Содержание
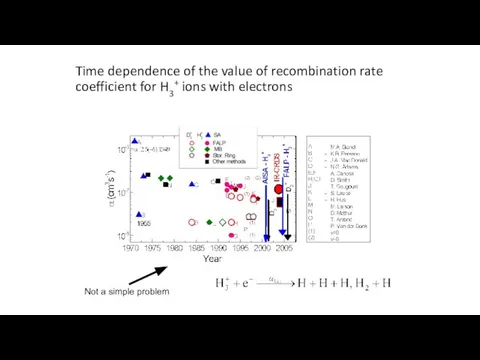
- 2. Time dependence of the value of recombination rate coefficient for H3+ ions with electrons Not a
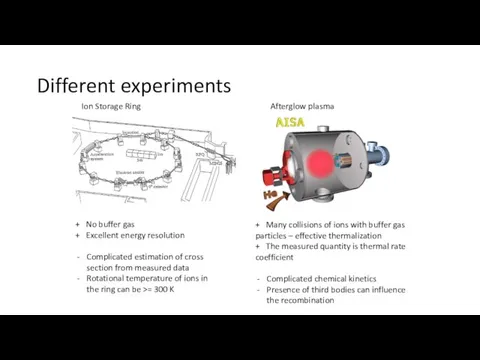
- 3. Different experiments Ion Storage Ring Afterglow plasma + No buffer gas + Excellent energy resolution Complicated
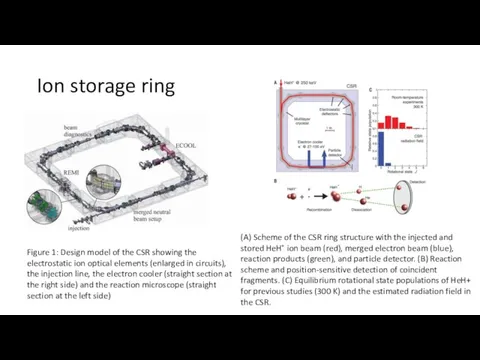
- 4. Ion storage ring Figure 1: Design model of the CSR showing the electrostatic ion optical elements
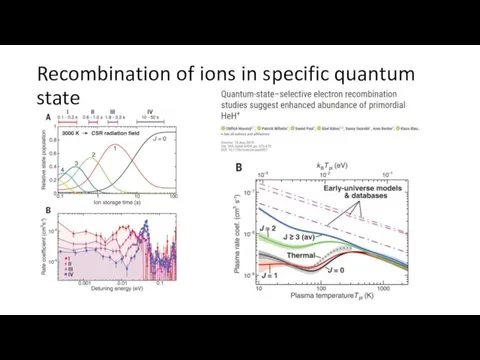
- 5. Recombination of ions in specific quantum state
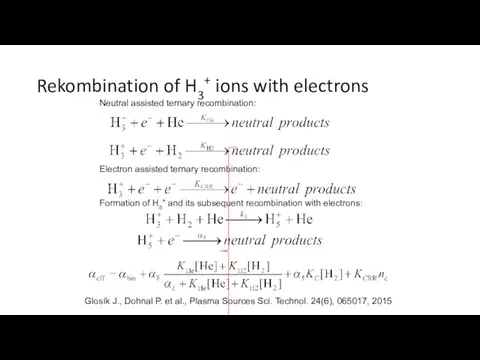
- 6. Rekombination of H3+ ions with electrons Neutral assisted ternary recombination: Electron assisted ternary recombination: Formation of
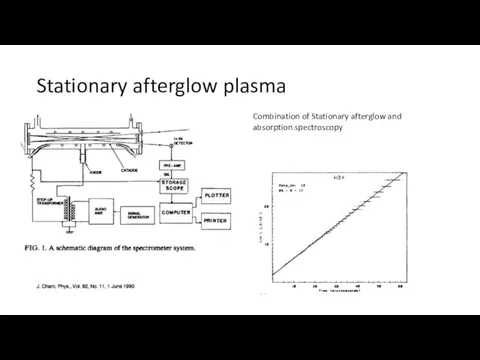
- 7. Stationary afterglow plasma Combination of Stationary afterglow and absorption spectroscopy
- 8. Stationary afterglow plasma AISA – Advanced Integrated Stationary Afterglow Mass spectrometer + Langmuir probe diagnostics Phys.
