Слайд 2Pharmaceutical Products
A drug or medicine is any chemical which:
Alters sensory sensations
Alters mood

or emotions
Alters physiological state (consciousness, activity level, or coordination)
Слайд 3Placebo effect:
A pharmacologically inert substance (often a sugar pill) produces a significant

reaction because the patient expects, desires, or was told it would happen
Used as a control in clinical trials
Highlights the body’s natural healing powers
Слайд 4Research and Development:
Development of a new drug is a very costly, lengthy

process controlled by the government:
In 1970, 3620 drugs were tested. 16 came on the market at an average cost of $20 million
Only 1 in 2000 drugs eventually make it to the market
Phase I: Initial clinical trials on volunteers after the drug has proven safe when given to animals
Phase II: Thorough clinical investigation to eliminate investigator bias
Phase III: Extended clinical evaluation
Слайд 5Thalidomide
Early 1960’s given to pregnant women to treat morning sickness
Later found

to cause major birth defects
One isomer controls morning sickness, the other leads to birth defects (optical isomers)
Слайд 6Methods of Administering Drugs:
Orally
Effect varies because absorption is affected by stomach content

and drug concentration
Primary site of absorption is the small intestine
Rectally
Effective if a drug cannot be taken orally or if a drug is pH sensitive
Inhalation
Rapid, systemic administration due to extensive network of blood vessels in lungs
Слайд 7Parenteral (injection)
Subcutaneous
Beneath the skin
Slow absorption
Intra-muscular
Used when
immediate response is not required
Used for
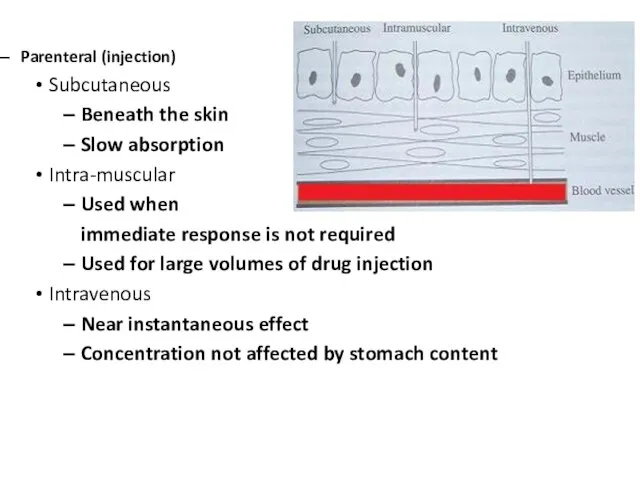
large volumes of drug injection
Intravenous
Near instantaneous effect
Concentration not affected by stomach content
Слайд 8More about drugs
Fat-soluble drugs are more easily absorbed, since blood vessels contain

a fatty layer
Capillaries of brain are denser and prevent diffusion of many substances into the brain (blood-brain barrier)
Drugs are broken down by the kidneys and liver
Half-life is the time required for half of the drug to be eliminated
Слайд 9Toxicity
LD50 is the dose (in mg of substance per kg of body

mass) that is lethal to to 50% of laboratory animals
The lower the LD50, the more toxic the substance
Lowest LD50 rating known as of yet: botulism toxin (BoTox) – most toxic substance known LD50 of roughly 0.005-0.05 µg/kg
Слайд 10Tolerance and Dependence
Drugs may result in physical or psychological dependence
Tolerance means that

over time, an individual requires an increased amount of the drug to achieve the same physiological effect
Слайд 11Antacids
Bases (metal oxides, metal hydroxides, metal carbonates, or metal hydrogencarbonates) that react

with excess stomach acid to adjust pH
Stomach acid helps suppress growth of harmful bacteria and aids in digestion
Often combined with alginates and anti-foaming agents to prevent reflux
Consumption of too much antacid results in alkalosis (basic stomach)
Слайд 12Analgesics
Pain relievers act by interfering with pain receptors
Mild analgesics work by blocking

the production of prostaglandins
Prostaglandins:
Constrict blood vessels
Affect hypothalamus (region of brain controlling heat regulation
Increase permeability of capillaries to allow for swelling
Strong analgesics work by binding to receptors in the brain
Prevents transmission of pain impulses without depressing the central nervous system
Слайд 13Mild analgesics
Aspirin (acetyl salicylic acid or ASA) produced from salicylic acid (relatively
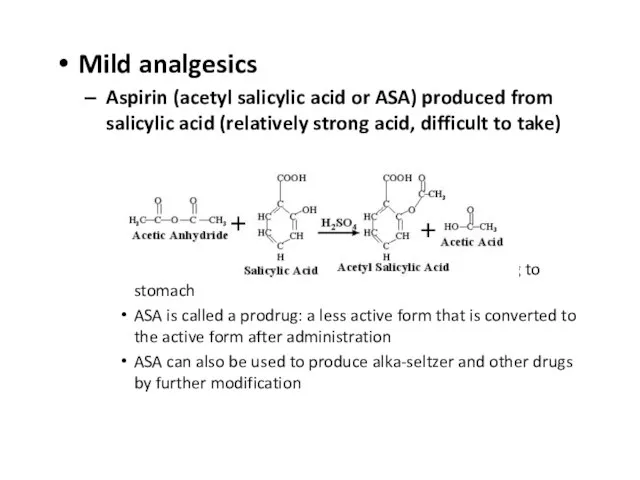
strong acid, difficult to take)
Addition of acetyl group lowers acidity – less irritating to stomach
ASA is called a prodrug: a less active form that is converted to the active form after administration
ASA can also be used to produce alka-seltzer and other drugs by further modification
Слайд 14Uses of salicylic acid and its derivatives:
Relief from minor aches and pains
Fever

