Содержание
- 2. Objectives I. Pharmacokinetics Absorption Bioavailability Distribution Drug metabolism Excretion II. Combined action of drugs
- 3. Pharmacokinetics Pharmacokinetics (from Greek pharmakon - medicine, kineo - move) Pharmacokinetics is the part of pharmacology
- 4. Pharmacokinetics Based on the hypothesis that the action of a drug requires presence of a certain
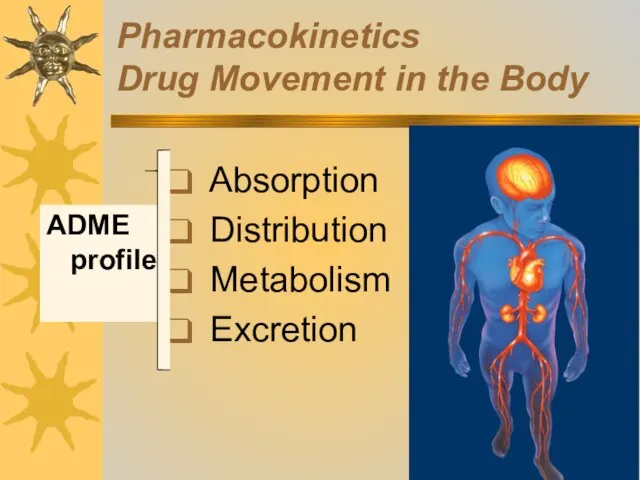
- 5. Pharmacokinetics Drug Movement in the Body Absorption Distribution Metabolism Excretion ADME profile
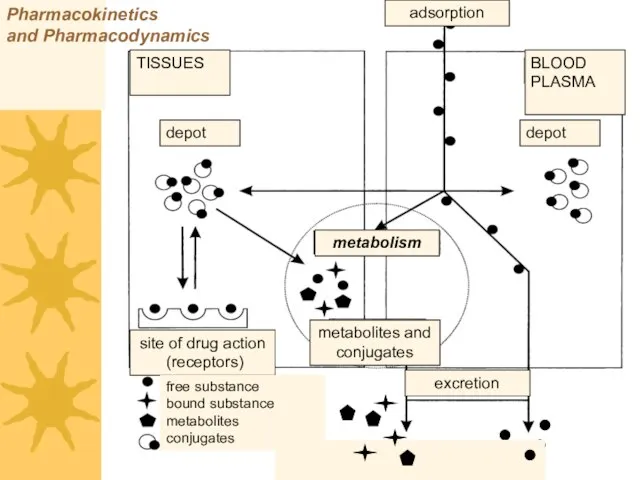
- 6. Pharmacokinetics and Pharmacodynamics TISSUES BLOOD PLASMA depot depot metabolism site of drug action (receptors) metabolites and
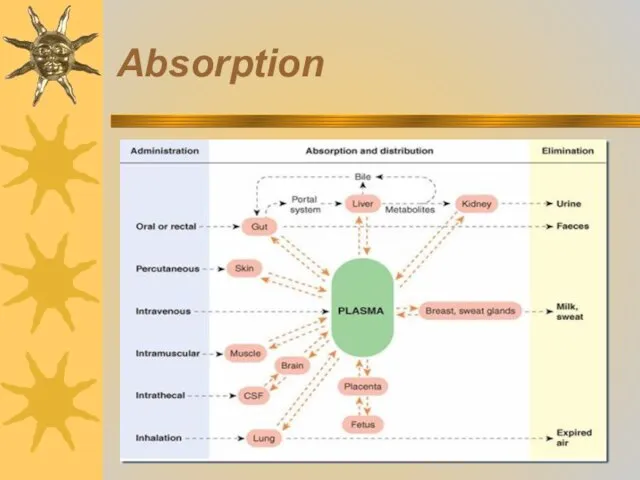
- 7. Absorption The usage of drugs starts with their administration into the organism or application onto body
- 8. Absorption
- 9. Absorption
