Содержание
- 2. http://www.earthday.gov/ Today is Earth Day, April 22nd 2003
- 3. It isn’t the pollution that’s harming the environment. It’s the impurities in our air and water
- 4. ENERGY STAR - Energy-efficient choices can save families about a third on their energy bill with
- 5. Learn to Conserve Water in Your Home - You can also take a virtual tour that
- 6. Ecosystems an assemblage of different species and their physical environment, all organized in a way that
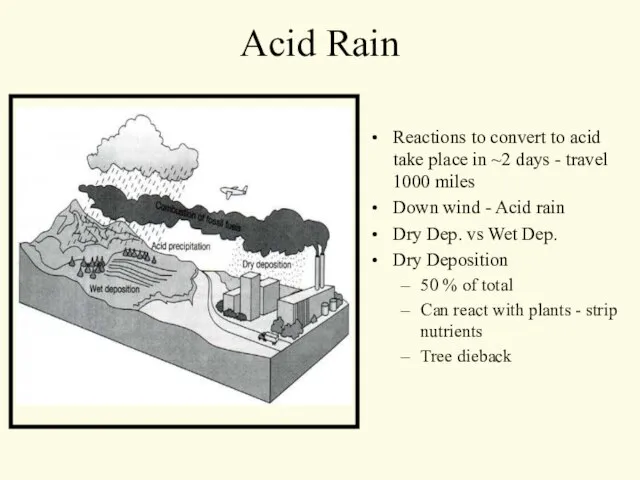
- 7. Acid Rain Reactions to convert to acid take place in ~2 days - travel 1000 miles
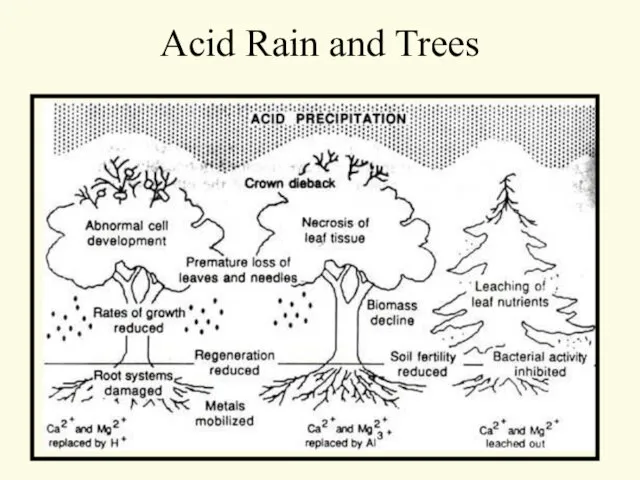
- 9. Acid Rain and Trees
- 10. Forests affected by Acid Rain Northeast US Canada Northern Europe Asia
- 11. Acid Rain and Buildings Many buildings are made of concrete and or stone These compounds act
- 12. Europe The US Capitol