reduction (antipyretic)
Anti-inflammatory agent
Anti-clotting agent
Disadvantages of aspirin:
Can cause upset stomach and ulceration
Risk of severe gastrointestinal bleeding following alcohol consumption
Small risk of allergy (.5% of population)
Accidental infant poisoning; small correlation to Reye’s syndrome in children
Слайд 15Aspirin substitutes
Acetaminophen ( paracetemol)
Does not upset stomach or cause bleeding
NOT an anti-inflammatory
Safe
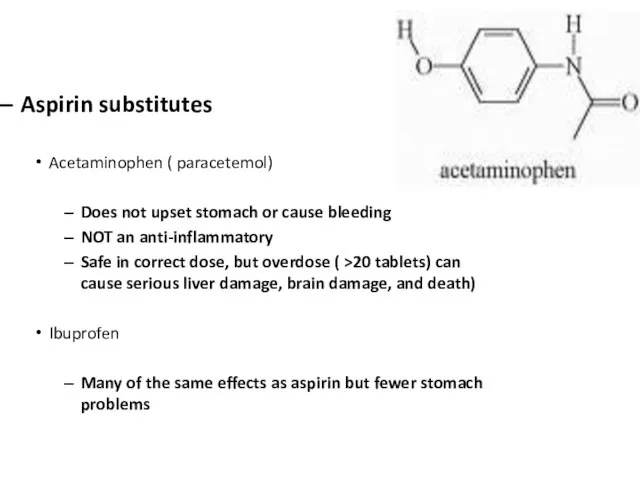
in correct dose, but overdose ( >20 tablets) can cause serious liver damage, brain damage, and death)
Ibuprofen
Many of the same effects as aspirin but fewer stomach problems
Слайд 16Strong analgesics
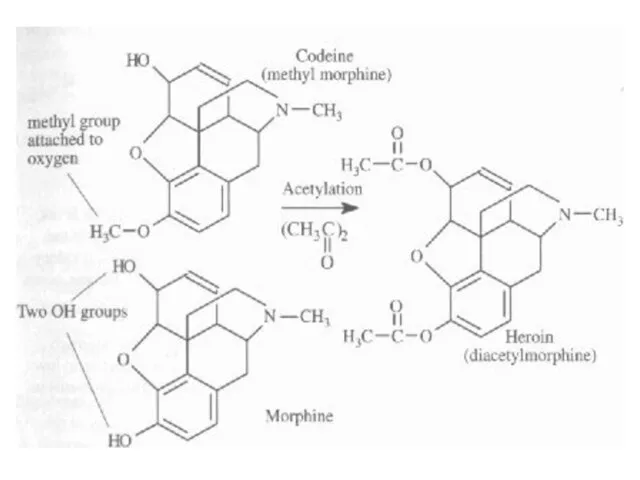
Opium alkaloids (morphine, heroin, codeine)
Belong to “opiate” class (drug that exerts

actions on the body similar to morphine) or “narcotics” (drug that produces a narcotic (sleep-inducing) effect as well as an analgesic (pain relieving) effect)
Morphine is principal alkaloid, making up about 10% by mass of raw opium
Codeine is about .5% of raw opium
Heroin is synthesized from morphine (semi-synthetic drug) via a simple acetylation
Слайд 18Advantages of Opiates:
Pharmacological effects
Major effects on:
Nervous system
The eye
GI tract
Uses:
Strong analgesic for relieving

severe pain
Treatment of diarrhea (produces constipation)
Cough suppressant
Слайд 19Disadvantages:
Psychological effects
Drowsiness, mood change, mental fogginess, nausea and vomiting
Anxiety, fear, lethargy, sedation,

lack of concern, inability to concentrate
Tolerance and Dependence
Cross-tolerance can occur (users tolerant to one opiate will be tolerant to other opiates)
Users may not function properly without the drug, experience withdrawal symptoms (addiction)
Слайд 20Depressants
Drugs that calm and relax the central nervous system
Tranquilizers
Alcohol, valium, librium (Reduce

distress but do not produce sleep)
Sedatives
Barbiturates (Reduce distress but do not produce sleep, stronger than tranquilizers)
Hypnotics
Chloral hydrate (produces sleep in larger doses)
Слайд 21Alcohol
Small, fat-soluble organic molecule – readily penetrates cell membrane and is easily

absorbed from the GI tract
Social effects:
Costs
Sickness and death associated with abuse
Crime and traffic costs
Physiological effects
Short term:
Reduces anxiety and inhibitions
Impairs attention, judgment, and control
Violent or aggressive behavior
Loss of motor function
Effect depends on body mass and concentration of alcohol in the blood
Слайд 22Long-term
Alcoholism is caused by an inability to reduce alcohol intake
Withdrawal symptoms (nausea,

sweating, anxiety, hypertension
Tolerance
Cirrhosis (scarring) and cancer of the liver (the major detoxification organ)
Heart disease
Hypertension
Strokes
Gastritis
Ulcers
Depression
Birth defects
Слайд 23Alcohol interacts with other drugs
Can produce coma or death when combined with

