Содержание
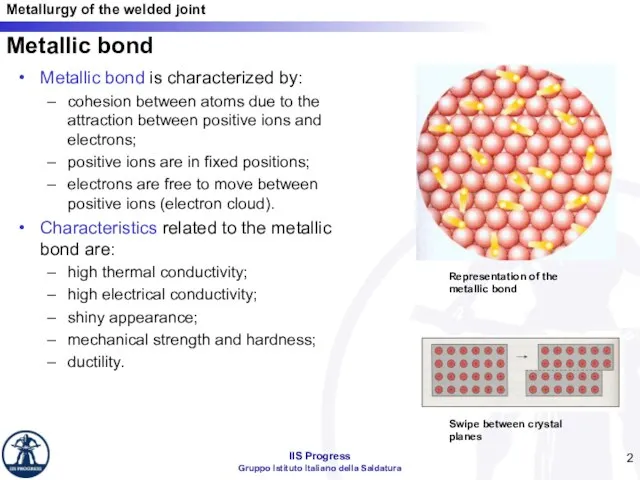
- 2. Metallic bond Metallic bond is characterized by: cohesion between atoms due to the attraction between positive
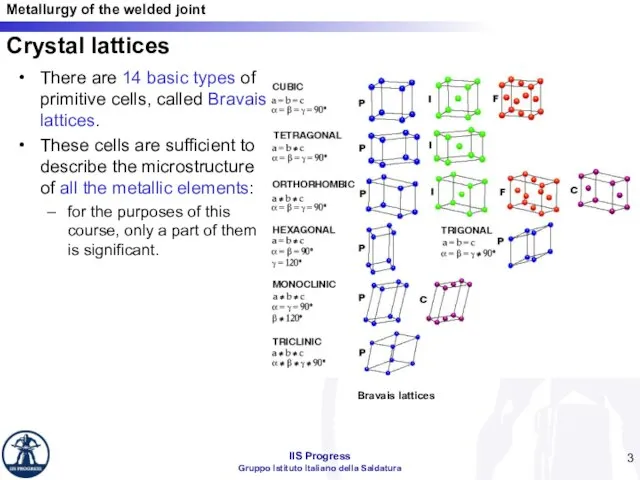
- 3. There are 14 basic types of primitive cells, called Bravais lattices. These cells are sufficient to
- 4. Monomorphic and polymorphic metallic materials The metallic elements can be divided into: monomorphic elements: always have
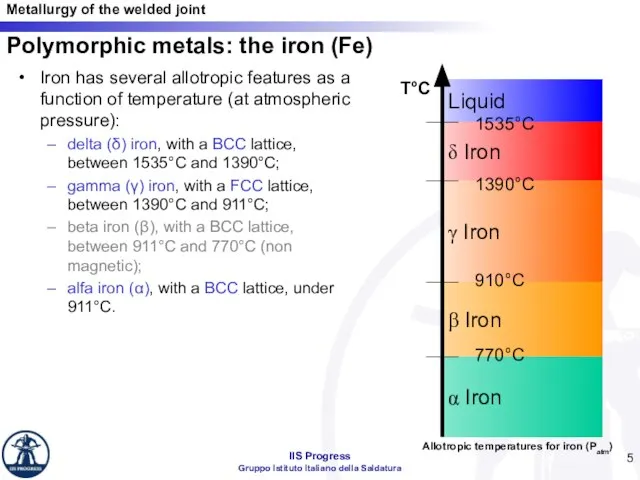
- 5. Iron has several allotropic features as a function of temperature (at atmospheric pressure): delta (δ) iron,
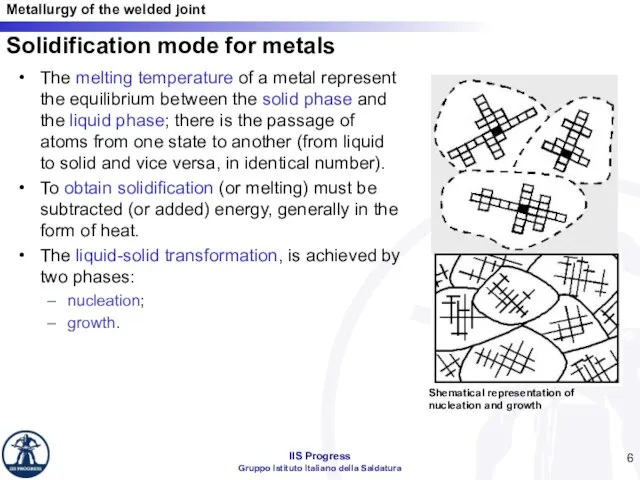
- 6. Solidification mode for metals The melting temperature of a metal represent the equilibrium between the solid
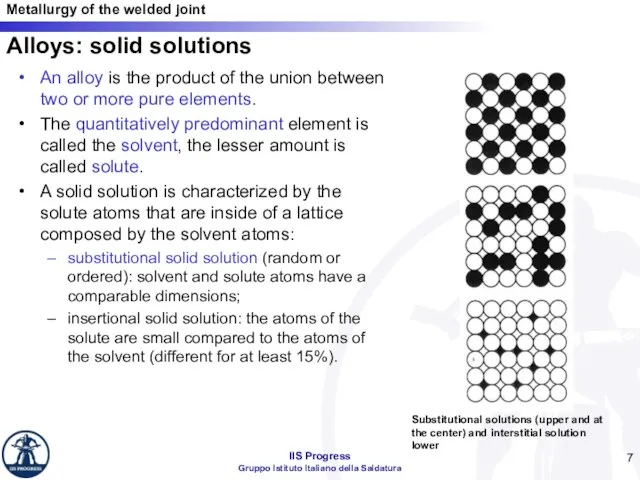
- 7. Alloys: solid solutions An alloy is the product of the union between two or more pure
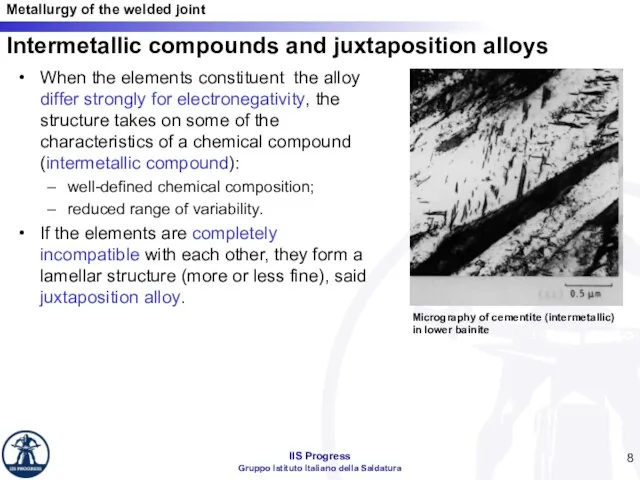
