Содержание
- 2. The sub-atomic particles: protons, neutrons, electrons Элементарные частицы: протоны, нейтроны, электроны
- 3. New terms and definitions:
- 4. Atomic Structure Learning Objectives: Do I know .. The structure of an atom? About the relative
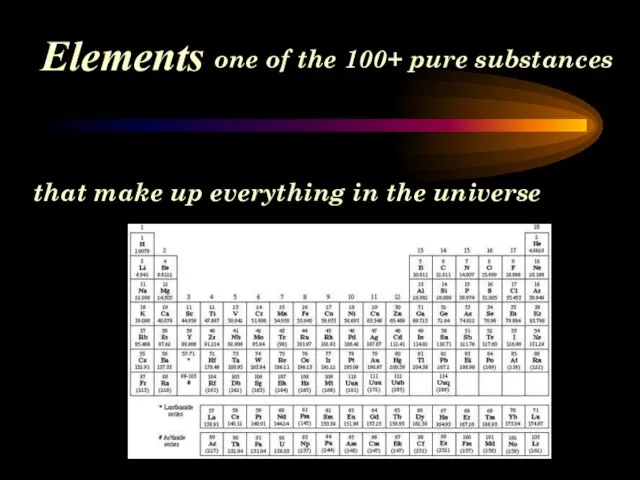
- 5. Elements one of the 100+ pure substances that make up everything in the universe
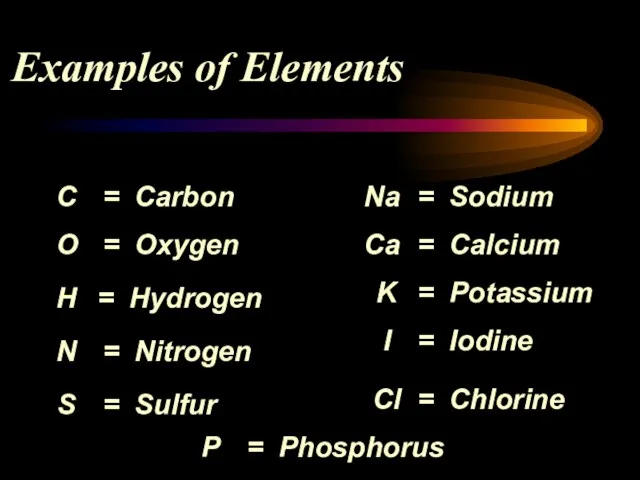
- 6. Examples of Elements
- 7. Working in pairs complete the following: Draw an atom It must include all the subatomic particles,
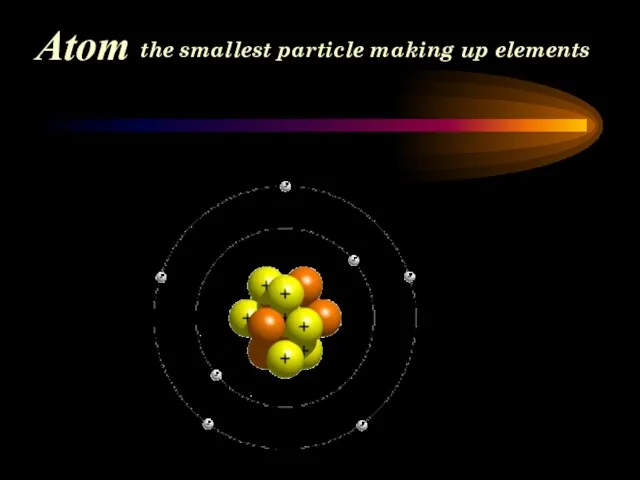
- 8. Atom the smallest particle making up elements
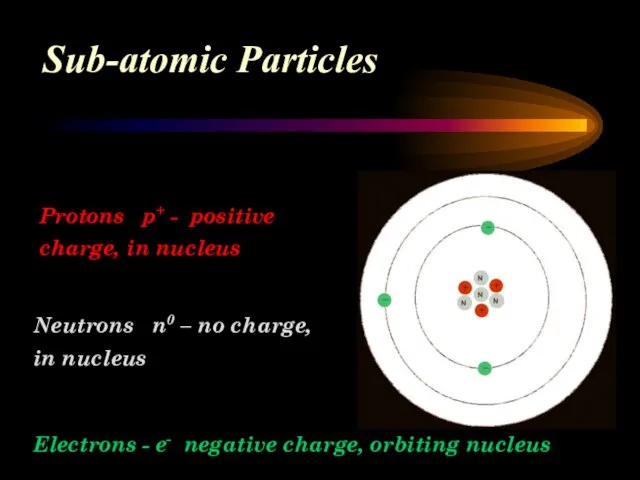
- 9. Sub-atomic Particles Protons p+ - positive charge, in nucleus Electrons - e- negative charge, orbiting nucleus
- 10. http://www.pil-network.com/resources/tools
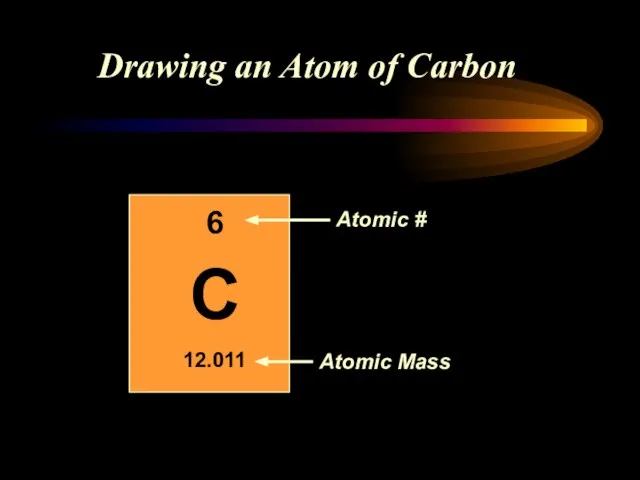
- 11. Drawing an Atom of Carbon
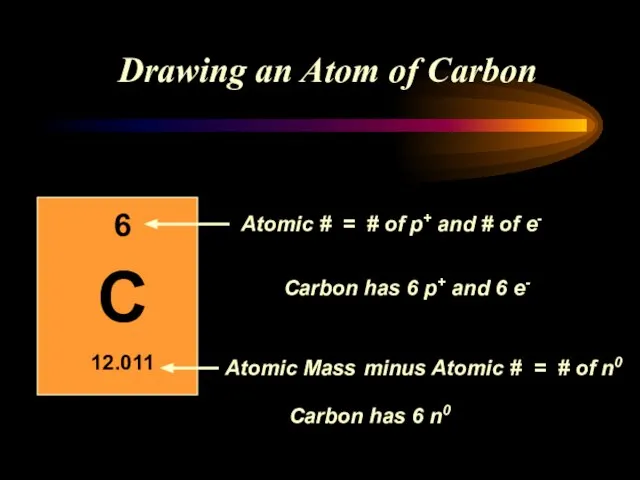
- 12. Drawing an Atom of Carbon minus Atomic # = # of n0 = # of p+
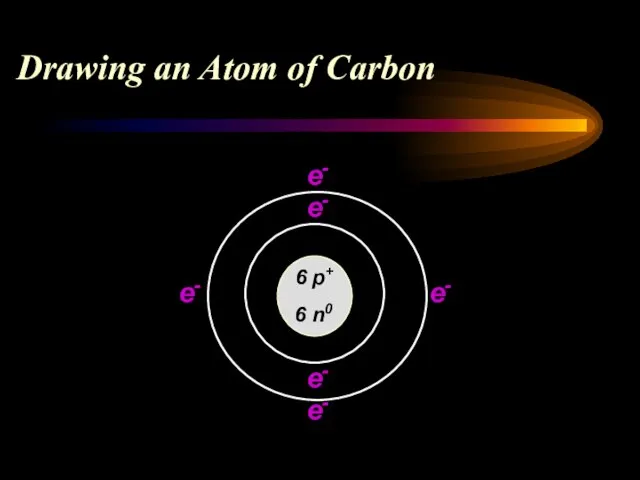
- 13. Drawing an Atom of Carbon
- 14. exercises Task 1. Determine the number of protons and electrons in the atoms of iron and
- 15. exercises Task 2. An atom of an element has 10 neutrons in the nucleus of an
- 16. Complete the handout in pairs
- 17. Assessment for learning…. Using the mini white board answer the following questions individually
- 18. How many protons does Silicon have?
- 19. What makes up the atomic weight of an atom?
- 20. How many electrons does a neutral Calcium atom have?
- 21. What element has one less proton than Boron?
- 22. What is the atomic number and Atomic mass of Argon?
- 23. Chemistry Diga, diga, diga, diga, that’s all folks!
- 25. Скачать презентацию















 Место и роли психолога в организации. Задачи психолога на предприятии
Место и роли психолога в организации. Задачи психолога на предприятии Интернет-активность российских и зарубежных пользователей.