sleeping pills or barbiturates
Can cause stomach bleeding with aspirin
Can inhibit breakdown of other drugs
Measuring blood alcohol concentration (BAC)
Mass (g) of ethanol per 100 cm3 of blood
.08% is legal limit in US (.080 g per 100 cm3 of blood)
Ethanol is easily absorbed from the stomach to the blood, where it is exhaled by the lungs (ethanol is fairly volatile)
C2H5OH(l) ?? C2H5OH(g)
The alcohol vapor can be detected by a number of methods
Слайд 24Breathalyzer test
Subject breathes into an analyzer containing an oxidizing agent and a

detector
Potassium dichromate (K2CrO4)is the oxidizing agent
Oxidizes ethanol to ethanoic acid
This is an oxidation-reduction reaction that involves an electron transfer
This electron transfer generates an electric current which can be detected by the machine
Unreliable in legal cases
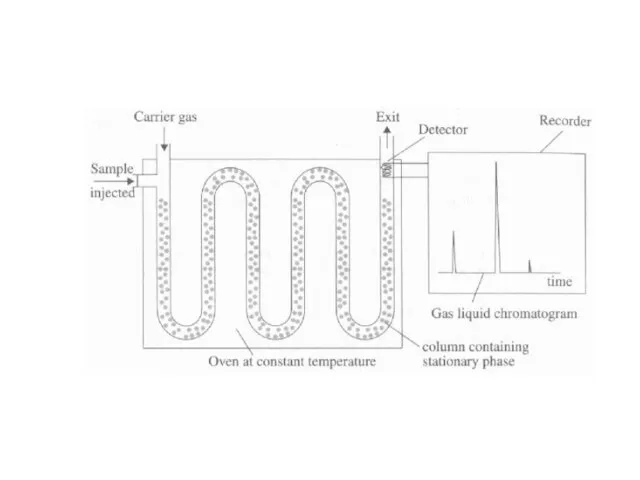
Слайд 25Gas Liquid Chromatography
More precise than breathalyzer
Uses a stationary phase (non-volatile liquid or

solid support) and a mobile phase (inert gas, like N2)
Breath components (CO2, H2O, and alcohol vapor) are injected into the machine and partitioned (divided) between the stationary and mobile phases
Components exit at different intervals (each substance has a different affinity and bond strength for the two phases, and thus move through at different rates)
Components are detected
Retention time for each component is measured (time taken for each component to pass through the column)
Blood alcohol’s retention time is compared to the retention time for a standard ethanol sample
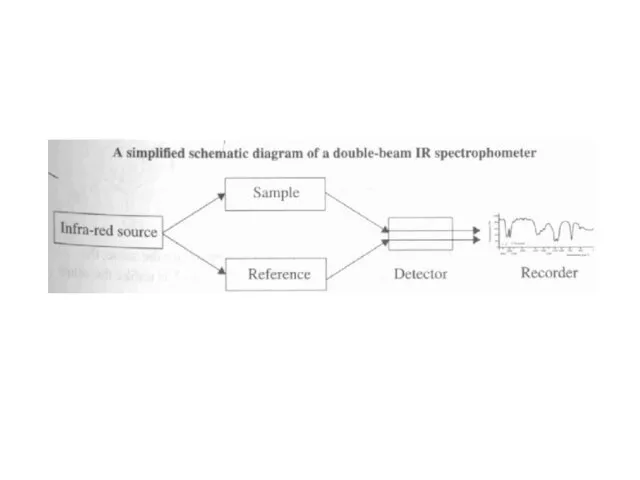
Слайд 27Infra-Red Spectroscopy
IR light does not promote electrons to higher levels, but does

provide enough energy to make molecules “vibrate”
Vibrational motion depends on the mass of the molecule and the types of bonds present
IR spectrum therefore depends on types of molecules present (“molecular fingerprint”)
Scale is based on wavenumber (1/wavelength)
Police use intoximeter (IR spectrometer) to confirm breathalyzer test
IR radiation is passed through breath sample
C-H group in alcohol absorbs a certain frequency of IR light
% transmittance of the C-H frequency is determined, indicating amount of alcohol present
Слайд 29Other Depressants
Diazepam (Valium) is a tranquilizer used to relieve anxiety and tension
Nitrazepam

(Mogadon) is a hypnotic drug used to induce sleep
Fluoxetine hydrochloride (Prozac) is used to treat mental depression by increasing activity of serotonin (a neurotransmitter)
Слайд 30Stimulants
Stimulate brain and central nervous system
Cause increased alertness and awareness
Include amphetamines, nicotine,

and caffeine
Слайд 31Amphetamines
Have structures
similar to
adrenaline
Both are
derived from
Phenylethylamine
Mimic the
actions of
adrenaline
(sympathomimetic)
Constrict
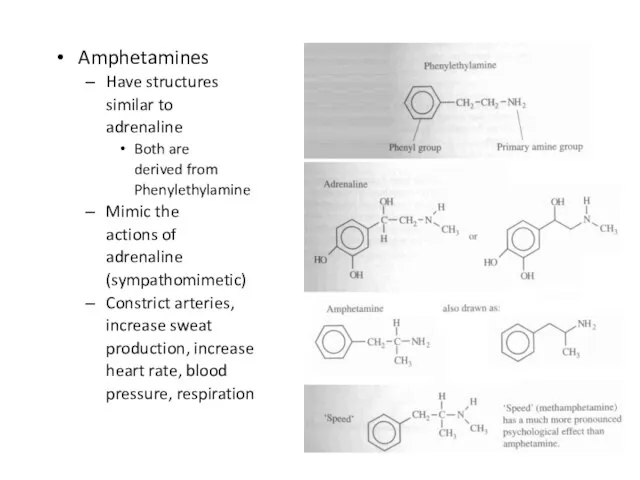
arteries,
increase sweat
production, increase
heart rate, blood
pressure, respiration
Слайд 32Nicotine
Initial stimulant effect, followed by depression, which encourages frequent use
Short term effects:
Increased