- 8. Intermetallic compounds and juxtaposition alloys When the elements constituent the alloy differ strongly for electronegativity, the
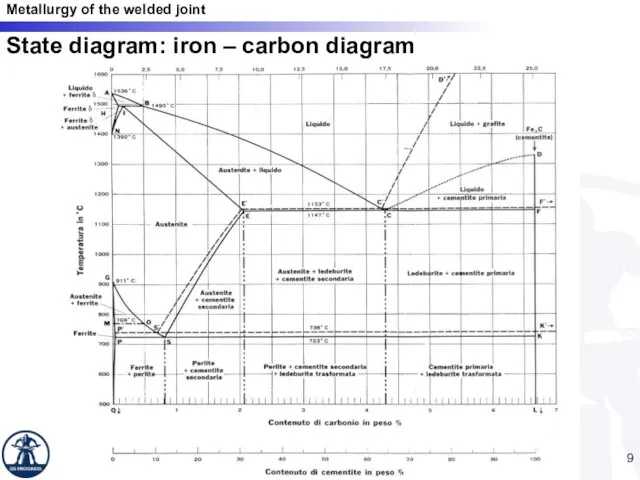
- 9. State diagram: iron – carbon diagram
- 10. Cooling speed It’s the main parameter that influences the transformations at the solid state. For carbon
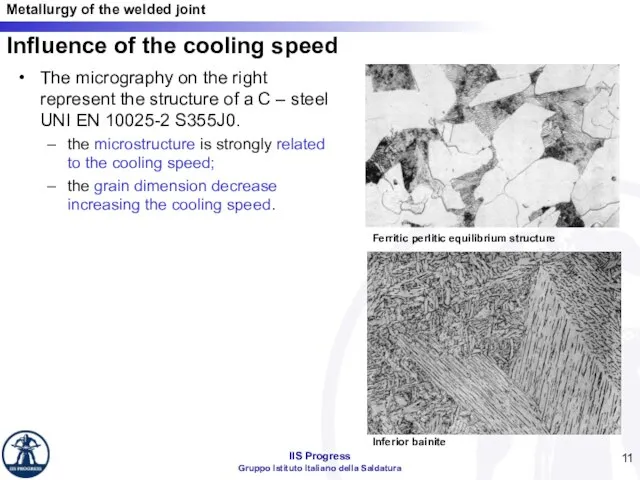
- 11. Influence of the cooling speed The micrography on the right represent the structure of a C
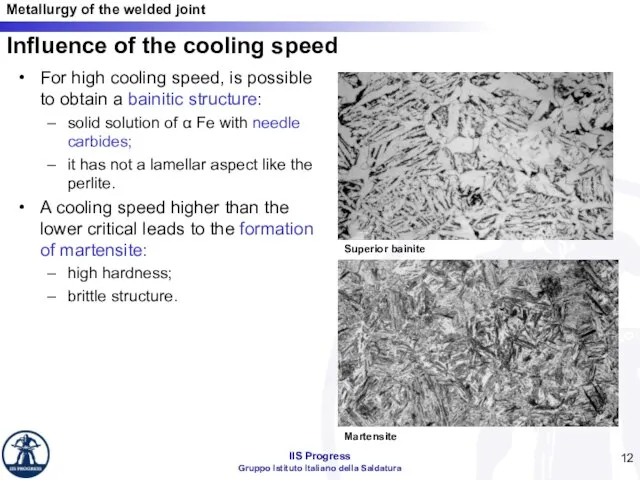
- 12. For high cooling speed, is possible to obtain a bainitic structure: solid solution of α Fe
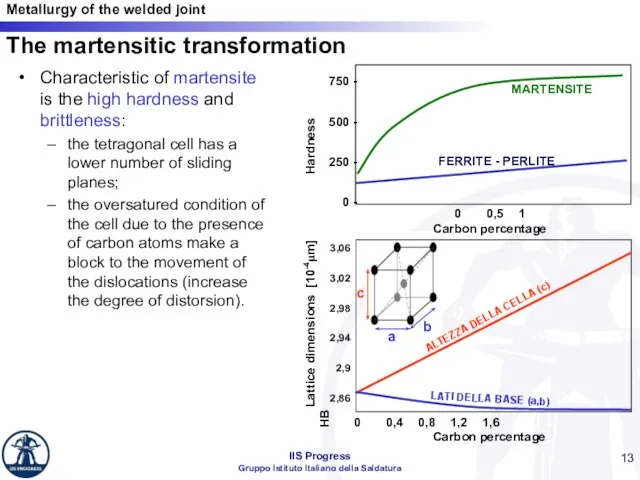
- 13. Characteristic of martensite is the high hardness and brittleness: the tetragonal cell has a lower number
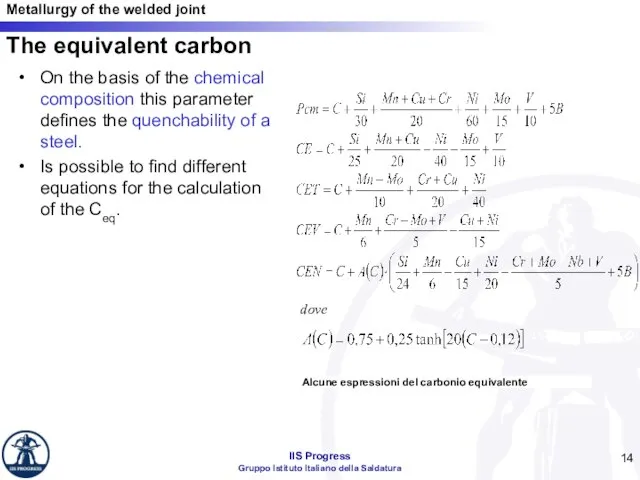
- 14. The equivalent carbon On the basis of the chemical composition this parameter defines the quenchability of
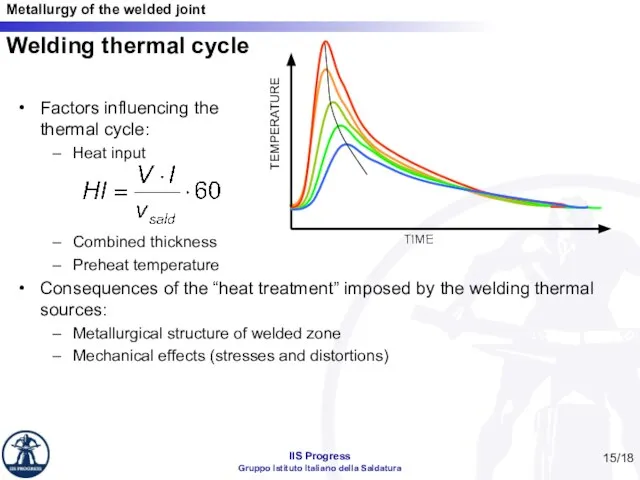