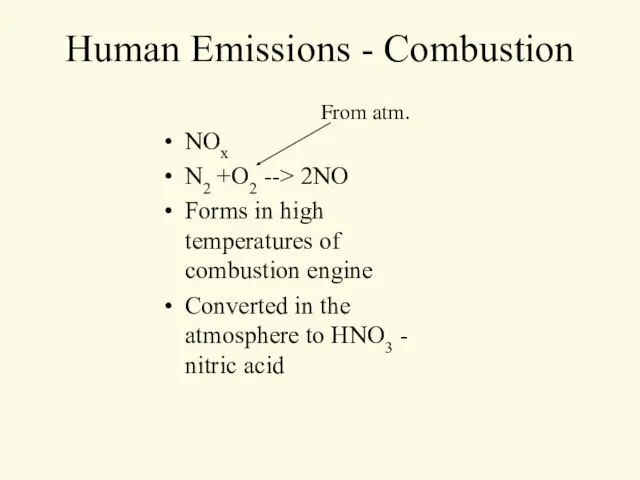
- 13. Human Emissions - Combustion NOx N2 +O2 --> 2NO Forms in high temperatures of combustion engine
- 14. Human Emissions - Fertilizer N2 +Energy H+--> NH3 Formed by the Haber process Added to fields
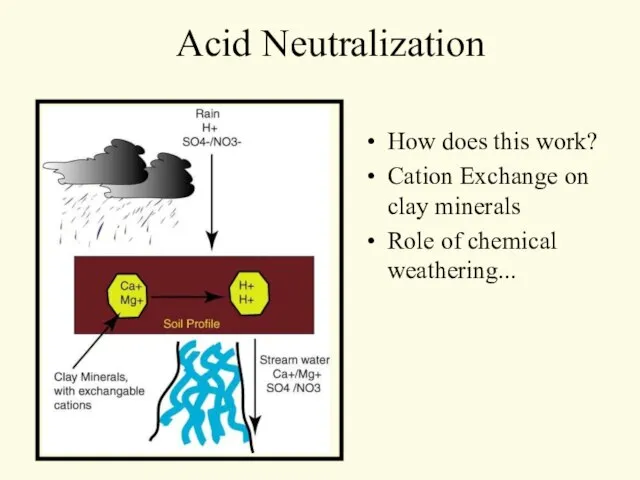
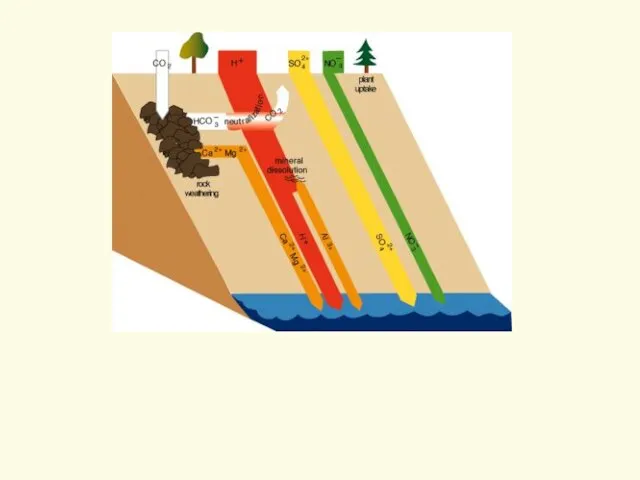
- 15. Acid Neutralization How does this work? Cation Exchange on clay minerals Role of chemical weathering...
- 16. Where do N emissions originate? ~ 55% come from agriculture ~ 25% come from industry –
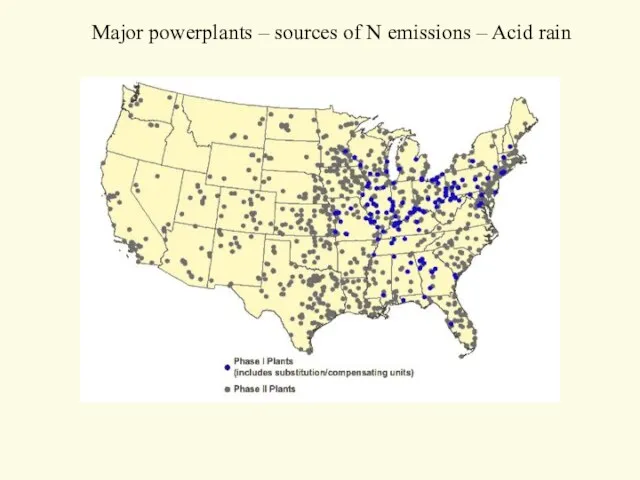
- 17. Major powerplants – sources of N emissions – Acid rain
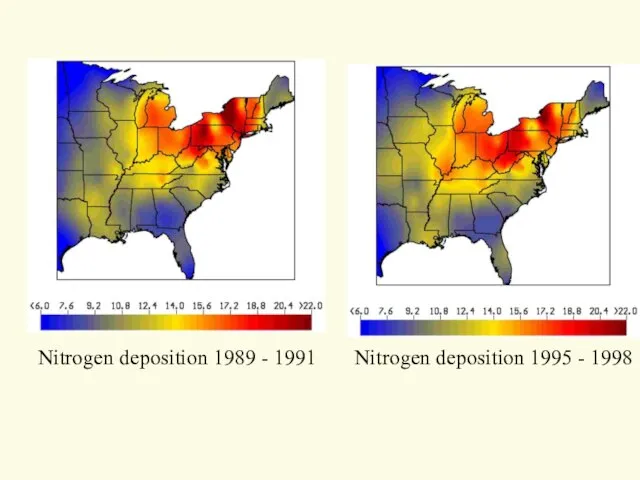
- 18. Nitrogen deposition 1989 - 1991 Nitrogen deposition 1995 - 1998
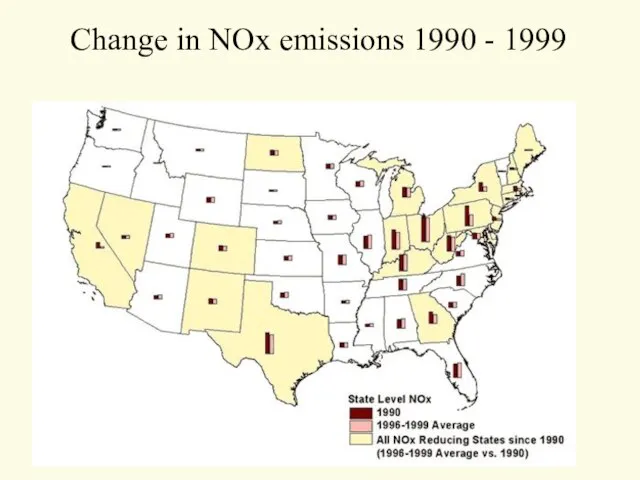
- 19. Change in NOx emissions 1990 - 1999
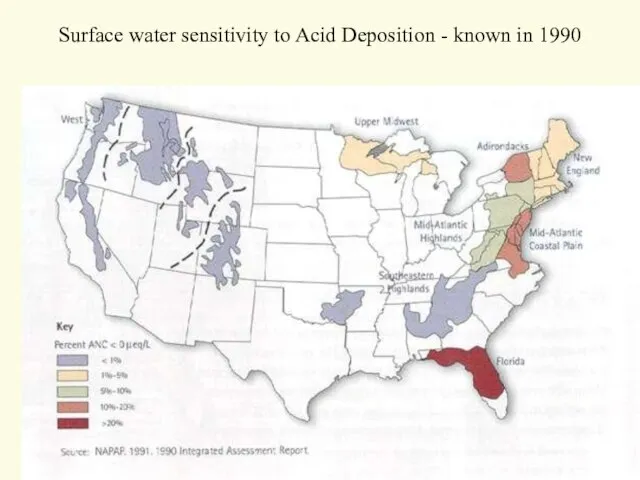
