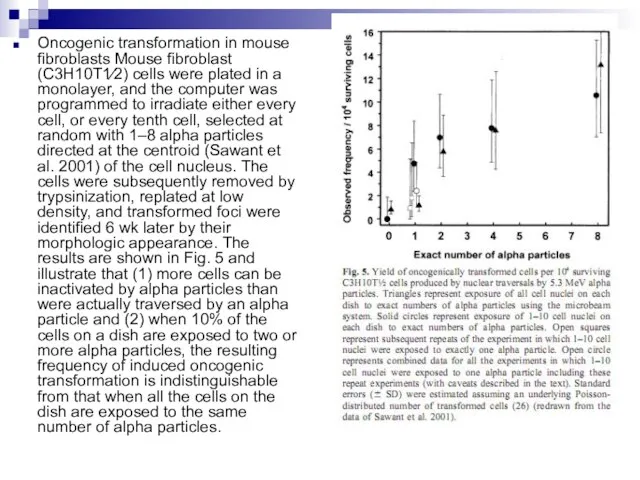
- 89. Oncogenic transformation in mouse fibroblasts Mouse fibroblast (C3H10T1⁄2) cells were plated in a monolayer, and the
- 90. It is important to note that the experimental results discussed in this paper involve laboratory model
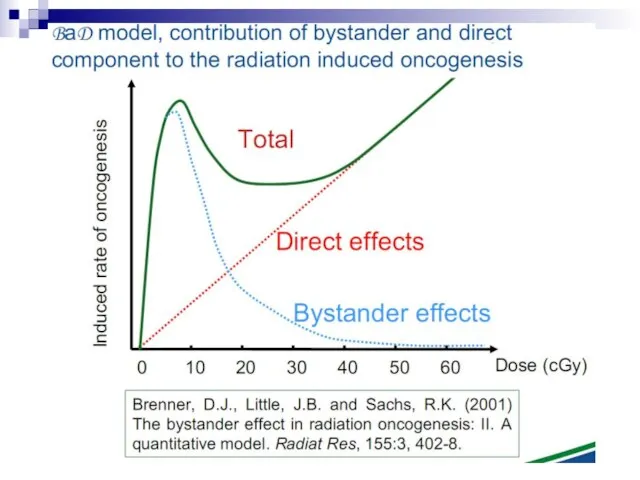
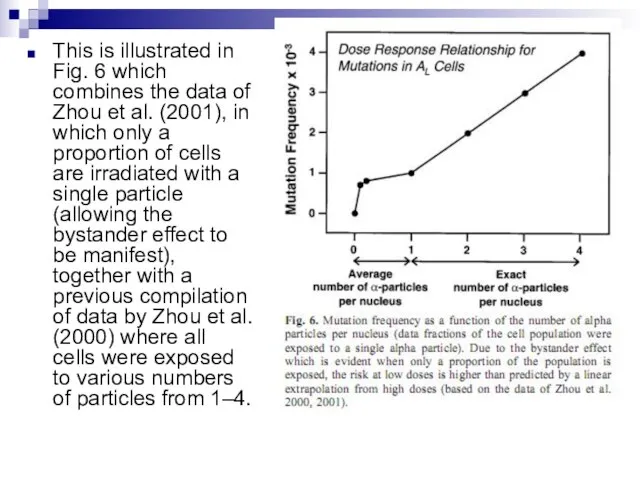
- 91. This is illustrated in Fig. 6 which combines the data of Zhou et al. (2001), in
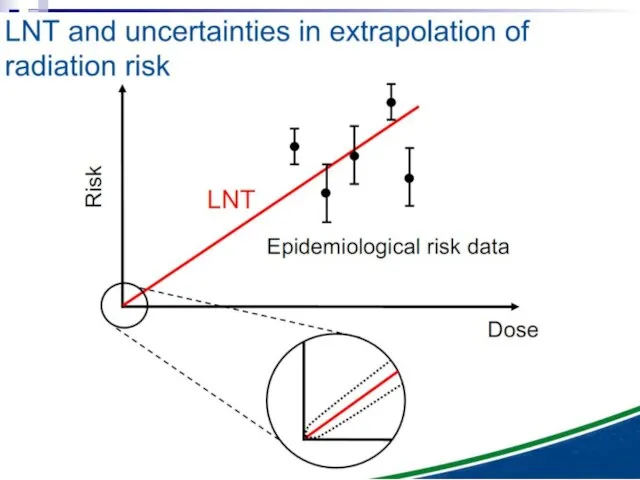
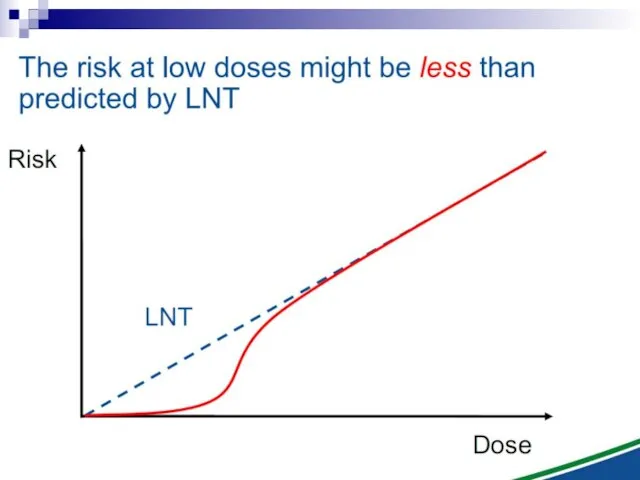
- 92. Under these experimental conditions, it is evident that a linear extrapolation of risks from high doses
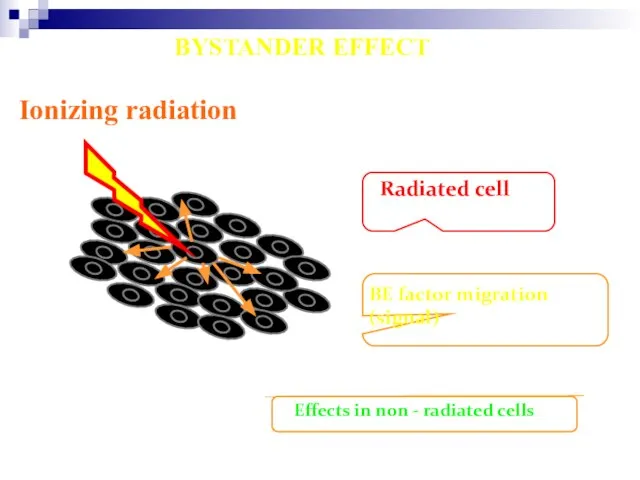
- 93. Ionizing radiation Radiated cell BE factor migration (signal) Effects in non - radiated cells BYSTANDER EFFECT
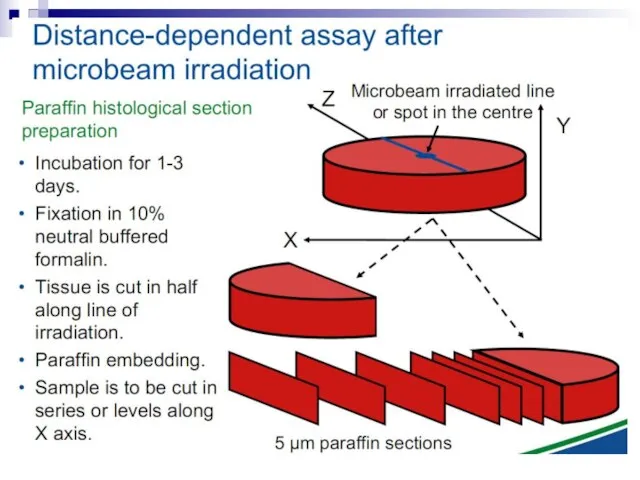
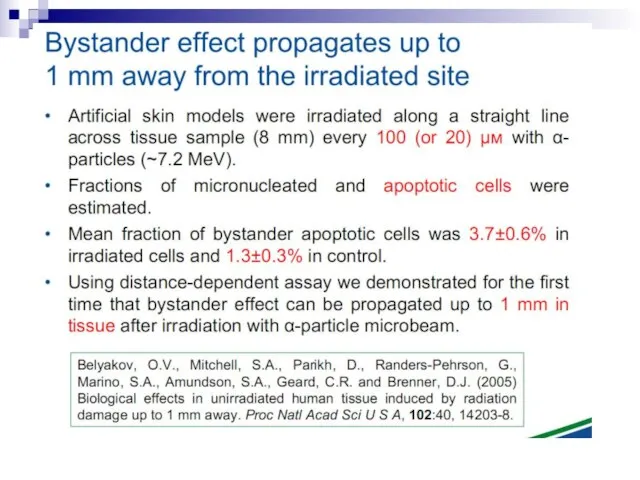
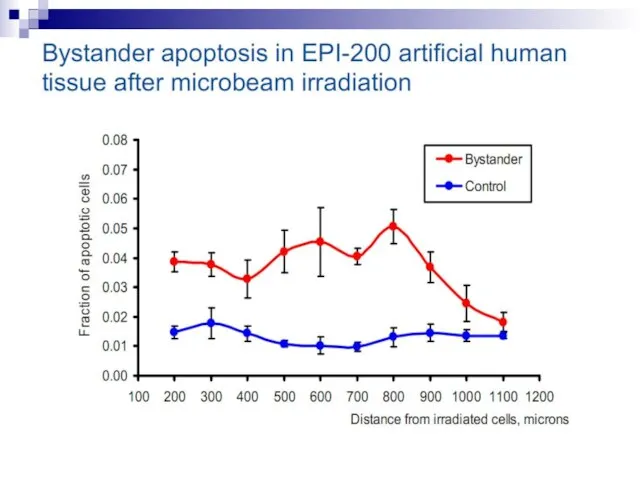