- 10. Absorption of drugs from the gastrointestinal tract through the skin, respiratory and vascular walls is connected
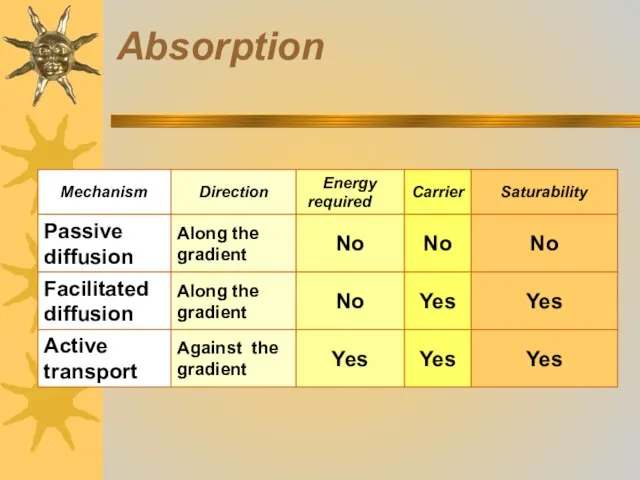
- 11. Passive diffusion: substances move from the area with high concentration to the area with low concentration
- 12. Absorption
- 13. Passive transport
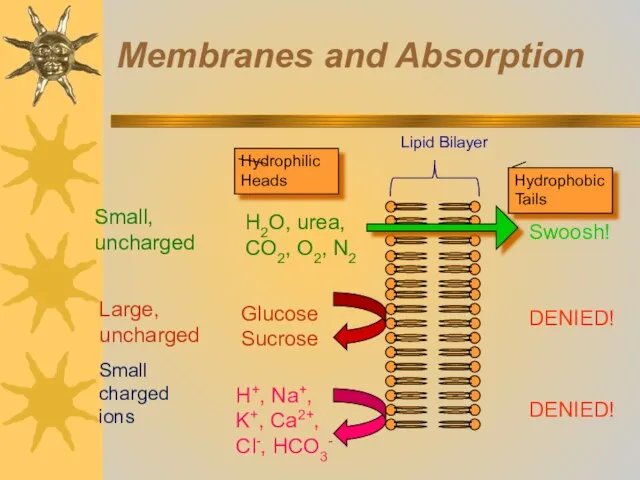
- 14. Membranes and Absorption
- 15. Facilitated transport
- 16. Active transport
- 17. Pinocytosis
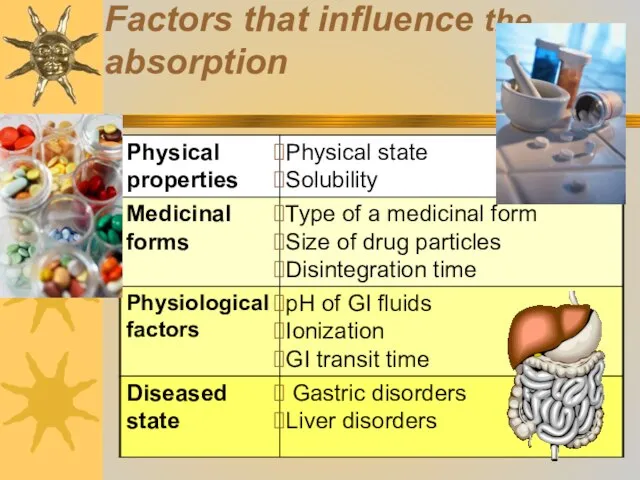
- 18. Factors that influence the absorption
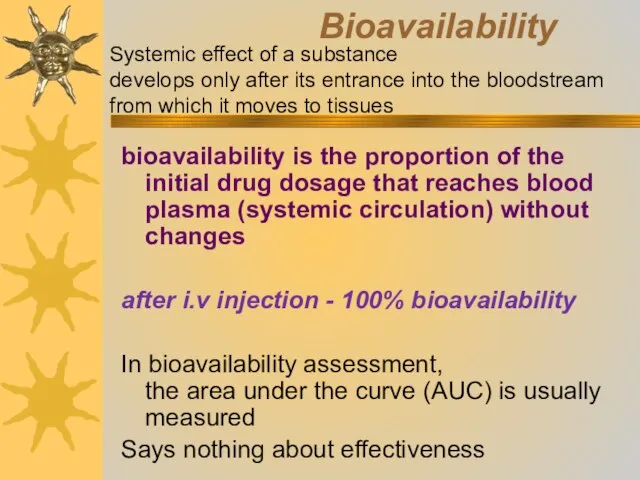
- 19. Bioavailability bioavailability is the proportion of the initial drug dosage that reaches blood plasma (systemic circulation)
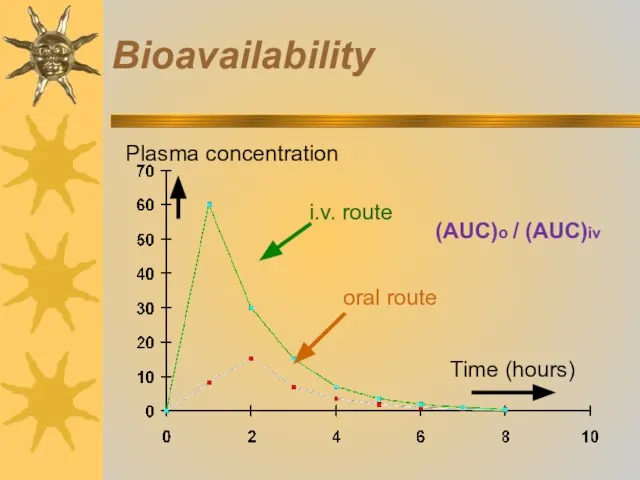
- 20. Bioavailability
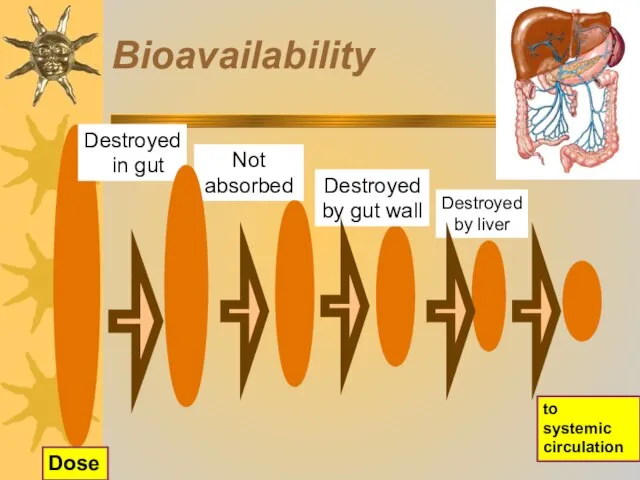
- 21. Bioavailability Not absorbed Destroyed by gut wall to systemic circulation Destroyed by liver Dose Destroyed in
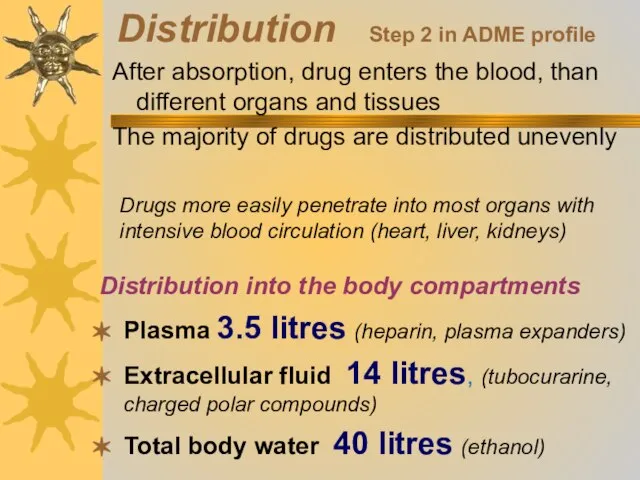