- 20. Surface water sensitivity to Acid Deposition - known in 1990
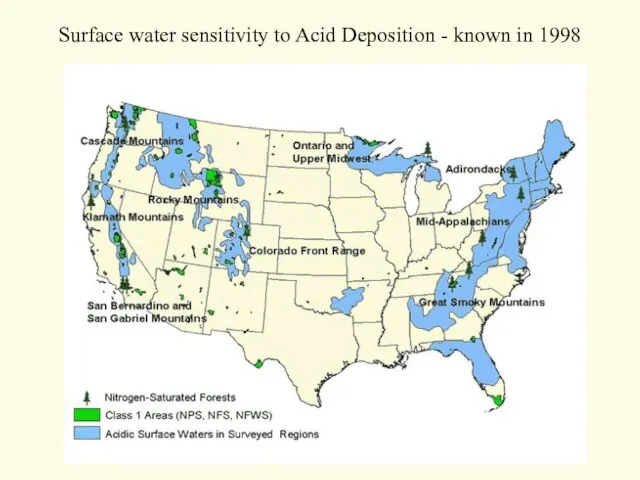
- 21. Surface water sensitivity to Acid Deposition - known in 1998
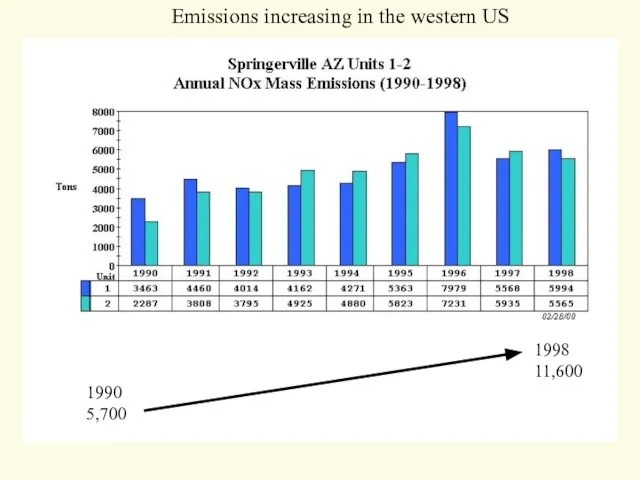
- 22. 1990 5,700 1998 11,600 Emissions increasing in the western US
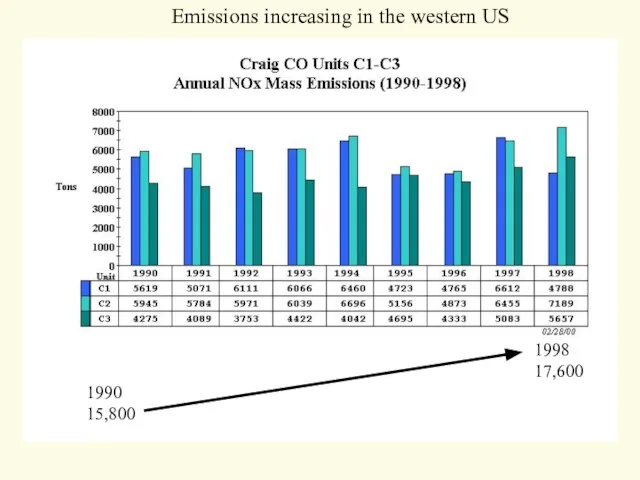
- 23. 1990 15,800 1998 17,600 Emissions increasing in the western US
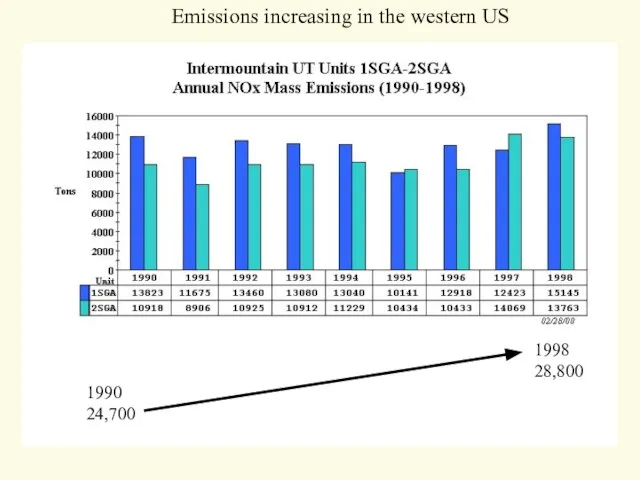
- 24. 1990 24,700 1998 28,800 Emissions increasing in the western US
- 25. Recent and current policies to reduce acid precipitation and Nitrogen emissions are shifting the problem from
- 26. Other types of air pollution The difference between stratospheric and tropospheric ozone Photochemical smog Inversion layers
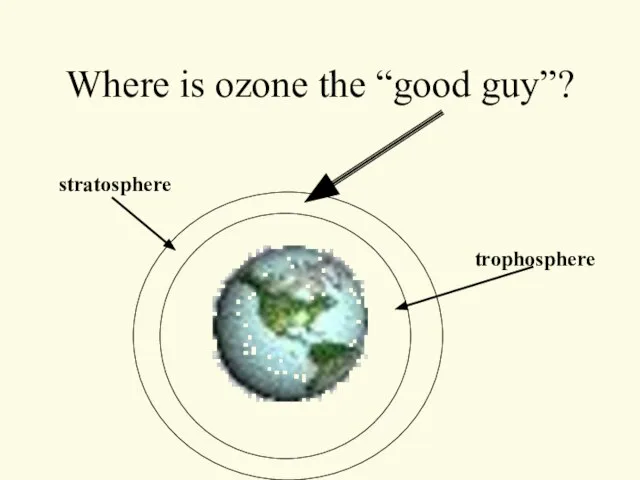
- 27. Where is ozone the “good guy”? trophosphere stratosphere