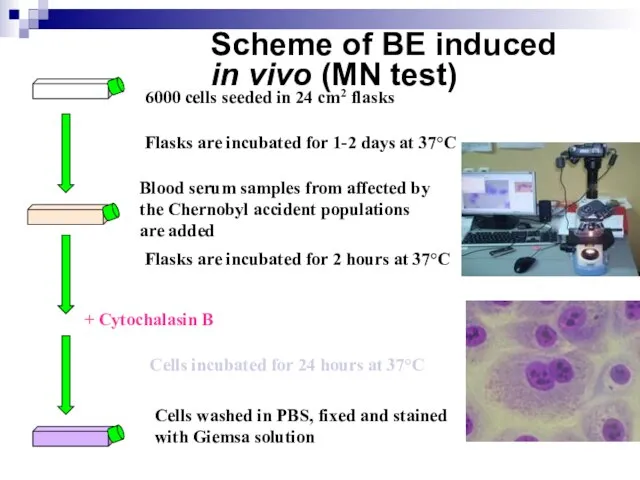
- 94. Scheme of BE induced in vivo (MN test) 6000 cells seeded in 24 cm2 flasks Blood
- 96. Killing Non-transduced Tumor Cells via Bystander Effect The bystander effect was first reported by Moolten (1986)
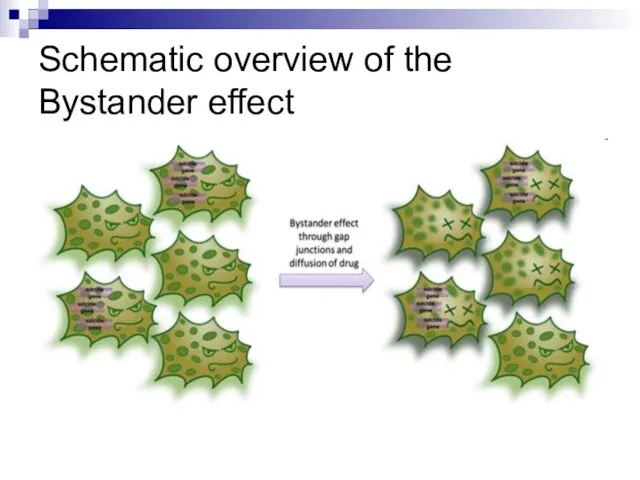
- 97. Schematic overview of the Bystander effect
- 98. Our understanding of how radiation kills normal and tumour cells has been based on an intimate
- 99. When ionizing radiation interacts with biological material, energy is deposited and chemical bonds are broken. In
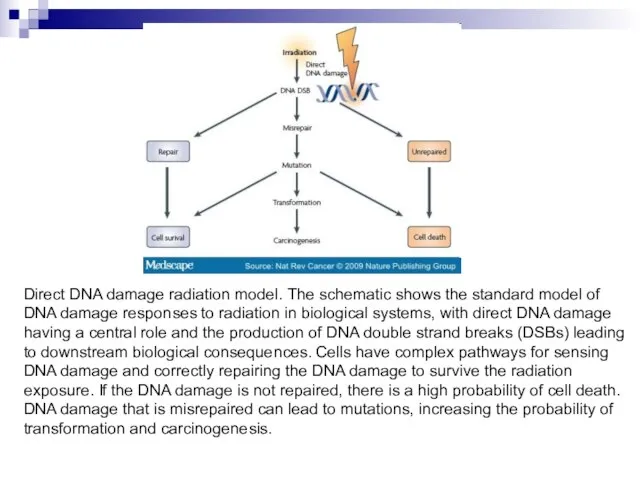
- 100. Direct DNA damage radiation model. The schematic shows the standard model of DNA damage responses to
- 101. The mechanisms underpinning DNA damage and repair processing in irradiated cells have been extensively studied since
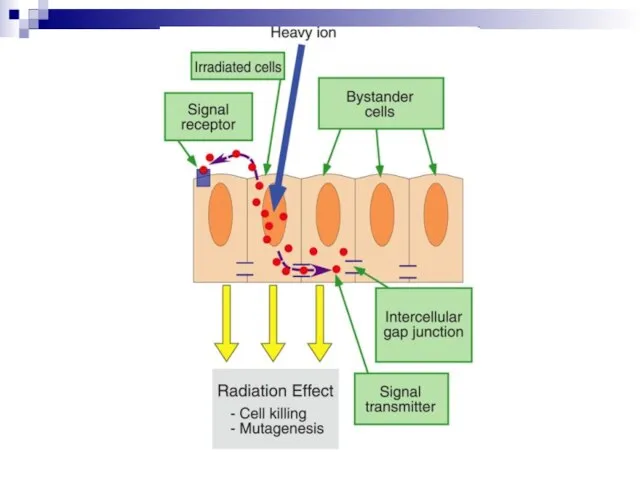
- 102. Evidence now shows that, as well as these direct DNA damage-dependent effects, irradiated cells also send