- 9. Stationary afterglow plasma Microwave diagnostics + mass spectrometry
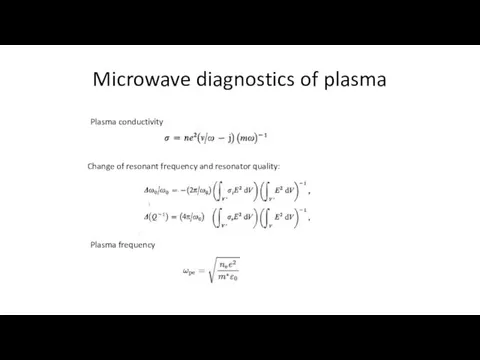
- 10. Microwave diagnostics of plasma Plasma conductivity Change of resonant frequency and resonator quality: Plasma frequency
- 11. Microwave diagnostics of plasma Sicha et al., Czech. J. Phys. B 20, 684, 1970 From the
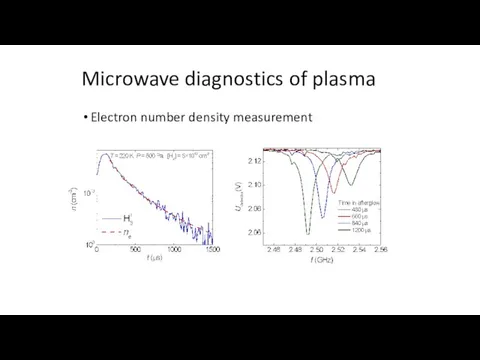
- 12. Electron number density measurement Microwave diagnostics of plasma
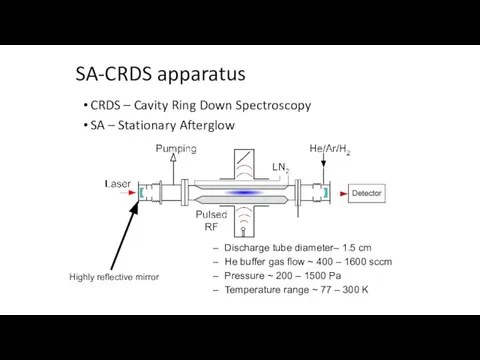
- 13. SA-CRDS apparatus CRDS – Cavity Ring Down Spectroscopy SA – Stationary Afterglow Highly reflective mirror Discharge
- 14. Cavity ringdown spectroscopy First used for mirror reflectivity determination (Herbelin et al. 1980). Later, the dependence
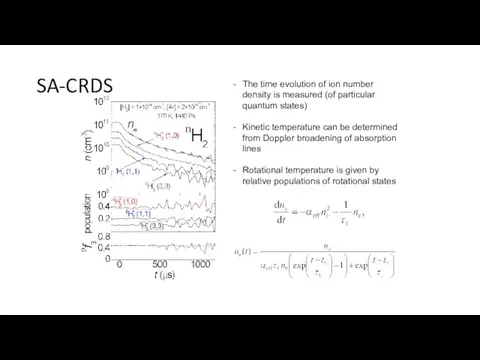
- 15. SA-CRDS The time evolution of ion number density is measured (of particular quantum states) Kinetic temperature
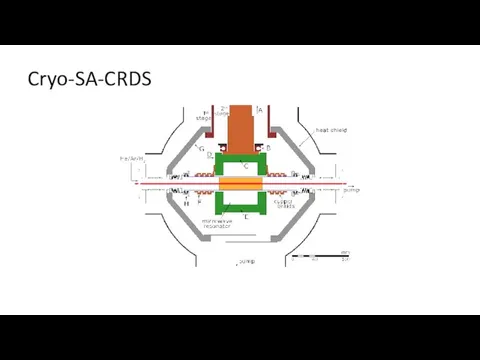
- 16. Cryo-SA-CRDS
- 17. Cryo-SA-CRDS
- 18. Cryo-SA-CRDS Plašil, R; Dohnal, P; Kálosi, Á; Roučka, Š; Shapko, D; Rednyk, S; Johnsen, R; Glosík,
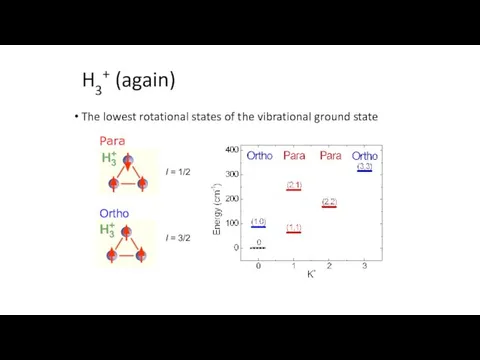
- 19. H3+ (again) The lowest rotational states of the vibrational ground state Para I = 1/2 I