- 22. Distribution Step 2 in ADME profile After absorption, drug enters the blood, than different organs and
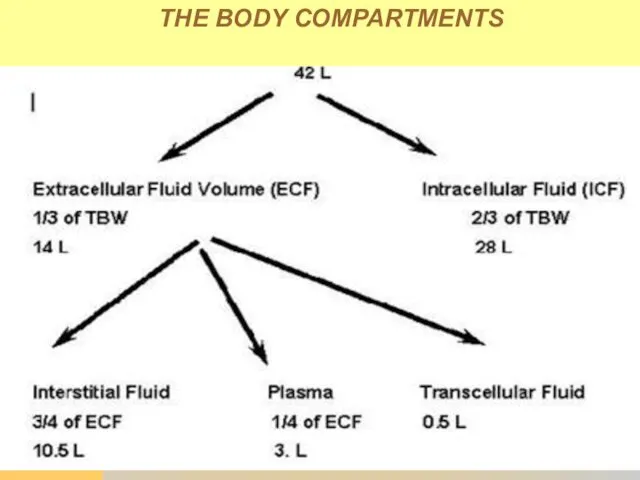
- 23. THE BODY COMPARTMENTS
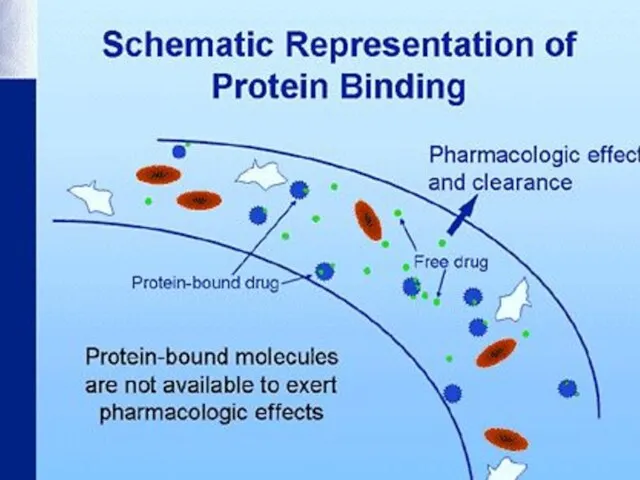
- 25. Distribution of drugs in the body In the body drugs partially bind to other molecules and
- 27. Distribution
- 28. Important for clinical pharmacology It is the presumed volume of liquid in which a drug can
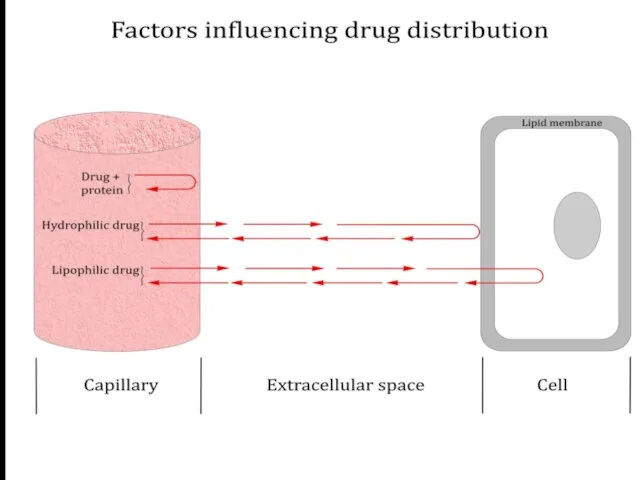
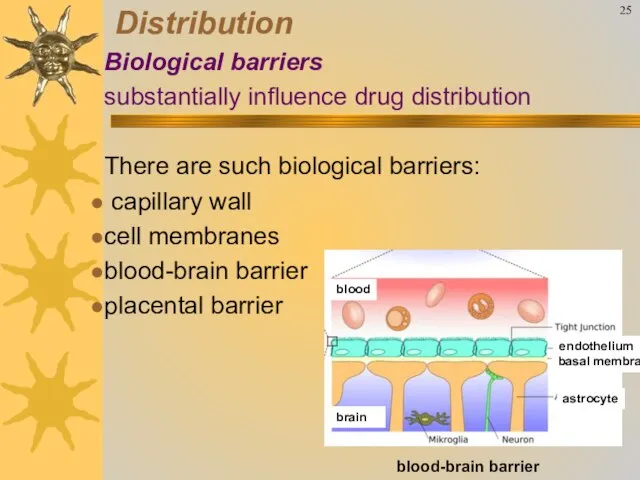
- 29. 25 Distribution Biological barriers substantially influence drug distribution There are such biological barriers: capillary wall cell
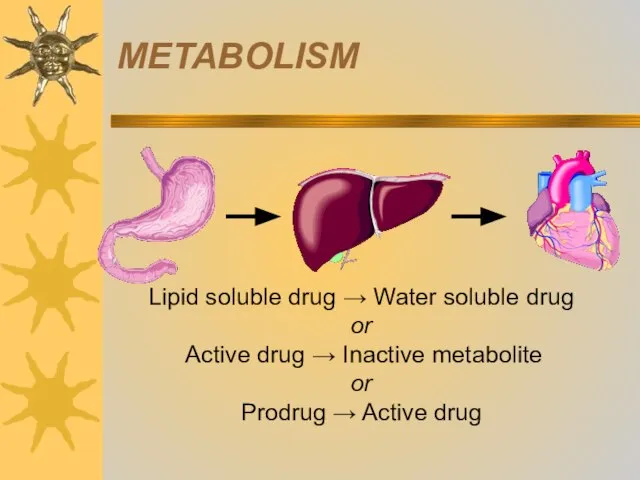
- 30. Biotransformation of drugs (drug metabolism) is the process of drugs (xenobiotics) conversion into metabolites that are
- 31. METABOLISM
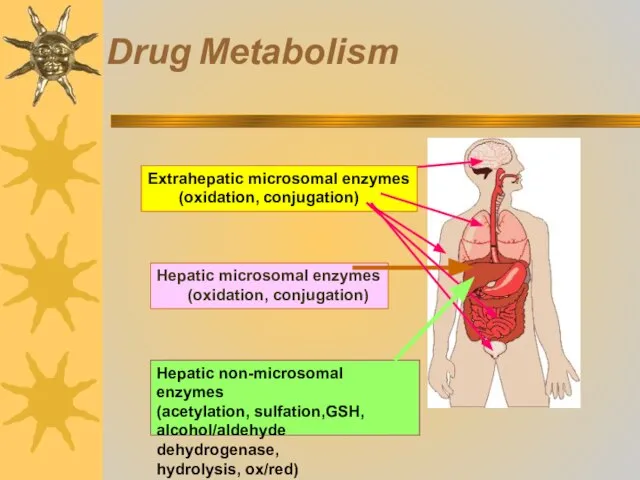
- 32. Drug Metabolism