Интернет-активность российских и зарубежных пользователей. Welcome to the Christmas game
Welcome to the Christmas game Вопрос А30. Лексическое значение слова
Вопрос А30. Лексическое значение слова 37318
37318 Нестандартные приёмы решения квадратных уравнений
Нестандартные приёмы решения квадратных уравнений Школьный проектМакаревич АлександрРуководитель регионального представительства Softline г. Калининград
Школьный проектМакаревич АлександрРуководитель регионального представительства Softline г. Калининград История космонавтики МОУ СОШ 2
История космонавтики МОУ СОШ 2 20170320_zap_sibir
20170320_zap_sibir Причины и начало ВФР
Причины и начало ВФР Роман Гончарова «Обломов» 10 класс
Роман Гончарова «Обломов» 10 класс Презентация на тему Скорость сближения и удаления
Презентация на тему Скорость сближения и удаления Teoria_nasilia
Teoria_nasilia К доске
К доске 2.1_
2.1_ квитанция
квитанция Организация дистанционного образования детей-инвалидов в 2011-2012 учебном году
Организация дистанционного образования детей-инвалидов в 2011-2012 учебном году Искусство сравнивать
Искусство сравнивать Старинные меры длинны
Старинные меры длинны Искусство Новгорода. Тест
Искусство Новгорода. Тест Новый год шагает по планете
Новый год шагает по планете Отчёт о расходовании средствблаготворительного фонда 2010 - 2011
Отчёт о расходовании средствблаготворительного фонда 2010 - 2011 Тригонометрические функции. Синус
Тригонометрические функции. Синус Птица – образ весны и тепла
Птица – образ весны и тепла Исаакиевский собор
Исаакиевский собор Конкурсная документация на грантовое финансирование молодых ученых по научным проектам на 2022-2024 годы
Конкурсная документация на грантовое финансирование молодых ученых по научным проектам на 2022-2024 годы Театральные этюды
Театральные этюды Словарное богатство русского языка. Синонимы. Антонимы
Словарное богатство русского языка. Синонимы. Антонимы