heart rate and blood pressure, putting stress on the heart
Reduces urine output
Long term effects
Increased risk of heart disease and blood clot (thrombosis)
Inhibits oxygen-carrying capacity of blood
Increased risk of peptic ulcers
Слайд 33Smoking can also lead to
Lung cancer
Cancer of the larynx and mouth
Heart and

blood vessel disease
Empyhsema
Chronic bronchitis
Air pollution
Fires!!
Stained fingers and teeth
Bad breath
Very easy to develop dependence on nicotine compared to alcohol or barbiturates
Withdrawal symptoms: weight gain, nausea, insomnia, irritability, fatigue, depression, and inability to concentrate
Слайд 34Caffeine
Increases rate of cellular metabolism and therefore respiration
In low doses, enhances wellbeing,

alertness, energy, and motivation
In large amounts, physical coordination and timing are affected, and sleeplessness may also result.
Weak diuretic (increases urine flow)
Tolerance occurs, but no physical dependence
Vasoconstrictor (blood vessel constriction), so can help in treating migraines
Can help newborn babies to breathe as it increases respiration
Слайд 35Caffeine, like nicotine, contains a tertiary amine group (nitrogen atom attached to
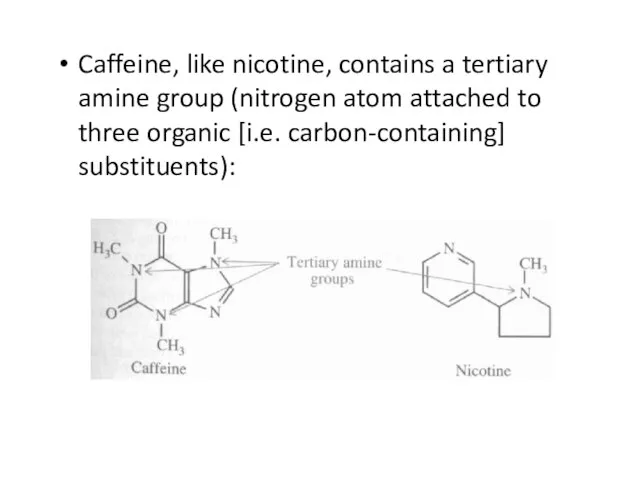
three organic [i.e. carbon-containing] substituents):
Слайд 36Antibacterials
Antibacterials are selective: they attack infectious bacteria rather than human cells
Can be
Bacteriostatic

(inhibit bacterial cell division) or
Bacteriocidal (directly kill bacteria)
Normally ineffective against viruses because viruses live within host cell, which are unaffected by most antibiotics
Слайд 37Penicillins:
Produced from fungi (penicillium genus)
Accidentally discovered by Alexander Fleming, who noticed that