- 103. For example, the archetypal gene therapy model is the herpes simplex virus-thymidine kinase (HSV-TK) system. In
- 104. Radiation-induced bystander responses have been observed in a range of cell types, tissue models and in
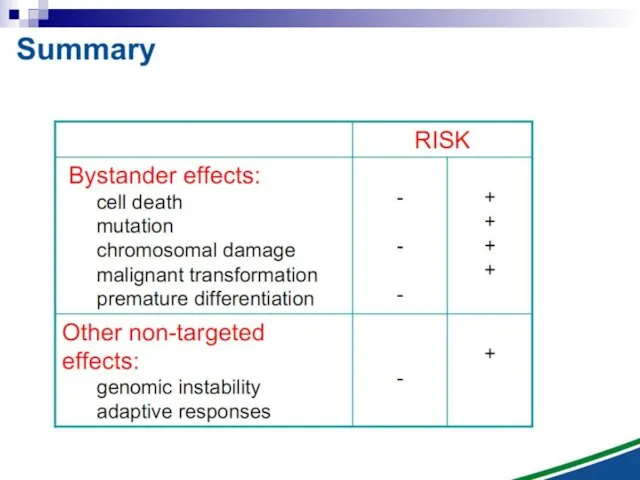
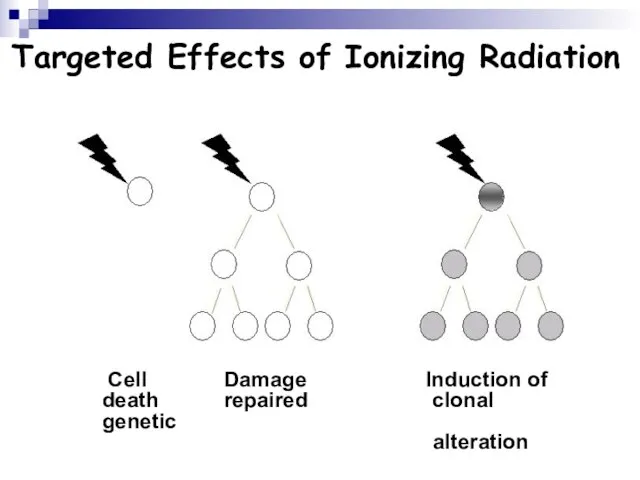
- 105. Targeted Effects of Ionizing Radiation Cell Damage Induction of death repaired clonal genetic alteration
- 106. Untargeted Effects of Exposure to Ionizing Radiation Effects in unexposed cells and their progeny i.e. in
- 107. Radiation-induced Genomic Instability micronucleus chromosome aberration cell death gene mutation mitotic failure aneuploidy
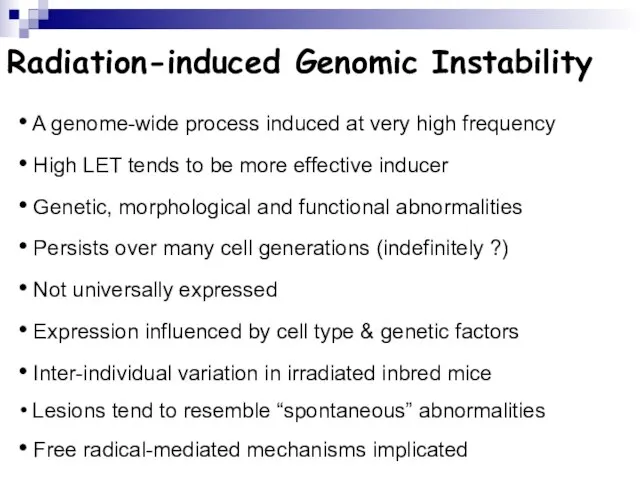
- 108. Radiation-induced Genomic Instability A genome-wide process induced at very high frequency High LET tends to be
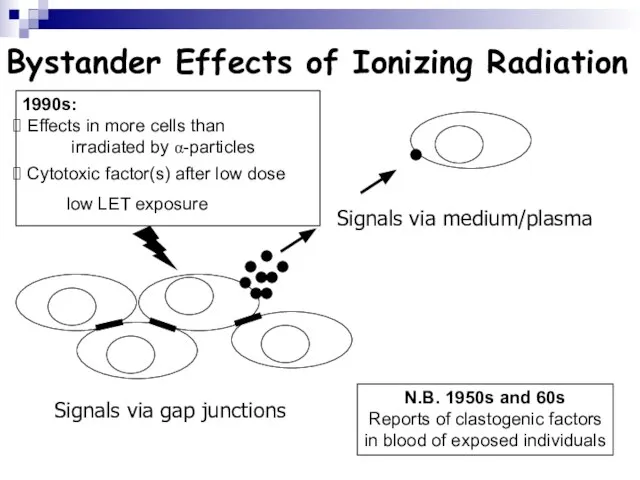