- 33. 1. Metabolic transformation occurs through oxidation, reduction, hydrolysis In these reactions groups with active hydrogen atoms
- 34. Biotransformation of drugs (drug metabolism) METABOLISM deactivation
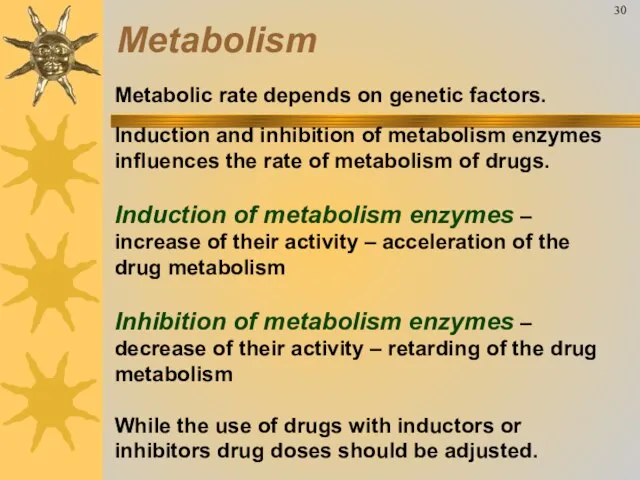
- 35. Metabolic rate depends on genetic factors. Induction and inhibition of metabolism enzymes influences the rate of
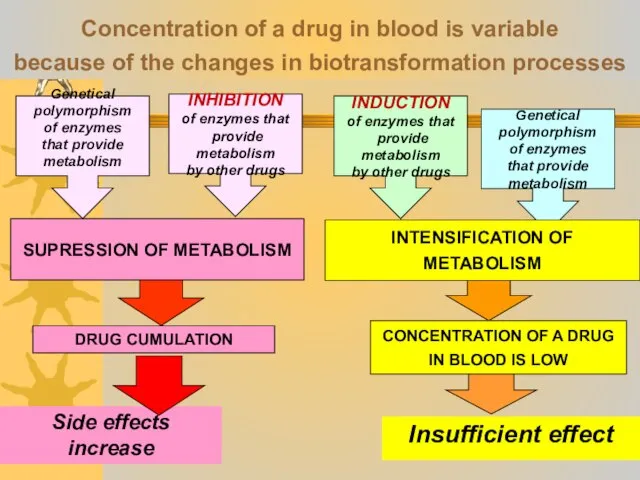
- 36. Concentration of a drug in blood is variable because of the changes in biotransformation processes SUPRESSION
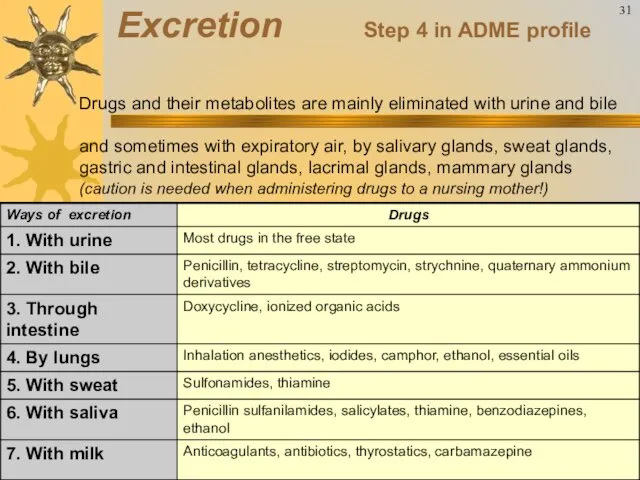
- 37. Drugs and their metabolites are mainly eliminated with urine and bile and sometimes with expiratory air,
- 38. Important for clinical pharmacology It shows the time necessary to decrease drug concentration in blood plasma
- 39. EXCRETION
- 40. Drug interactions Based on the change in drugs pharmacokinetics Based on the change in drugs pharmacodynamics