bacteria did not grow around a spot of penicillium notatum mold on a culture plate
Fleming could not isolate the “penicillin,” and later gave up the research
Florey and Chain, at Oxford, renewed the research and started administering the drug to humans
Awarded the Nobel Prize
Thousands of lives were saved during WWII
Слайд 38Structure
Penicillins all have a certain structural feature in common, the 6-APA group
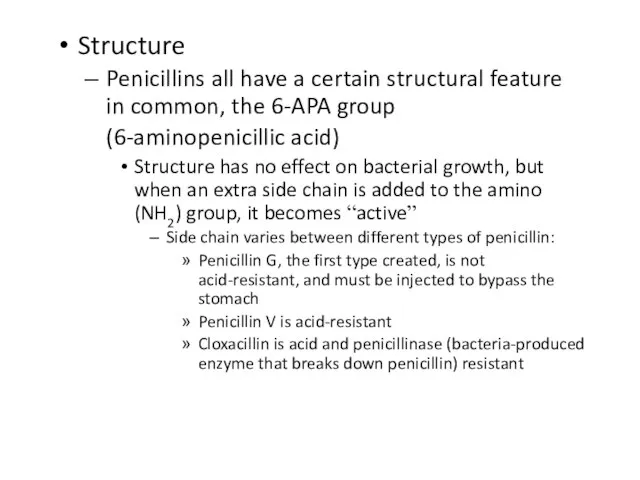
(6-aminopenicillic acid)
Structure has no effect on bacterial growth, but when an extra side chain is added to the amino (NH2) group, it becomes “active”
Side chain varies between different types of penicillin:
Penicillin G, the first type created, is not acid-resistant, and must be injected to bypass the stomach
Penicillin V is acid-resistant
Cloxacillin is acid and penicillinase (bacteria-produced enzyme that breaks down penicillin) resistant
Слайд 39Penicillins differ only in their type of side chain
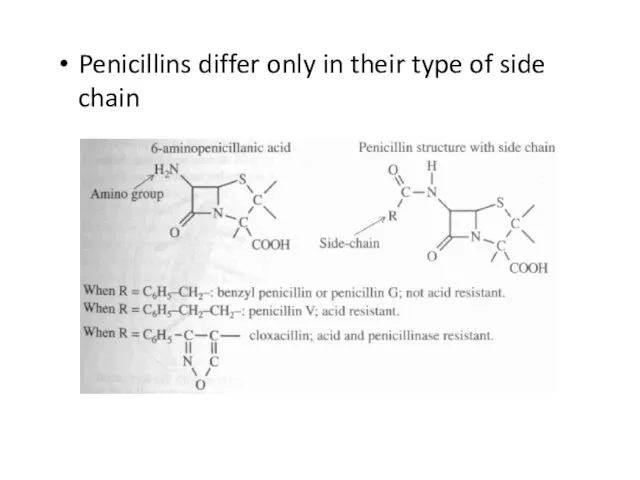
Слайд 40Penicillins function by interfering with the cross-links that connect separate layers of
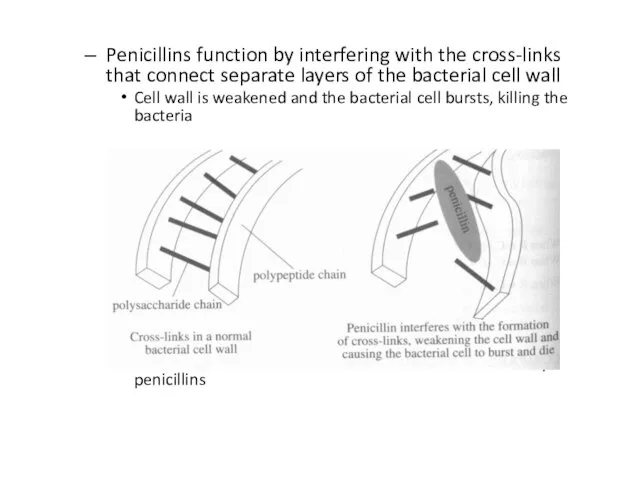
the bacterial cell wall
Cell wall is weakened and the bacterial cell bursts, killing the bacteria
Humans do not have cell walls and are thus unaffected by penicillins
Слайд 41Disadvantages of penicillins
About 10% of the population is allergic
Side effects include fever,

body rash, shock, and death
Overprescription can result in destruction of harmless bacteria in the digestive tract, allowing harmful bacteria to colonize
Overprescription leads to genetics resistance over time, rendering the antibiotic eventually useless
Thus, antibiotics should only be prescribed when there is no other option that can reduce suffering or save a life
Слайд 42Broad vs. Narrow Spectrum Antibiotics:
Broad spectrum
Effective against a wide variety of bacteria
Tetracyclines
(Aureomycin, Terramycin)
Repeated use may wipe out harmless bacteria in the digestive tract, which may be replaced by harmful strains
Narrow spectrum
Effective against only certain types of bacteria
Penicillins
Typically, a broad spectrum is initially prescribed until the bacteria can be identified, at which point a narrow spectrum is prescribed
Слайд 43Antibiotics in animal feed
Antibiotics are added to animal feed to prevent the

spread of infection throughout livestock
However, this can encourage the development of drug-resistant bacteria that humans will eventually be exposed to
Слайд 44Antivirals
Viruses are submicroscopic, non-cellular infectious particles that can only reproduce inside a
living host cell
Unlike bacteria, which have a cellular structure, viruses have no nucleus, cytoplasm, or cell membrane
This limits the effectiveness of antibacterial drugs on viruses
Слайд 45Controlling viruses
Antibacterials may be effective if they block the transfer of genetic