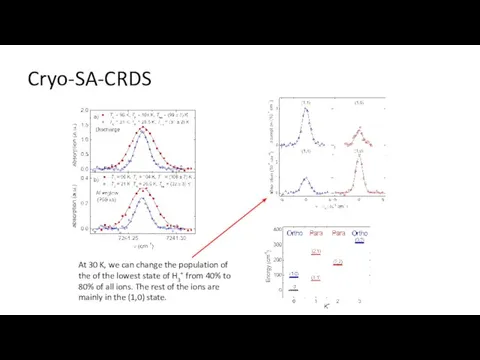
- 20. Cryo-SA-CRDS At 30 K, we can change the population of the of the lowest state of
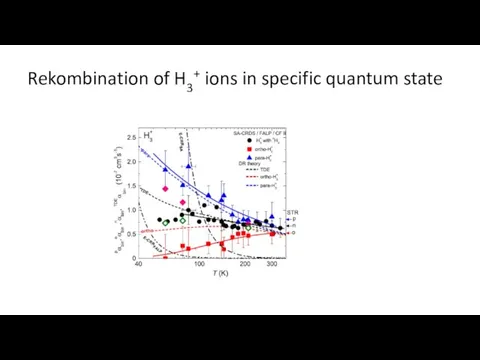
- 21. Rekombination of H3+ ions in specific quantum state
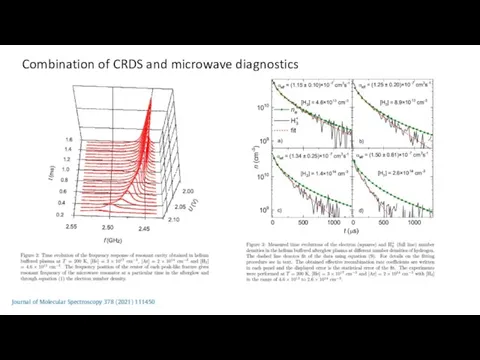
- 22. Combination of CRDS and microwave diagnostics
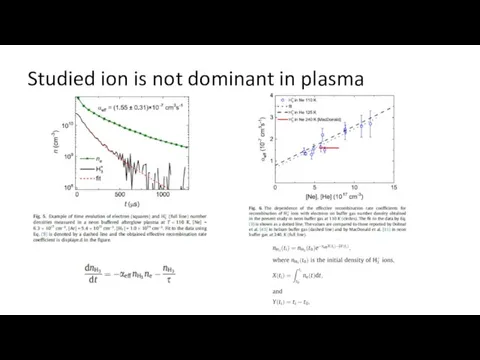
- 23. Studied ion is not dominant in plasma
- 24. Flowing afterglow plasma
- 25. Flowing afterglow plasma
- 26. FALP, SIFT, SIFDT FALP – Flowing Afterglow with Langmuir Probe SIFT – Selective Ion Flow Tube
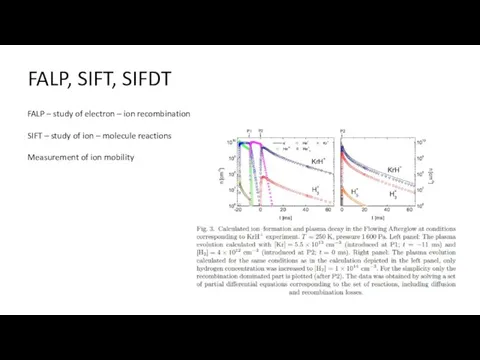
- 27. FALP, SIFT, SIFDT FALP – study of electron – ion recombination SIFT – study of ion
- 28. Cryo-FALP II Cryogenic Flowing Afterglow with Langmuir Probe Flowtube diameter – 5 cm He buffer gas
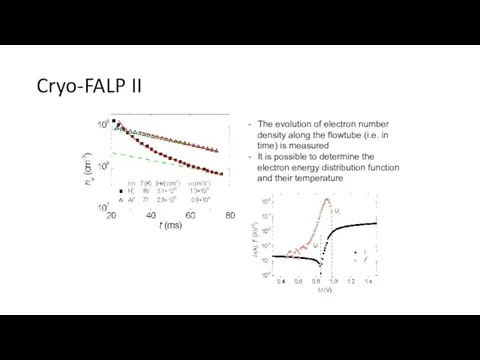
- 29. Cryo-FALP II The evolution of electron number density along the flowtube (i.e. in time) is measured
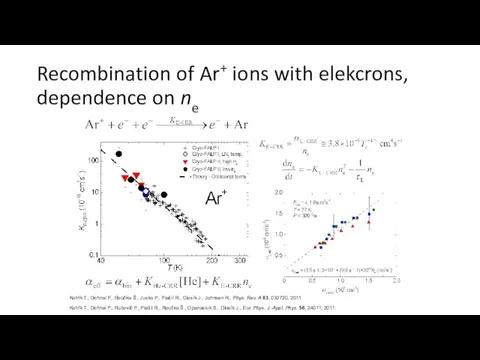
- 30. Recombination of Ar+ ions with elekcrons, dependence on ne Kotrík T., Dohnal P., Roučka Š., Jusko
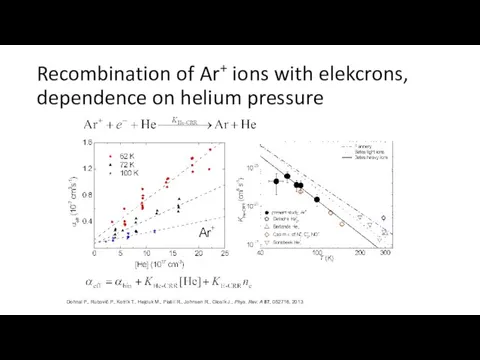
- 31. Recombination of Ar+ ions with elekcrons, dependence on helium pressure Dohnal P., Rubovič P., Kotrík T.,