- 41. Synergism (from Greek syn – together, ergos – work) increase in effect of drugs used at
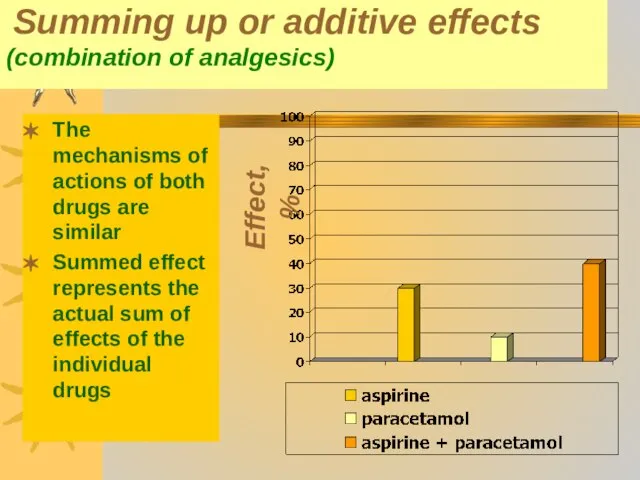
- 42. Summing up or additive effects (combination of analgesics) The mechanisms of actions of both drugs are
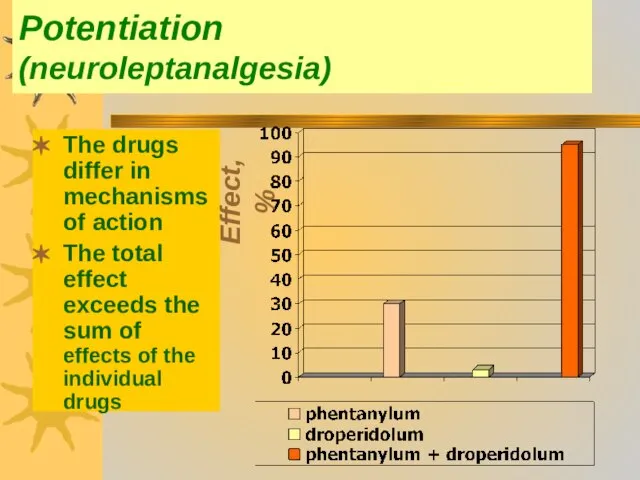
- 43. Potentiation (neuroleptanalgesia) The drugs differ in mechanisms of action The total effect exceeds the sum of
- 44. Antagonism (from Greek antagōnisma – struggle, conflict) the ability of drug to decrease the effect of
- 45. Antagonism Types of antagonism - two-way and one-way antagonism A blocker reliably eliminates the effects of
- 46. Drug incompatibility Incompatibility is the weakening, full loss or change in the pharmacotherapeutic effect, intensification of
- 48. Скачать презентацию























 Изображения изделий и их обозначения при выполнении графических конструкторских документов
Изображения изделий и их обозначения при выполнении графических конструкторских документов Использование функций
Использование функций Математические задачи от русских, советских и зарубежных писателей
Математические задачи от русских, советских и зарубежных писателей Каникулы в международном детском лагере
Каникулы в международном детском лагере Язык есть исповедь народа
Язык есть исповедь народа Работа по стимулированию юридических лиц и индивидуальных предпринимателей к трудоустройству безработных
Работа по стимулированию юридических лиц и индивидуальных предпринимателей к трудоустройству безработных Физико-географическое районирование Северной Евразии
Физико-географическое районирование Северной Евразии  Узоры “тетёрки ” на печенье
Узоры “тетёрки ” на печенье Презентация на тему Постоянные магниты
Презентация на тему Постоянные магниты Весенний фестиваль для учащихся
Весенний фестиваль для учащихся 23 февраля. Поздравляем!