information, although few do
Vaccination is primary method of prevention
Patient is exposed to weakened or inert viral particles to stimulate immune system
Immune system produces antibodies, crucial in the immune response, specific to that virus
Future exposure to active viral particles is more easily controlled because antibodies have already been produced against it
Слайд 46Many antiviral drugs work to inhibit the function of replication-specific enzymes
Latent viruses
are viruses that inject their genetic material into a host cell, but the material is not expressed until a later date
Herpes simplex virus, certain types of cancer
AIDS virus
Attacks immune system by binding to a receptor glycoprotein (CD4) on T4 immune cells
Difficult to fight because of:
its ability to mutate (thus rendering a previous treatment ineffective)
Its metabolism is similar to human cells
Слайд 47Stereochemistry in Drug Action and Design (HL only)
Stereoisomers are isomers with the
same molecular formula AND the same structural formula, but a different arrangement of atoms in space.
Geometric isomers:
If a pair of stereoisomers contains a double bond, cis and trans arrangements can exist:
cis: substituents are on the same side of the double bond
trans: substituents are on opposite sides of the double bond
Слайд 48Geometric isomers have:
different physical properties, including polarity, boiling point, melting point,
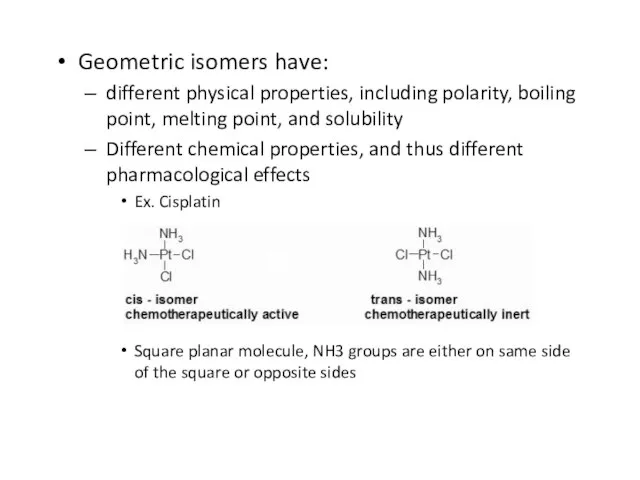
and solubility
Different chemical properties, and thus different pharmacological effects
Ex. Cisplatin
Square planar molecule, NH3 groups are either on same side of the square or opposite sides
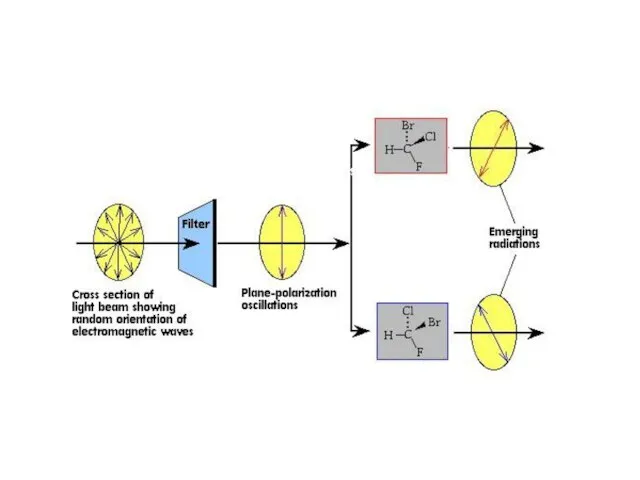
Слайд 49Optical isomers:
Different from geometric isomers:
The molecules are chiral (asymmetric, meaning that there
are four different groups around a central atom)
The isomers are non-superimposable mirror images of one another
Each isomers differs in its optical activity (the ability to rotate the plane of polarized light)
One isomer (enantiomer) rotates the plane of polarized clockwise (+ form), the other rotates it counterclockwise (- form)
Слайд 51An equimolar mixture of both enantiomers (racemic mixture) will not rotate the

plane and is said to be optically inactive
Drugs from natural sources are usually chiral and are generally found as a single enantiomer
Ex. Penicillin V
Opposite enantiomer can only be produced artificially and is pharmacologically inactive
Слайд 52Synthetic drugs, when chiral, are usually produced as racemic mixtures
Ex. : Ibuprofen
One

enantiomer is pharmacologically inactive
Drug still produced as a racemic mixture to reduce costs
Thalidomide
One enantiomer alleviates morning sickness, the other can cause birth defects
Unknown before it was prescribed in the 1970’s
Racemic mixture (“bad” and “good” enantiomers) can still be sold as a treatment for leprosy
Слайд 53Synthesis of non-racemic mixtures is difficult, as both enantiomers are chemically identical
in relation to non-chiral reagents
“chiral auxiliaries” (helping-hands) are used to produce a desired enantiomer from a non-chiral molecule
Attaches itself to non-chiral “building block” to create the stereochemical conditions necessary to force the reaction to follow a certain stereospecific path
Auxiliary can be removed and reused once the desired enantiomer has been formed
Eliminates the need to separate a racemic mixture
Слайд 54Combinatorial chemistry
As drug R & D is very costly and time-consuming, most

drug research begins with a “lead compound,” (not lead as in metal, but “leed) whose main structure is left unaltered but other parts are changed to produce more effective drugs.
Combinatorial chemistry (combi-chem) involves creating a large number of molecules and quickly testing them for desirable biological activity
Sometimes compounds are “virtually tested” by computer simulation
Combi-chem involves reacting a set of starting materials in all possible combinations
Uses same methods as basic organic synthesis, but uses technology and computers to make very large libraries of related chemicals
Increases the chances of finding better drugs
Слайд 55Libraries of a vast amount of related compounds are produced using robotics