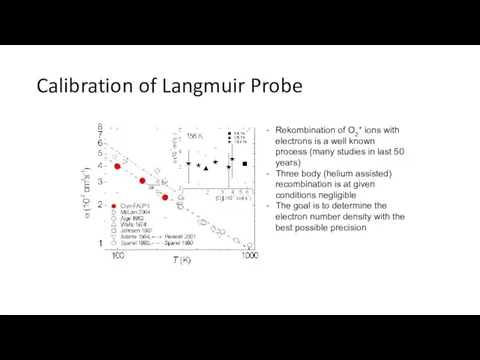
- 32. Calibration of Langmuir Probe Rekombination of O2+ ions with electrons is a well known process (many
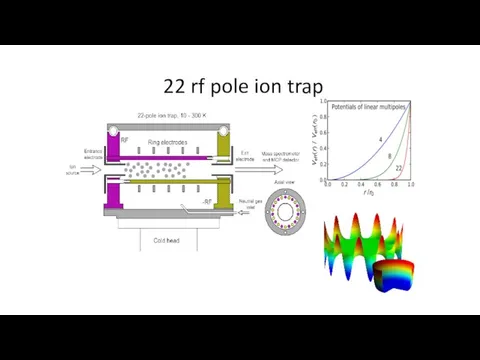
- 33. 22 rf pole ion trap Many configurations for different experiments Cold Heads at 22PT and H
- 34. 22 rf pole ion trap
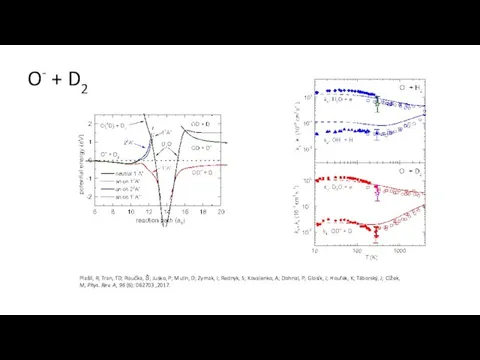
- 35. O- + D2 Plašil, R; Tran, TD; Roučka, Š; Jusko, P; Mulin, D; Zymak, I; Rednyk,
- 37. Скачать презентацию











 Законы Кирхгофа
Законы Кирхгофа Сопротивление материалов
Сопротивление материалов Устройство карданной передачи, разработка технологической карты
Устройство карданной передачи, разработка технологической карты Полупроводниковые приборы
Полупроводниковые приборы Электромагнитные явления
Электромагнитные явления Что такое кипение
Что такое кипение Презентация по физике "Механическое движение" -
Презентация по физике "Механическое движение" -  Работа и мощность
Работа и мощность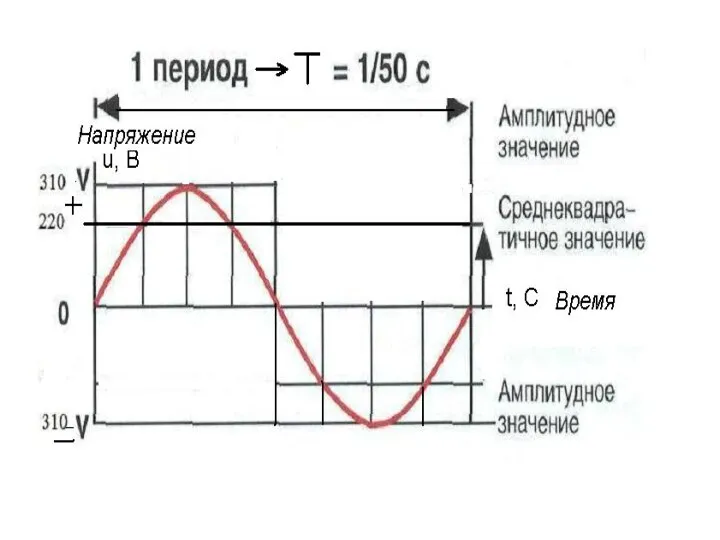 Действующее и среднее значение синусоидального тока
Действующее и среднее значение синусоидального тока Применение фотоэффекта
Применение фотоэффекта Невагомiсть