- 109. Bystander Effects of Ionizing Radiation Signals via gap junctions Signals via medium/plasma N.B. 1950s and 60s
- 110. Bystander Effects of Ionizing Radiation Increases in damage-inducible proteins Decreases in damage-inducible proteins Increases in reactive
- 111. Bystander Effects of Ionizing Radiation Target for biological effects is larger than the cell Important implications
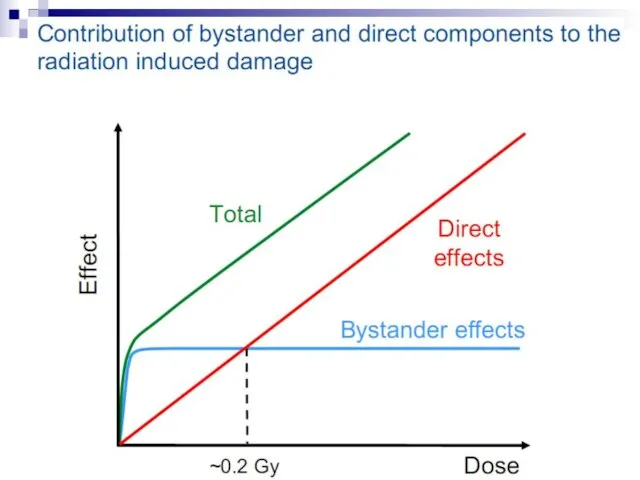
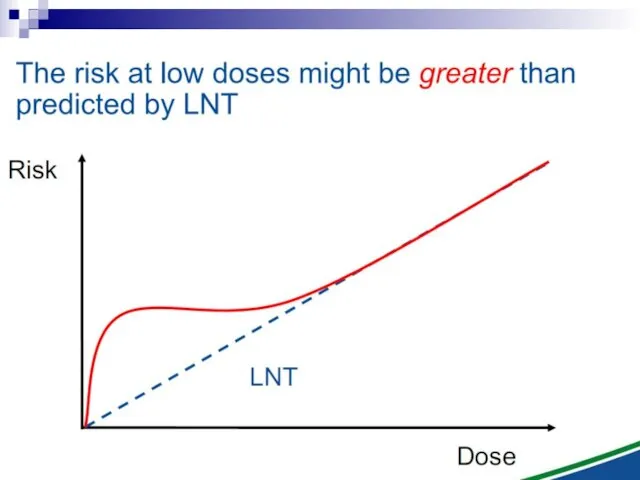
- 112. The Linear No Threshold Problem Induced Effect Dose Threshold supralinear Supralinear linear Threshold sublinear Hormesis Non-targeted
- 113. DNA Damage Genome stability
- 115. Скачать презентацию
























 Возрождение
Возрождение Презентация на тему Моя семья
Презентация на тему Моя семья Я тінь Анни Кареніної. Невід'ємна частинка її життя та смерті
Я тінь Анни Кареніної. Невід'ємна частинка її життя та смерті Изобразительное искусство