- 28. In the stratosphere…. Ozone blocks incoming Ultra-violet radiation Ultraviolet radiation Skin cancer Cataracts Plant Damage
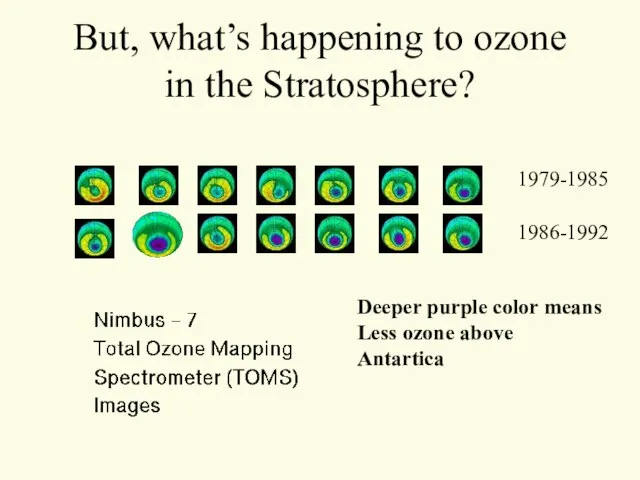
- 29. But, what’s happening to ozone in the Stratosphere? 1979-1985 1986-1992 Deeper purple color means Less ozone
- 30. Why? Chlorofluorocarbons (CFC) are very stable compounds that we produce at earth’s surface They migrate to
- 31. The Montreal Protocol has reduced use of CFC’s, but… Their long life span means that they
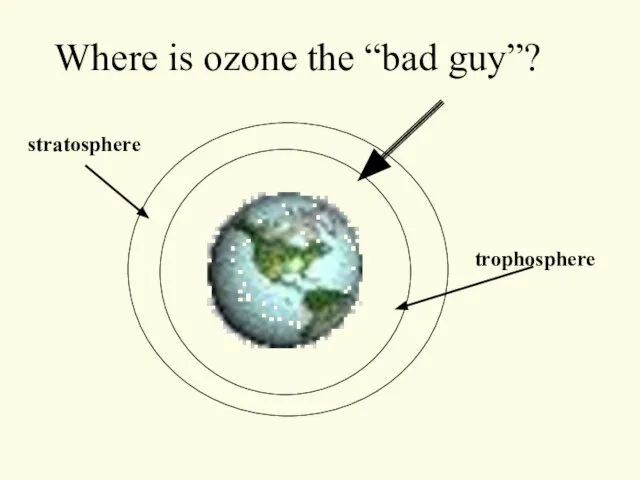
- 32. Where is ozone the “bad guy”? trophosphere stratosphere
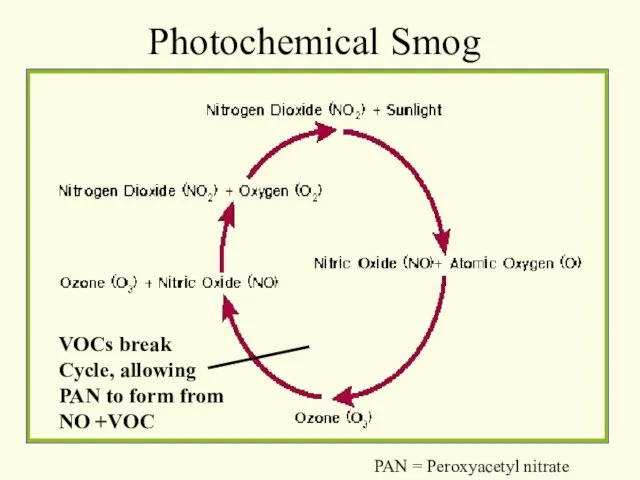
- 33. Photochemical Smog VOCs break Cycle, allowing PAN to form from NO +VOC PAN = Peroxyacetyl nitrate
- 34. Examples of Smog
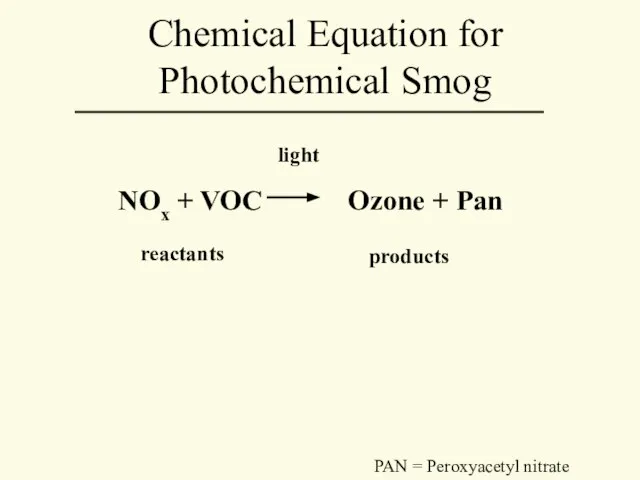