to perform repetitive work (ex. adding a fixed volume of a substance to a collection of chemicals) (parallel synthesis)
Products of these reactions are then tested, without animals, by studying their effects on enzymes
Слайд 56Combi-chem began in the 1960’s
Most importantly: Solid-phase synthesis:
Peptide bond is created between
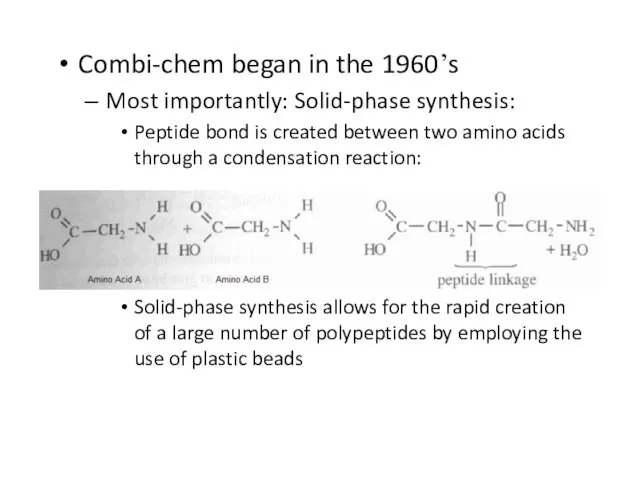
two amino acids through a condensation reaction:
Solid-phase synthesis allows for the rapid creation of a large number of polypeptides by employing the use of plastic beads
Слайд 57
“linking group” is attached to a plastic bead
In vessel 1, amino acid
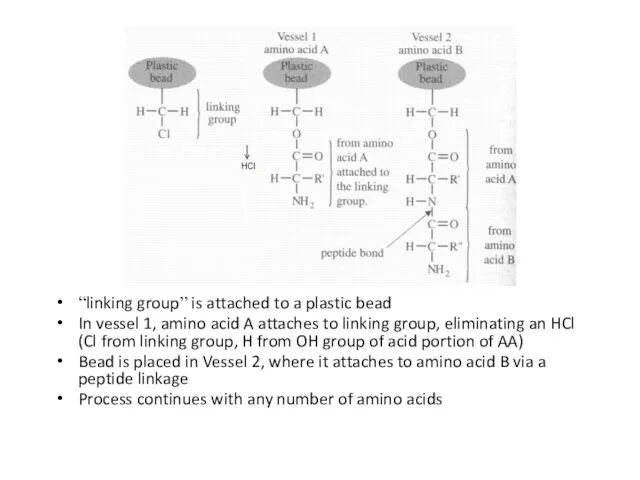
A attaches to linking group, eliminating an HCl (Cl from linking group, H from OH group of acid portion of AA)
Bead is placed in Vessel 2, where it attaches to amino acid B via a peptide linkage
Process continues with any number of amino acids
Слайд 58Procedure can be extended so that the first step reacts two amino

acids, A and B, to produce bead A and bead B
These can be split into separate containers so that each now contains beads A and B, in a half and half mixture
In the second stage, one container is reacted with amino acid A to produce bead A-A and bead B-A
the other container is reacted with amino acid B to produce bead A-B and bead B-B
This two amino acid, two stage process produces 4 (22 ) amino acids (A-A, B-A, A-B, and B-B)
Starting with 3 amino acids in a 2 stage process would produce 32 (9) peptides, 10 amino acids in a 4 stage process would produce 104 (10,000 polypeptides) etc.
A large polypeptide library can therefore be quickly produced
Process can also be extended to other molecules besides peptides to produce very extensive chemical libraries
Слайд 59Anaesthetics
Local vs. General
Local anaesthetics block pain in a specific area (injected under

the skin or applied topically)
Cocaine, procaine, benzococaine, lidocaine
Block nerve conduction and decrease blood supply
Procaine and lidocaine do not affect the brain, but cocaine does
Слайд 60Cocaine, procaine, and lidocaine all contain a benzene ring and a tertiary
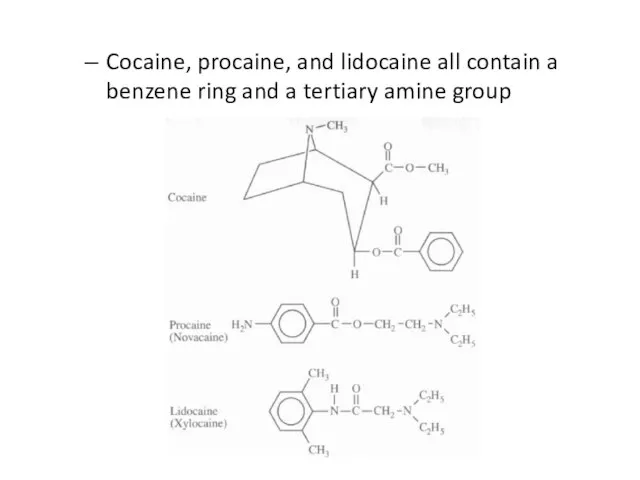
amine group
Слайд 61Cocaine, besides acting as a local anaesthetic, can also stimulate the central

nervous system
Only used medically as a surface application in oral surgery, extremely dangerous when injected because it is a vasoconstrictor
Produces a strong psychological addiction, although no physical dependence or tolerance
Procaine gives prolonged pain relief and immediate loss of feeling prior to dental surgery
Applied through injection and is short-lasting
Lidocaine produces loss of feeling and is applied topically
More potent than procaine
Itching and swelling are side effects
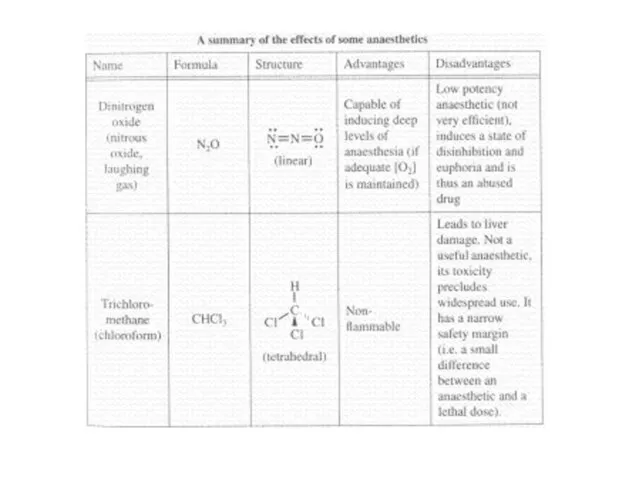
Слайд 62General anaesthetics act on the brain and produce unconsciousness, which can be

