Содержание
- 2. Chemistry is a science that studies the properties of substances and how substances react with each
- 3. Who uses chemistry? Many people use chemistry as part of their work. Cooks use chemistry all
- 4. Where do the chemists work? People who have trained as chemists work in hospital laboratories, in
- 5. MATTER and states of matter Matter is anything that has mass and takes up a space.
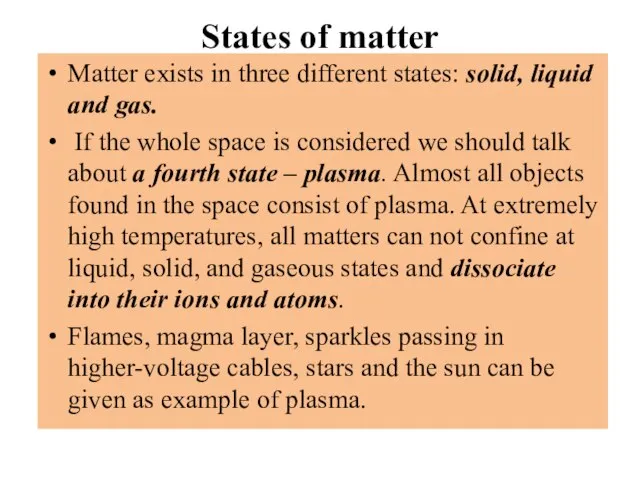
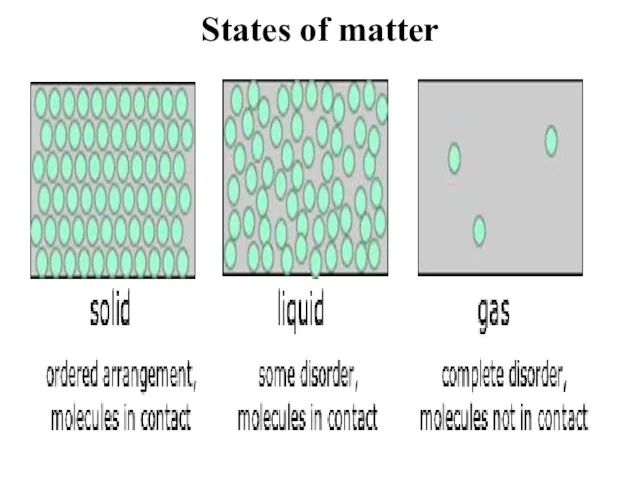
- 6. States of matter Matter exists in three different states: solid, liquid and gas. If the whole
- 7. States of matter
- 8. ‘substances’ Scientist also use the word ‘substances’. This means a particular type of matter, which you
- 9. ELEMENTS An element is one of a group of fundamental substances that cannot be broken down
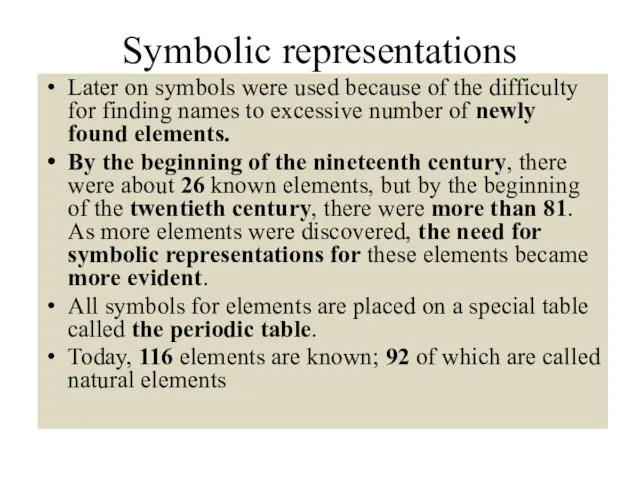
- 10. Symbolic representations Later on symbols were used because of the difficulty for finding names to excessive
- 11. THE MODERN PERIODIC TABLE The modern periodic table appeared as a function of the physical and
- 12. USAGE OF SOME ELEMENTS Hydrogen: A rocket fuel Production of hydrogen bomb Being the lightest of
- 13. Sodium: Production of electricity in nuclear reactors by transferring excess heat to the vapor turbines Its
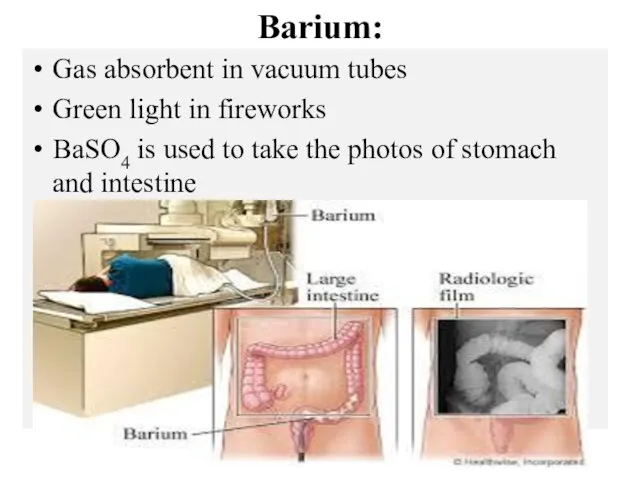
- 14. Barium: Gas absorbent in vacuum tubes Green light in fireworks BaSO4 is used to take the
- 15. Physical and chemical changes When we look around, in the world we live, we see some
- 16. Physical and chemical changes
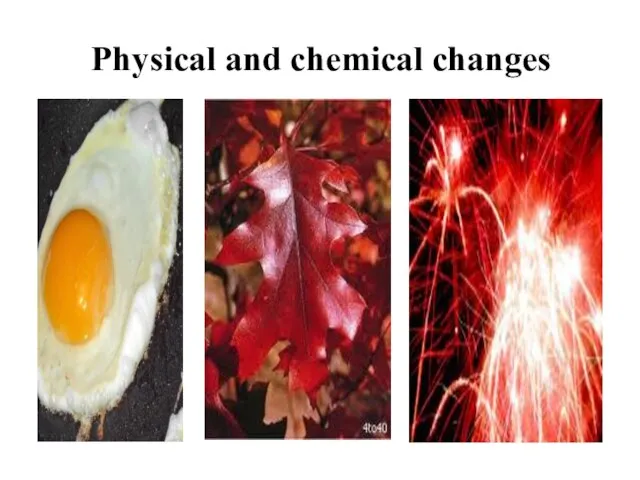
- 17. chemical changes Changes in the molecular structure of substances are called chemical changes. baking of cake