- 15. Welding thermal cycle Factors influencing the thermal cycle: Heat input Combined thickness Preheat temperature Consequences of
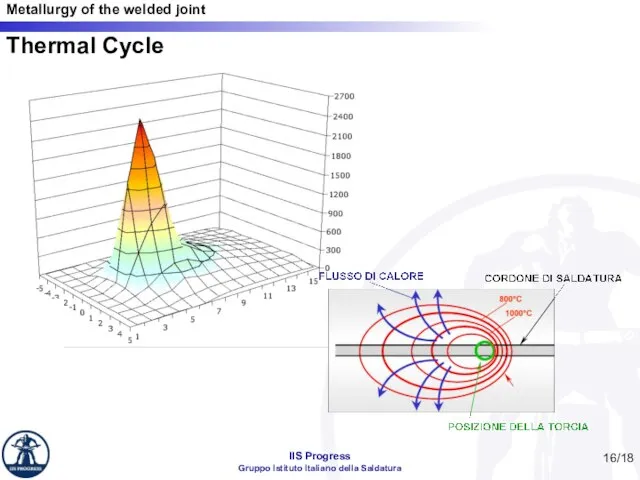
- 16. Thermal Cycle /18
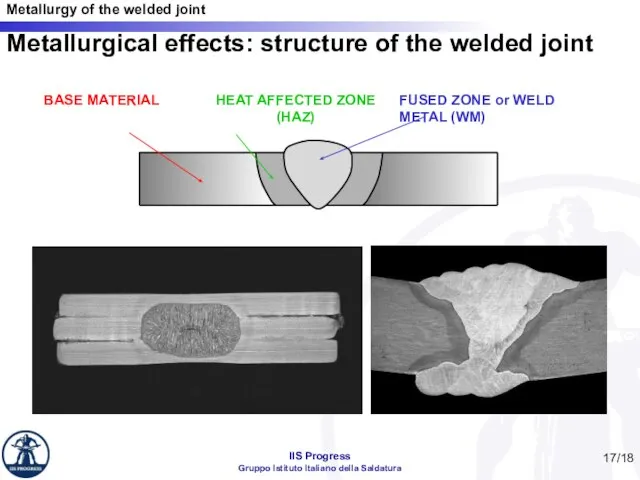
- 17. /18 Metallurgical effects: structure of the welded joint
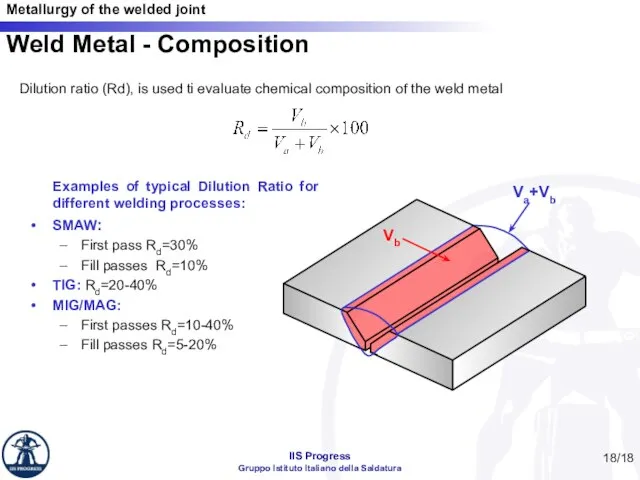
- 18. /18 Weld Metal - Composition Dilution ratio (Rd), is used ti evaluate chemical composition of the
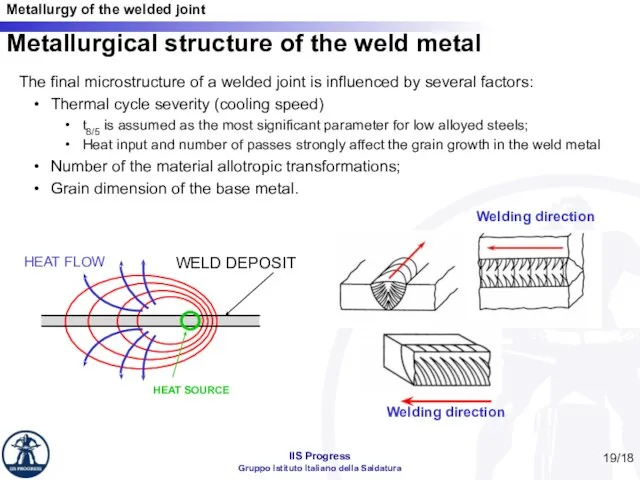
- 19. /18 Metallurgical structure of the weld metal Welding direction Welding direction The final microstructure of a
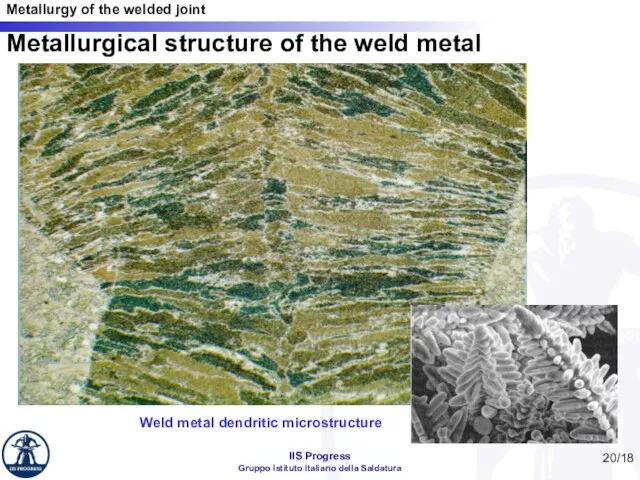
- 20. /18 Metallurgical structure of the weld metal Weld metal dendritic microstructure
- 21. /18 Heat Affected Zone The heat-affected zone, includes those regions that are measurably influenced by the
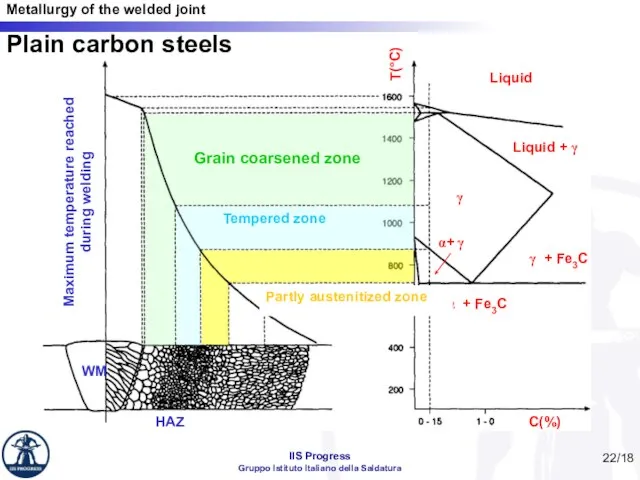
- 22. /18 C(%) T(°C) Liquid Liquid + γ γ γ + Fe3C α + Fe3C α+ γ