- 35. NOx + VOC Ozone + Pan light reactants products Chemical Equation for Photochemical Smog PAN =
- 36. Where reactants come from NOx primarily from transportation VOC from a variety of sources, including refining,
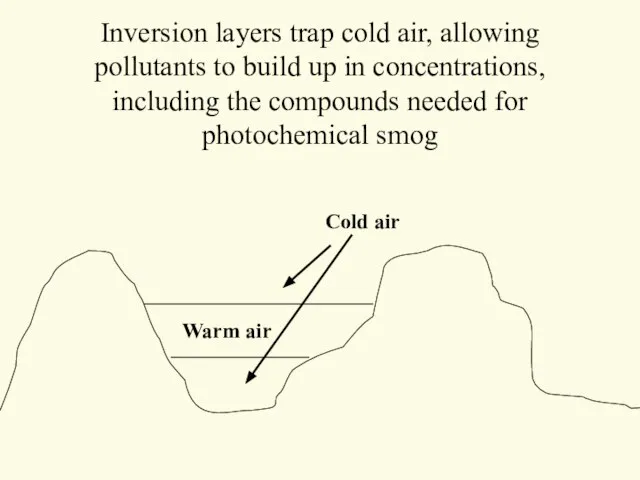
- 37. Inversion layers trap cold air, allowing pollutants to build up in concentrations, including the compounds needed
- 38. Ozone’s bad features Extremely reactive will burn leaves, lungs, synthetic compounds (e.g. rubbers, plastics) Because of
- 39. Humans depend on very small reservoirs of water for all our needs These reservoirs cycle/ turnover
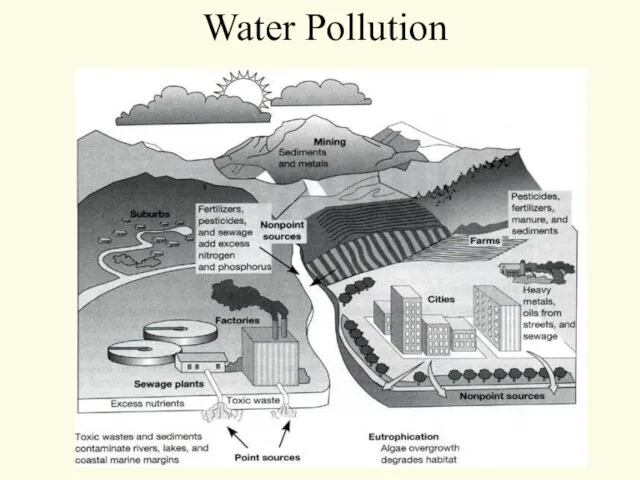
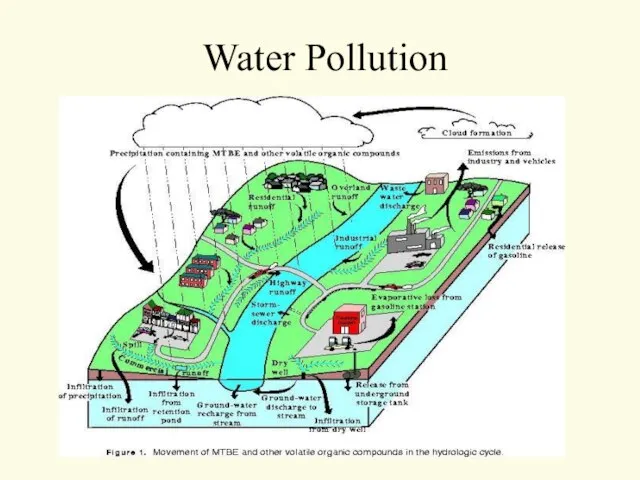
- 40. Water Pollution
- 41. Water Pollution
- 42. Water Pollution Two major classifications Point Source Non-point Source
- 43. Point Sources Single large source Can localize it to one spot Industrial Plants - Sewage pipes