23 февраля. Поздравляем! Российский государственный университет нефти и газа имени И.М.Губкина Кафедра машин и оборудования нефтяной и газовой промышленн
Российский государственный университет нефти и газа имени И.М.Губкина Кафедра машин и оборудования нефтяной и газовой промышленн Звук и свет в лирике И. Анненского
Звук и свет в лирике И. Анненского Книжка-раскраска
Книжка-раскраска Взаимодействие научного руководителя со студентами через личный кабинет с использование сервиса Антиплагиат
Взаимодействие научного руководителя со студентами через личный кабинет с использование сервиса Антиплагиат Зачем живые организмы запасают питательные вещества?
Зачем живые организмы запасают питательные вещества? Особенности приема 2020 (колледж). Дистанционная подача документов
Особенности приема 2020 (колледж). Дистанционная подача документов Ресурсный Центр «Кристина»
Ресурсный Центр «Кристина» Проверка правильности кирпичной кладки
Проверка правильности кирпичной кладки Смешанное обучение – blended learning
Смешанное обучение – blended learning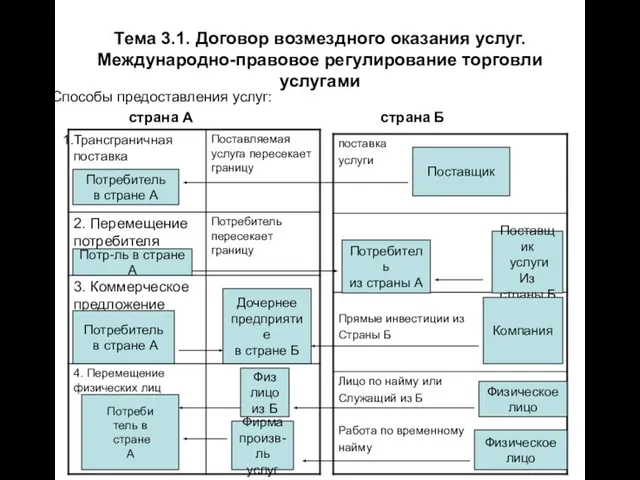 Тема 3.1. Договор возмездного оказания услуг. Международно-правовое регулирование торговли услугами Способы предоставления услуг
Тема 3.1. Договор возмездного оказания услуг. Международно-правовое регулирование торговли услугами Способы предоставления услуг Конвенция о правах ребенка
Конвенция о правах ребенка Богомолова Роза Александровна
Богомолова Роза Александровна Renault Assistance. Служба маркетинга запасных частей и послепродажного обслуживания
Renault Assistance. Служба маркетинга запасных частей и послепродажного обслуживания Принцип действия жидкокристаллических дисплеев
Принцип действия жидкокристаллических дисплеев Правила работы с обучающей презентацией
Правила работы с обучающей презентацией плакат
плакат ОП по Гагинскому району МО МВД России Большеболдинский: история, сегодняшнее состояние и перспективы развития
ОП по Гагинскому району МО МВД России Большеболдинский: история, сегодняшнее состояние и перспективы развития