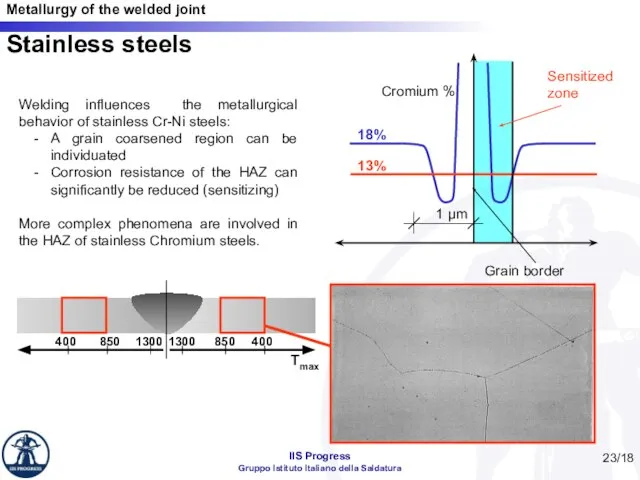
- 23. /18 Stainless steels Welding influences the metallurgical behavior of stainless Cr-Ni steels: A grain coarsened region
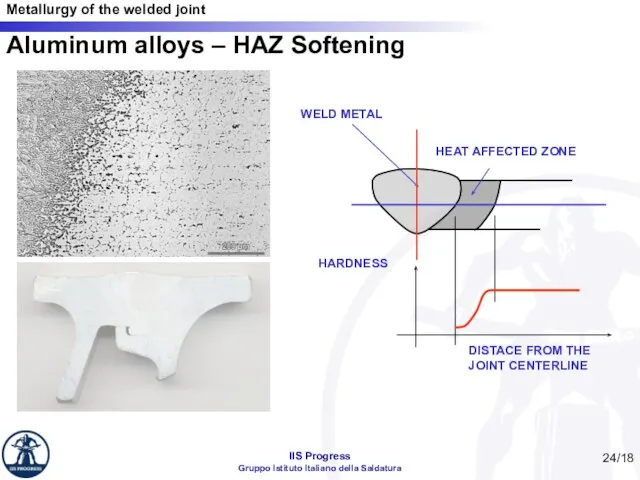
- 24. /18 Aluminum alloys – HAZ Softening
- 25. The feasibility of welding a particular metal or alloy. A number of factors affect weldability including
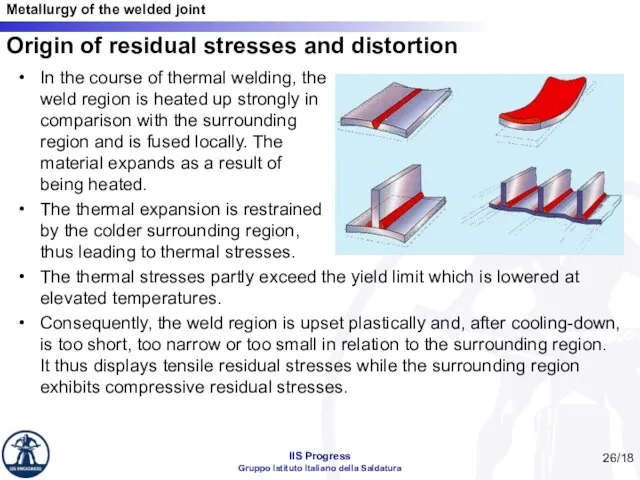
- 26. Origin of residual stresses and distortion In the course of thermal welding, the weld region is
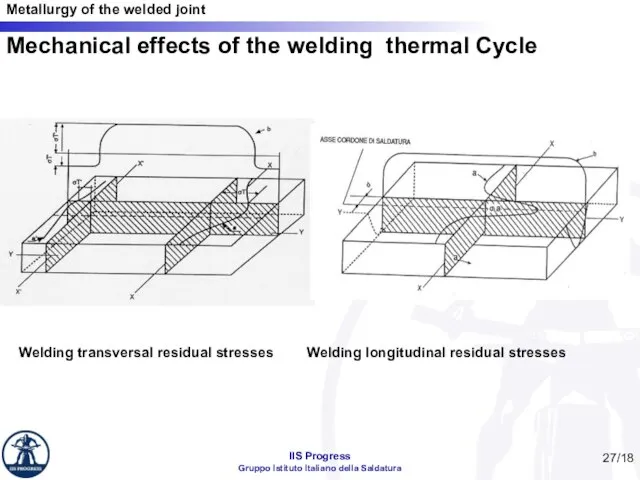
- 27. Mechanical effects of the welding thermal Cycle Welding transversal residual stresses Welding longitudinal residual stresses /18
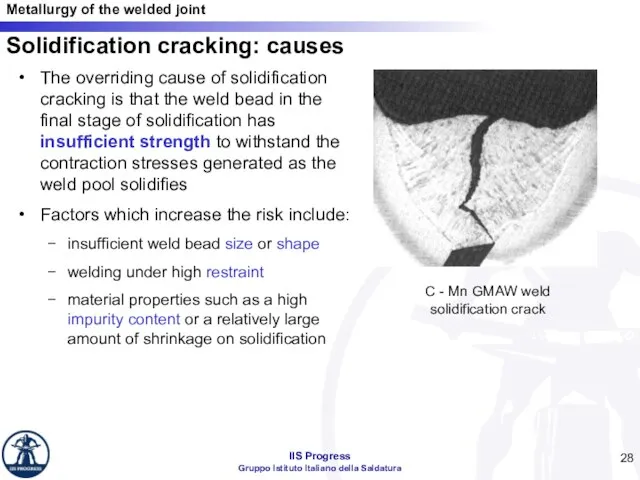
- 28. Solidification cracking: causes The overriding cause of solidification cracking is that the weld bead in the
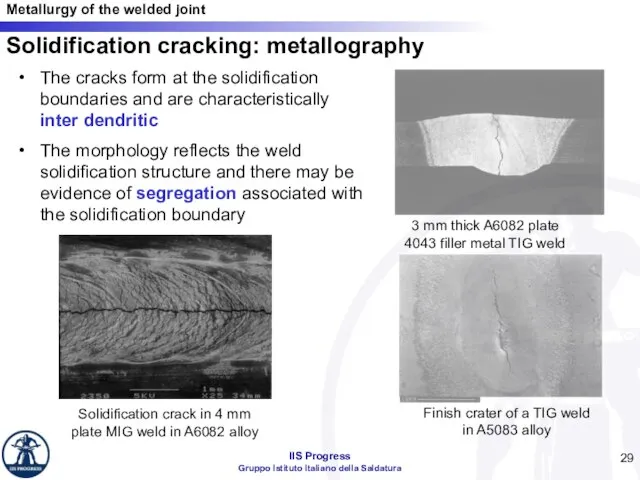
- 29. Solidification cracking: metallography The cracks form at the solidification boundaries and are characteristically inter dendritic The