Невагомiсть Перемещение при прямолинейном равноускоренном движении (9 класс)
Перемещение при прямолинейном равноускоренном движении (9 класс) Радионавигация
Радионавигация Преломление света
Преломление света Разработка и моделирование МЭМС-датчика давления воздушной среды
Разработка и моделирование МЭМС-датчика давления воздушной среды Измерение физических величин. 7 класс
Измерение физических величин. 7 класс Презентация на тему Опасна ли гроза
Презентация на тему Опасна ли гроза  Внутренняя энергия
Внутренняя энергия Кинематика. Занятие 4
Кинематика. Занятие 4 Применение фотоэффекта
Применение фотоэффекта Презентация на тему Тепловые двигатели
Презентация на тему Тепловые двигатели  Туннельный диод
Туннельный диод Понятие светового поля ( лекция 6 )
Понятие светового поля ( лекция 6 ) Магнитные материалы и компоненты. (Лекция 5)
Магнитные материалы и компоненты. (Лекция 5) Презентация на тему Механические явления в природе
Презентация на тему Механические явления в природе  Лекция №14. Интегралы, зависящие от параметров
Лекция №14. Интегралы, зависящие от параметров Физическая викторина. Игра
Физическая викторина. Игра Вероятность формулы. 11 класс, 10 задание
Вероятность формулы. 11 класс, 10 задание