- 44. Point Source - Example LUST - Leaky Underground Storage Tanks 22% of the 1.2 million UST
- 45. Point source examples
- 46. Non-point Sources Diffuse source or many smaller point sources Automobiles Fertilizer on fields
- 47. Non point source examples
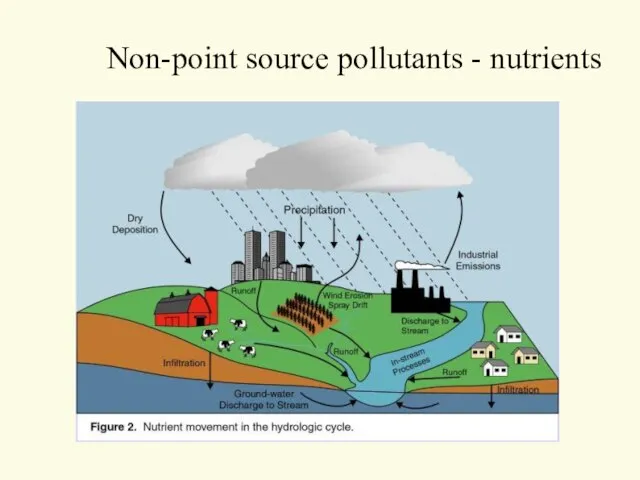
- 48. Non-point source pollutants - nutrients
- 49. End Lecture 4/22/03
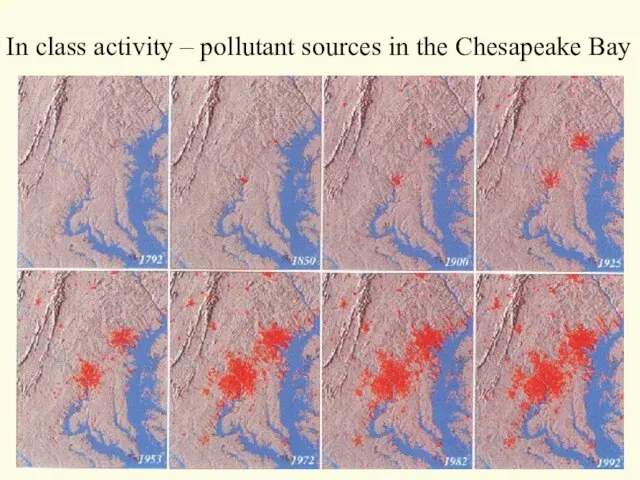
- 50. In class activity – pollutant sources in the Chesapeake Bay
- 51. The four main roles for class debate on 5/1/2003 Pacific Lumber Company /Maxxam Corporation The CEO’s
- 53. How does acid kill the fish? One way is mobilizing metals When all base cations are
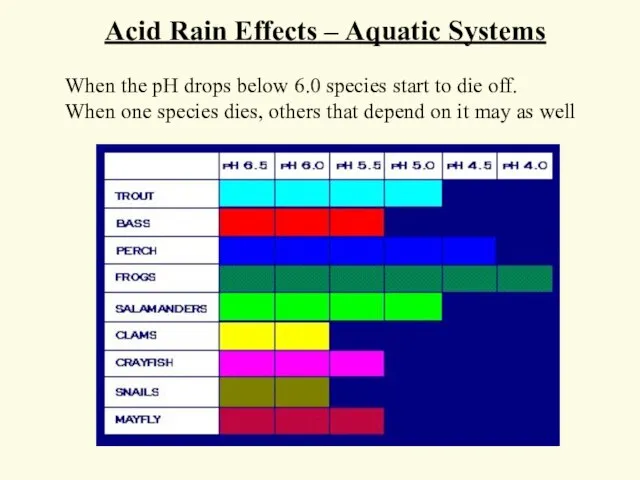
- 54. When the pH drops below 6.0 species start to die off. When one species dies, others
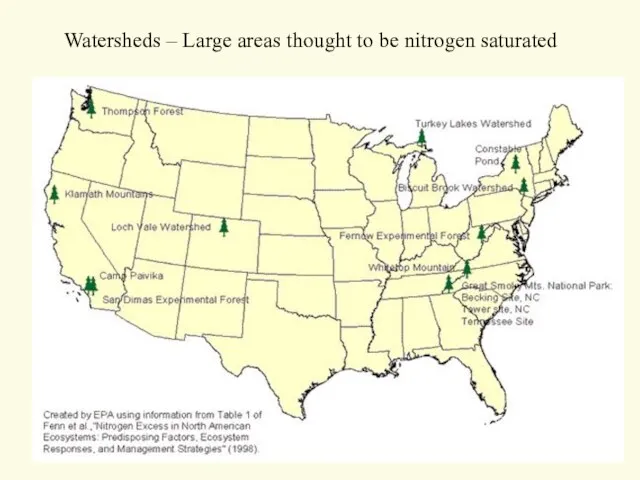