- 30. Hydrogen cold cracking Hydrogen cracking may also be called cold cracking or delayed cracking The principal
- 31. Lamellar tearing Lamellar tearing can occur beneath the weld especially in rolled steel plate which has
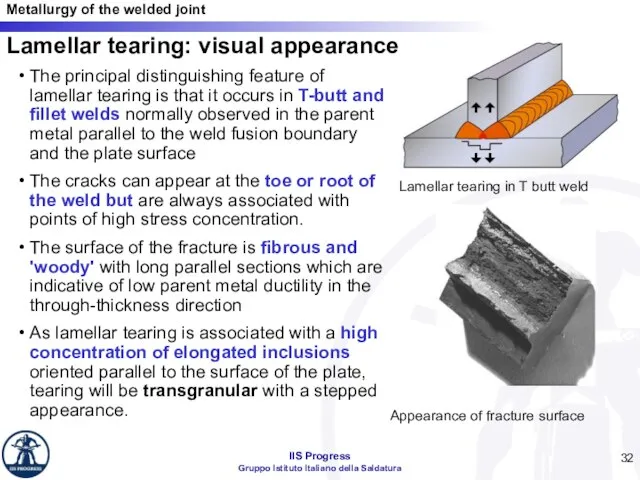
- 32. Lamellar tearing: visual appearance The principal distinguishing feature of lamellar tearing is that it occurs in
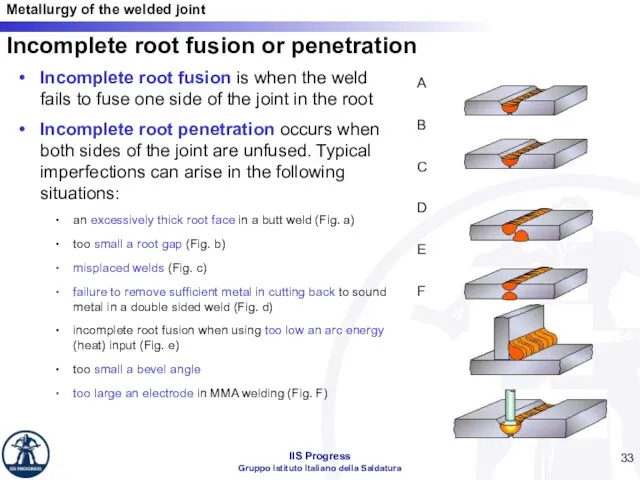
- 33. Incomplete root fusion or penetration Incomplete root fusion is when the weld fails to fuse one
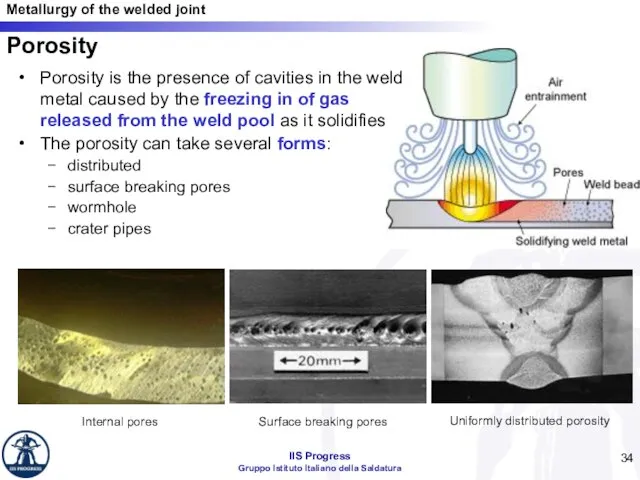
- 34. Porosity Porosity is the presence of cavities in the weld metal caused by the freezing in
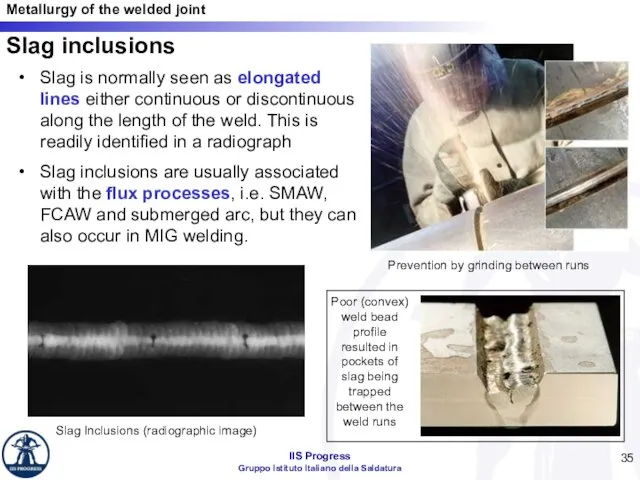
- 35. Slag inclusions Slag is normally seen as elongated lines either continuous or discontinuous along the length
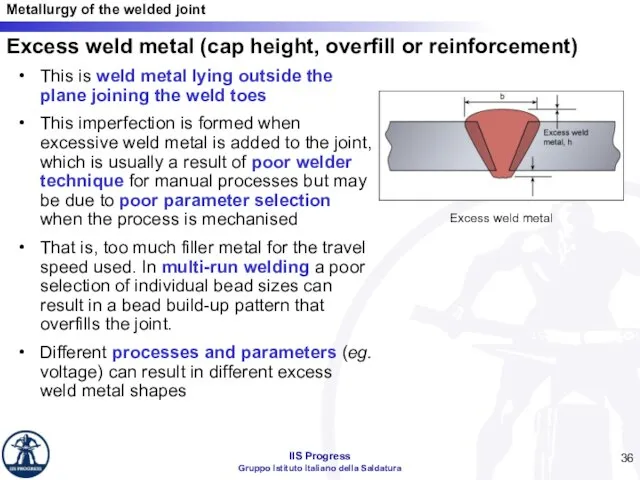