Изобразительное искусство Требования к степени очистки сточных вод при их отведении в поверхностные водные объекты
Требования к степени очистки сточных вод при их отведении в поверхностные водные объекты Бочки
Бочки 20170929_trebovaniya_k_kimam_-_kopiya
20170929_trebovaniya_k_kimam_-_kopiya Специальные конструкционные стали
Специальные конструкционные стали Фердинанд де Соссюр
Фердинанд де Соссюр Информационные процессы
Информационные процессы Факторы размещения предприятий черной и цветной металлургии
Факторы размещения предприятий черной и цветной металлургии Бытие как тема философии
Бытие как тема философии Авторское право и смежные права
Авторское право и смежные права Summer Dreams
Summer Dreams  Экскурсия на ТЭЦ-1 г. Казани
Экскурсия на ТЭЦ-1 г. Казани Нашествие с Востока Почему монголо – татары победили русских князей?
Нашествие с Востока Почему монголо – татары победили русских князей? Пример организации контроля доступа на территорию ЖК Триумф Палас
Пример организации контроля доступа на территорию ЖК Триумф Палас ОБЩЕУЧЕБНЫЕ УМЕНИЯ И НАВЫКИ КАК ЭЛЕМЕНТЫ ОЦЕНИВАНИЯ В НОВЫХ СТАНДАРТАХ ОБРАЗОВАНИЯ
ОБЩЕУЧЕБНЫЕ УМЕНИЯ И НАВЫКИ КАК ЭЛЕМЕНТЫ ОЦЕНИВАНИЯ В НОВЫХ СТАНДАРТАХ ОБРАЗОВАНИЯ Презентация на тему Грамматика со Смешариками
Презентация на тему Грамматика со Смешариками Применение нитратов
Применение нитратов Осударственный информационный ресурс бухгалтерской (финансовой) отчетности
Осударственный информационный ресурс бухгалтерской (финансовой) отчетности Консультирование истеричных личностей
Консультирование истеричных личностей Презентация Ветер
Презентация Ветер Деепричастный оборот
Деепричастный оборот Нормативно-правовое обеспечение ВФСК ГТО
Нормативно-правовое обеспечение ВФСК ГТО Презентация на тему Предмет философии
Презентация на тему Предмет философии Афганистан
Афганистан конструирование моделирование
конструирование моделирование