Слайд 2
Coordination compounds are the higher-order compounds which are stable in aqueous solutions

or dissociate insignificantly.
Слайд 3К4[Fe(CN)6]
Central atom (ion) or complexing agent takes central place in coordination compounds
![К4[Fe(CN)6] Central atom (ion) or complexing agent takes central place in coordination](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1005312/slide-2.jpg)
and is usually a positively charged ion
Fe2+
Ligands are ions of opposite charge or neutral molecules that are located (coordinated) around the complexing agent.
CN-
Слайд 4К4[Fe(CN)6]
Inner sphere (coordination entity) is formed by complexing agent and ligands.
[Fe(CN)6]4-
Outer sphere
![К4[Fe(CN)6] Inner sphere (coordination entity) is formed by complexing agent and ligands.](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1005312/slide-3.jpg)
is formed by Ions which are not included in the inner sphere.
K+
Слайд 5Classification of Coordination Compounds
Depending on the electric charge of the inner
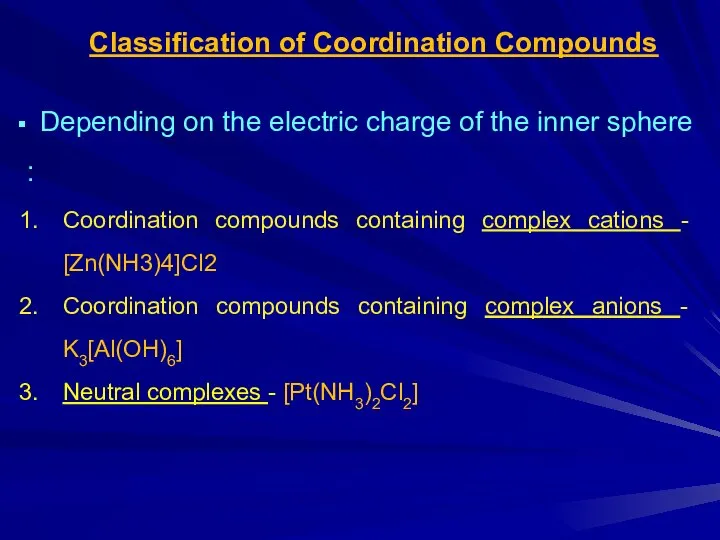
sphere :
Coordination compounds containing complex cations - [Zn(NH3)4]Cl2
Coordination compounds containing complex anions - K3[Al(OH)6]
Neutral complexes - [Pt(NH3)2Cl2]
Слайд 6Classification of Coordination Compounds
Depending on the nature of ligands:
Acid complexes -
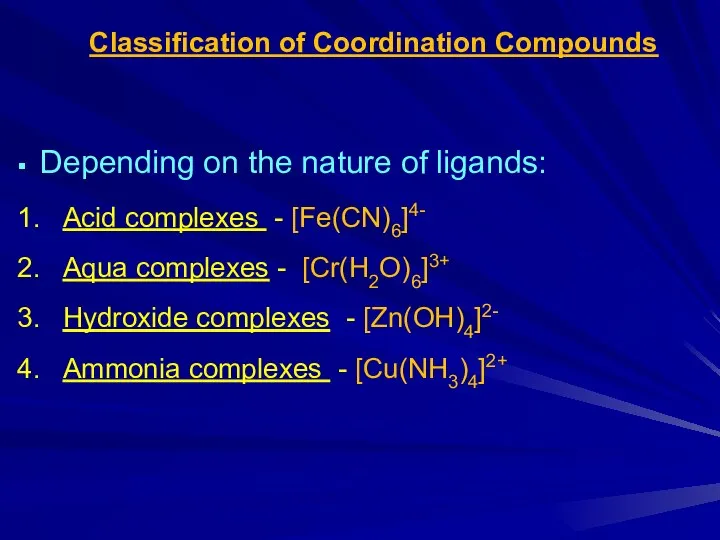
[Fe(CN)6]4-
Aqua complexes - [Cr(H2O)6]3+
Hydroxide complexes - [Zn(OH)4]2-
Ammonia complexes - [Cu(NH3)4]2+
Слайд 7Classification of Coordination Compounds
Depending on the chemical compounds class:
Acids - H[AuCl4]
Bases

- [Ag(NH3)2]OH
Salts - K2[HgI4]
Слайд 8Classification of Coordination Compounds
Depending on the quantity of central atoms:
Mononuclear -

[Cr(NH3)3(H2O)3]Cl3
Polynuclear - [Pt4(OH)4](ClO4)4
Слайд 9
When didentate or polydentate ligand uses its two or more donor atoms

to bind a single metal ion, it is said to be
a chelate ligand
The coordination compounds in which a ligand is bound with central atom by both donor-acceptor bond and ionic bond are called
chelates

![К4[Fe(CN)6] Central atom (ion) or complexing agent takes central place in coordination](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1005312/slide-2.jpg)
![К4[Fe(CN)6] Inner sphere (coordination entity) is formed by complexing agent and ligands.](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1005312/slide-3.jpg)





 Синичкин день
Синичкин день Хвойный лес
Хвойный лес Хромосомы. Кариотип. Жизненный цикл клетки
Хромосомы. Кариотип. Жизненный цикл клетки Исследования реакций адаптаций организма к высоким температурам
Исследования реакций адаптаций организма к высоким температурам Презентация на тему Зима в лесу
Презентация на тему Зима в лесу  Артериолы, венулы и капилляры м. мозговой оболочки кошки
Артериолы, венулы и капилляры м. мозговой оболочки кошки Модификационная изменчивость
Модификационная изменчивость Птицы
Птицы Презентация на тему Царство растений
Презентация на тему Царство растений  Огород в городе
Огород в городе Обитатели Черного моря
Обитатели Черного моря Prezentatsia_Organicheskie_veschestva_Uglevody_Belki
Prezentatsia_Organicheskie_veschestva_Uglevody_Belki Микроскоп. Части микроскопа
Микроскоп. Части микроскопа Общие вопросы. Задания 27. Часть 2-3
Общие вопросы. Задания 27. Часть 2-3 Развитие эволюционного учения (9)
Развитие эволюционного учения (9) Хеморецепция. Органы вкуса
Хеморецепция. Органы вкуса Презентация на тему ИСТОРИЯ РАЗВИТИЯ ГЕНЕТИКИ
Презентация на тему ИСТОРИЯ РАЗВИТИЯ ГЕНЕТИКИ  Времена года
Времена года Симметрия в природе
Симметрия в природе Головной мозг человека
Головной мозг человека Виртуальное путешествие в микромир
Виртуальное путешествие в микромир Двоякодышащие рыбы
Двоякодышащие рыбы Историческое прошлое людей
Историческое прошлое людей Прощание с ботаникой
Прощание с ботаникой Дружная семейка. Виртуальная выставка ко Дню любителей зоопарков
Дружная семейка. Виртуальная выставка ко Дню любителей зоопарков Тип членистоногие, класс насекомые, отряд стрекозы
Тип членистоногие, класс насекомые, отряд стрекозы Генетика пола
Генетика пола Углеводы: моносахариды, олигосахариды и полисахариды
Углеводы: моносахариды, олигосахариды и полисахариды