- 36. Excess weld metal (cap height, overfill or reinforcement) This is weld metal lying outside the plane
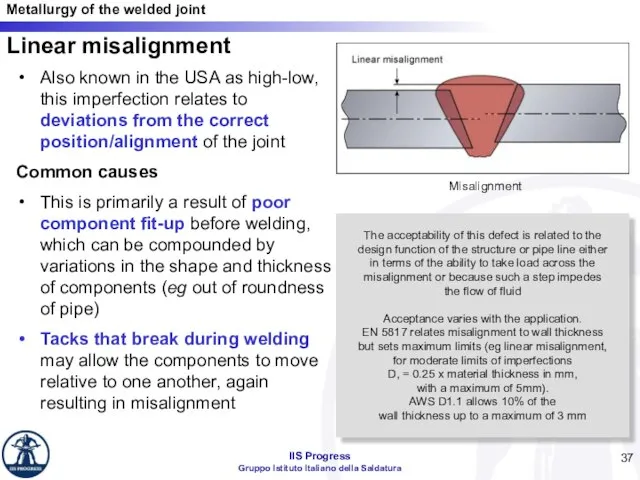
- 37. Linear misalignment Also known in the USA as high-low, this imperfection relates to deviations from the
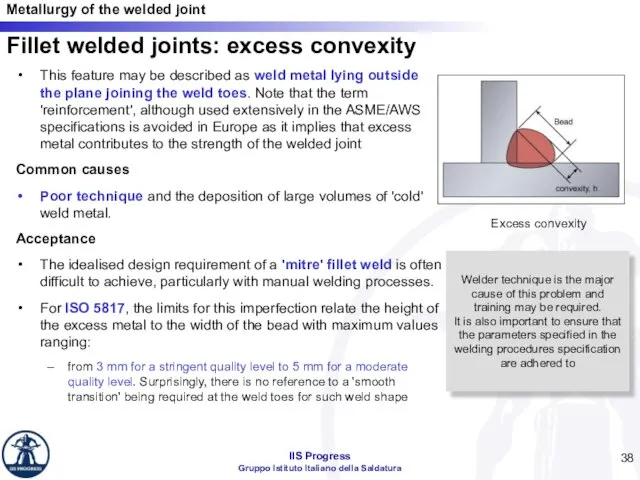
- 38. Fillet welded joints: excess convexity This feature may be described as weld metal lying outside the
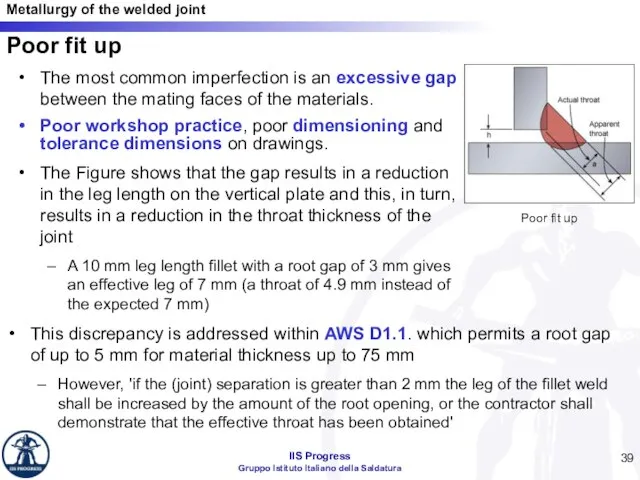
- 39. Poor fit up The most common imperfection is an excessive gap between the mating faces of
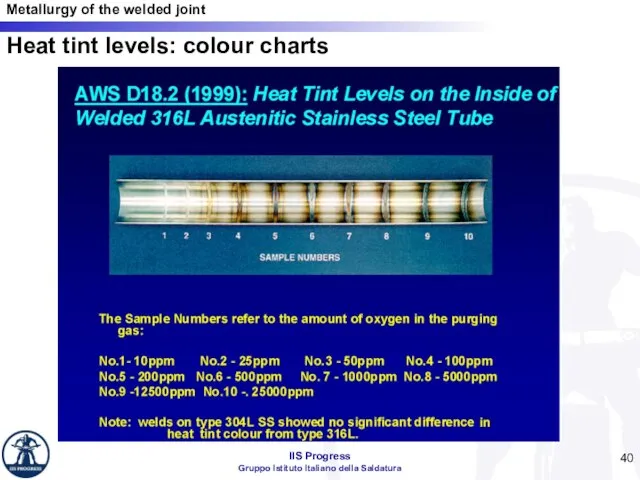
- 40. Heat tint levels: colour charts
- 42. Скачать презентацию




 Дымковские барышни
Дымковские барышни Эксклюзивный офис в Доме на Композиторской
Эксклюзивный офис в Доме на Композиторской Драма как род литературы
Драма как род литературы Лечение от табакокурения
Лечение от табакокурения Проведение Дня качества в регионах РФ
Проведение Дня качества в регионах РФ ГОСУДАРСТВЕННО-ЧАСТНОЕ ПАРТНЕРСТВО – ЧТО, ЗАЧЕМ, ДЛЯ ЧЕГО