- 18. physical changes Evaporation of water, melting ice, dissolving of sugar in water, powdering marble, breaking of
- 20. Скачать презентацию










 I can jump. Spotlight 2
I can jump. Spotlight 2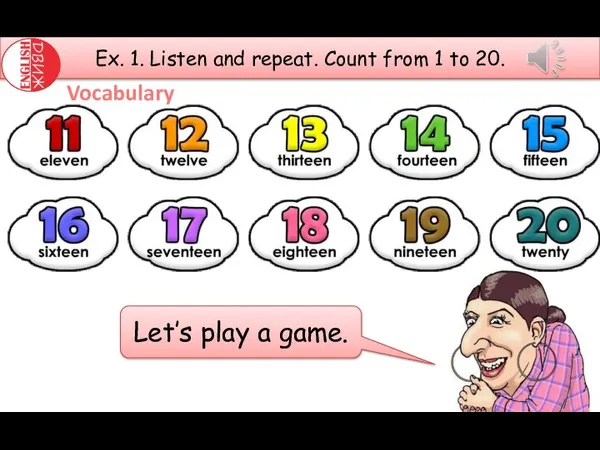 Count from 1 to 20. Let’s play a game
Count from 1 to 20. Let’s play a game Past Simple Memories
Past Simple Memories A day at the Airport
A day at the Airport Put the phrases in the correct order
Put the phrases in the correct order Раst Simple
Раst Simple Speaking English. Practice
Speaking English. Practice Ludwig Wittgenstein
Ludwig Wittgenstein Christmas anagramming
Christmas anagramming Articles a, an
Articles a, an When I was a child
When I was a child Problems of the environment
Problems of the environment Ancient mythical creatures
Ancient mythical creatures Write the name of showpalce and culture heritage on English
Write the name of showpalce and culture heritage on English Using technology to promote learner autonomy and good study habits
Using technology to promote learner autonomy and good study habits Установите соответствие тем текстам
Установите соответствие тем текстам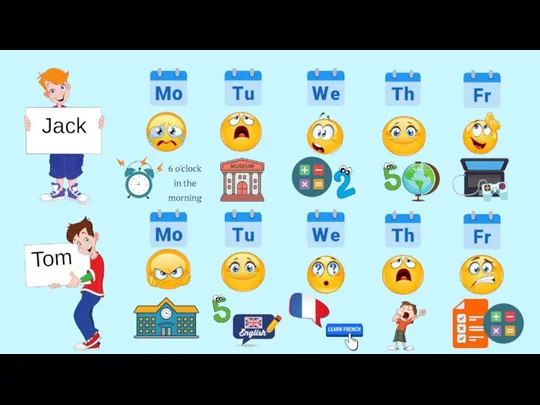 Days of the week
Days of the week Презентация на тему Healthy lifestyle (7 класс)
Презентация на тему Healthy lifestyle (7 класс)  Sweep, filthy, lather, rub
Sweep, filthy, lather, rub Checking up the hometask
Checking up the hometask Drought
Drought Summer course. Lesson 1
Summer course. Lesson 1 The adjectives
The adjectives On, in, under
On, in, under Fonetika Fairyland
Fonetika Fairyland Of time
Of time Singular - plural CW2
Singular - plural CW2 Points Tracker For online games and competitions
Points Tracker For online games and competitions