- 55. Watersheds – Large areas thought to be nitrogen saturated
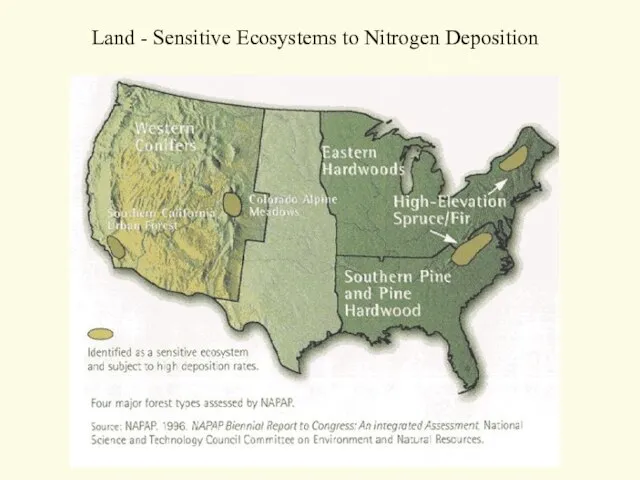
- 56. Land - Sensitive Ecosystems to Nitrogen Deposition
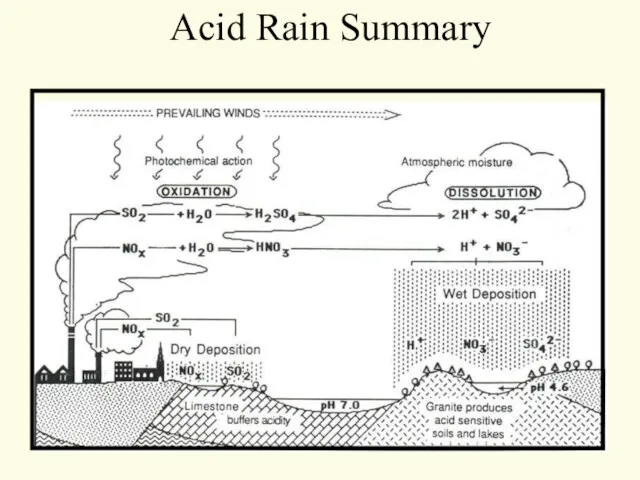
- 57. Acid Rain Summary
- 59. Скачать презентацию




























 техника безоп
техника безоп шиповое соединение
шиповое соединение Взаємодія України та Португалії
Взаємодія України та Португалії Основы конституционного строя России
Основы конституционного строя России Обсуждение художественного фильма Полосатый рейс с точки зрения трудового права
Обсуждение художественного фильма Полосатый рейс с точки зрения трудового права Планирование веб-релизовв условиях многопоточности задачсо скачущими приоритетами
Планирование веб-релизовв условиях многопоточности задачсо скачущими приоритетами Способы кодирования информации.