ГОСУДАРСТВЕННО-ЧАСТНОЕ ПАРТНЕРСТВО – ЧТО, ЗАЧЕМ, ДЛЯ ЧЕГО Международный маркетинг
Международный маркетинг Коты в китайской живописи
Коты в китайской живописи Перечень личного снаряжения для однодневного турпохода в зависимости от времени года (весна, осень)
Перечень личного снаряжения для однодневного турпохода в зависимости от времени года (весна, осень) Курение: дань моде, привычка, болезнь
Курение: дань моде, привычка, болезнь 6отношение организмов
6отношение организмов презентация+по+обществознанию+РЕЛИГИЯ
презентация+по+обществознанию+РЕЛИГИЯ Обеспечение АС ГКН информацией об адресах объектов капитального строительства
Обеспечение АС ГКН информацией об адресах объектов капитального строительства Урок-игра «Таинственное путешествие»
Урок-игра «Таинственное путешествие» ANTIBIOTICS
ANTIBIOTICS Презентация на тему Интерференция света 11 класс
Презентация на тему Интерференция света 11 класс  ПЛАН РАБОТЫ МИНИСТЕРСТВА ЭКОНОМИЧЕСКОГО РАЗВИТИЯ И ТОРГОВЛИ КАБАРДИНО-БАЛКАРСКОЙ РЕСПУБЛИКИ НА 2012 ГОД
ПЛАН РАБОТЫ МИНИСТЕРСТВА ЭКОНОМИЧЕСКОГО РАЗВИТИЯ И ТОРГОВЛИ КАБАРДИНО-БАЛКАРСКОЙ РЕСПУБЛИКИ НА 2012 ГОД Преимущества Hitechnic Android
Преимущества Hitechnic Android Красота и целесообразность 7 класс
Красота и целесообразность 7 класс Мы говорим твердое : «НЕТ – наркотикам!»
Мы говорим твердое : «НЕТ – наркотикам!» Презентация на тему Заболевания ногтей
Презентация на тему Заболевания ногтей Жилища животных 7 класс
Жилища животных 7 класс МАРКЕТОЛОГ – ЭТО:
МАРКЕТОЛОГ – ЭТО: Сказ о Святом Николае
Сказ о Святом Николае Колебания и волны
Колебания и волны Жрек артериялар мен веналарды рылысы жаса сай ерекшеліктері
Жрек артериялар мен веналарды рылысы жаса сай ерекшеліктері Технология продажи продовольственных товаров: крахмал, сахар, мед
Технология продажи продовольственных товаров: крахмал, сахар, мед Swot - анализ
Swot - анализ