Способы кодирования информации. Технология в жизни человека и общества
Технология в жизни человека и общества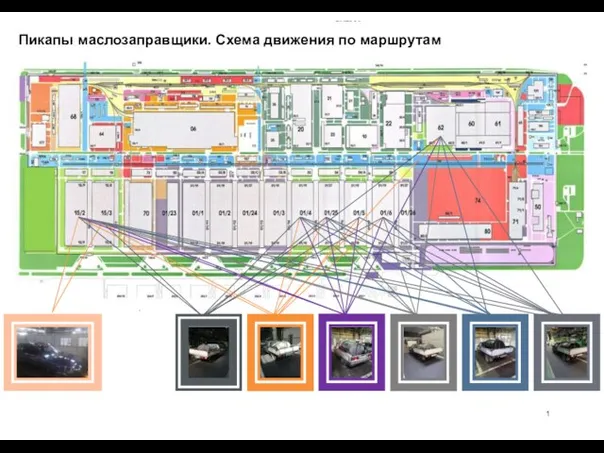 Пикапы маслозаправщики. Схема движения по маршрутам
Пикапы маслозаправщики. Схема движения по маршрутам «Магнетизм и его изучение». Учитель физики Балашова Н. А. Троицк 2011г.
«Магнетизм и его изучение». Учитель физики Балашова Н. А. Троицк 2011г.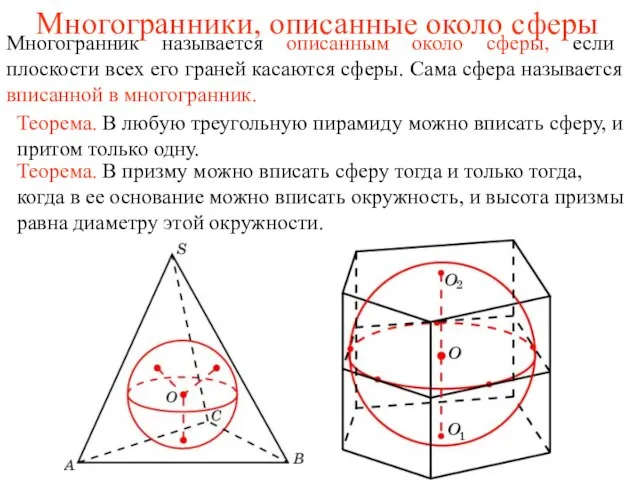 Многогранники, описанные около сферы
Многогранники, описанные около сферы Юношеский возраст. Лекция 8.2
Юношеский возраст. Лекция 8.2 Охрана земельных ресурсов – залог эффективного ведения сельского хозяйства
Охрана земельных ресурсов – залог эффективного ведения сельского хозяйства Элементы суггестивного управления в системе педагогической деятельности учителя
Элементы суггестивного управления в системе педагогической деятельности учителя Рязанские народные ремесла и промыслы
Рязанские народные ремесла и промыслы The best job in the world
The best job in the world  ИНФЕКЦИОННЫЙ КОНТРОЛЬ ИНФЕКЦИОННАЯ БЕЗОПАСНОСТЬ
ИНФЕКЦИОННЫЙ КОНТРОЛЬ ИНФЕКЦИОННАЯ БЕЗОПАСНОСТЬ Рекламное агентство
Рекламное агентство 1С-Битрикс: Сайт школы
1С-Битрикс: Сайт школы Презентация Microsoft Office PowerPoint
Презентация Microsoft Office PowerPoint Работа выполнена в рамках проекта «Повышения квалификаций различных категорий работников образования и формирование у них базов
Работа выполнена в рамках проекта «Повышения квалификаций различных категорий работников образования и формирование у них базов FN1_LessonOne
FN1_LessonOne Modal verb May/might
Modal verb May/might Образ ребенка в киноискусстве XX
Образ ребенка в киноискусстве XX Потребление воды и минеральных ресурсов
Потребление воды и минеральных ресурсов Эвдемонизм - это гуманизм
Эвдемонизм - это гуманизм 07_0___163
07_0___163 Презентация "Орфей и Эвридика" - скачать презентации по МХК
Презентация "Орфей и Эвридика" - скачать презентации по МХК