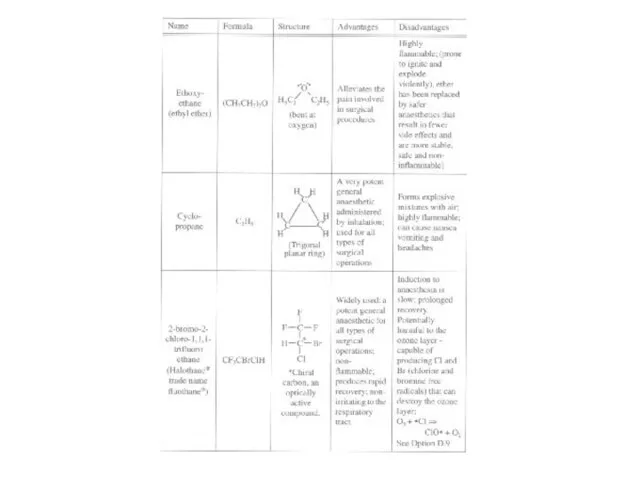
readily reversed
Nitrous oxide (N2O), diethyl ether (C2H5-O-C2H5), chloroform (CHCl3), cyclopropane (C3H6), and halothane (CHClBrCF3)
Some disadvantages:
Nitrous oxide is not very potent
Trichloromethane (chloroform) can lead to liver damage
Ethoxyethane and cyclopropane are highly flammable
Halothane is harmful to the ozone layer
Слайд 65Dalton’s Law of partial pressures can be used to calculate partial pressures
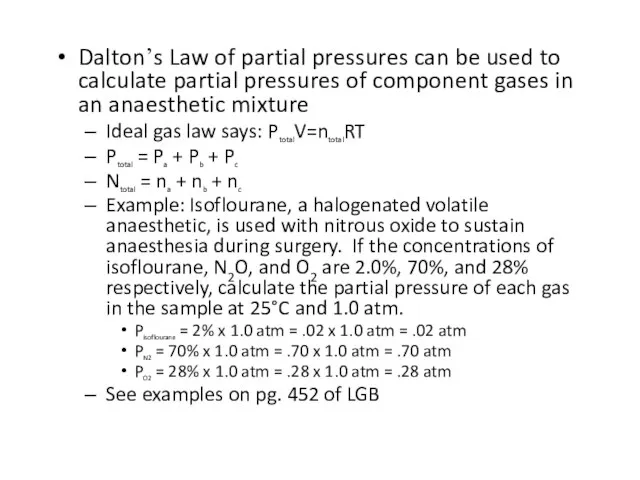
of component gases in an anaesthetic mixture
Ideal gas law says: PtotalV=ntotalRT
Ptotal = Pa + Pb + Pc
Ntotal = na + nb + nc
Example: Isoflourane, a halogenated volatile anaesthetic, is used with nitrous oxide to sustain anaesthesia during surgery. If the concentrations of isoflourane, N2O, and O2 are 2.0%, 70%, and 28% respectively, calculate the partial pressure of each gas in the sample at 25°C and 1.0 atm.
Pisoflourane = 2% x 1.0 atm = .02 x 1.0 atm = .02 atm
PN2 = 70% x 1.0 atm = .70 x 1.0 atm = .70 atm
PO2 = 28% x 1.0 atm = .28 x 1.0 atm = .28 atm
See examples on pg. 452 of LGB
Слайд 66Mind-altering drugs
Psychedelic drugs or psychotomimetics (simulate madness)
Cause hallucinations and distortion of senses
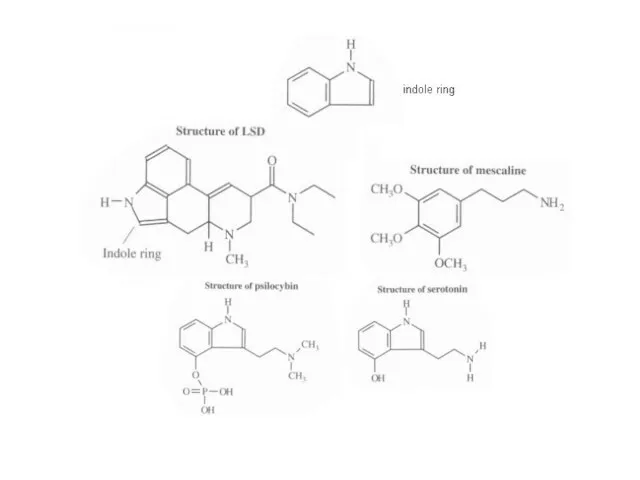
LSD

(lysergic acid)
Mescaline
Psilocybin (peyote mushrooms)
THC (tetrahydrocannabinol in marijuana)
Слайд 67LSD
Powerful hallucinogen
Effect depends on:
Dose
Physiological condition
Psychological condition
Expectations
Magnifies perception
Destroys sense of judgment
Produces flashbacks without

taking LSD
Does not produce physical addition but can produce tolerance and psychological addition
Слайд 68Mescaline
Produces color hallucinations
Lasts approximately 12 hours
Psilocybin
Magnified perception
Low doses produce relaxation, high doses

produce effects similar to LSD
THC (marijuana)
Mild hallucinogen
Causes silliness and excitement at low doses
As dosage increases, perception changes and hallucinations result
Can cause extreme anxiety, depression, uneasiness, panic attack and fearfulness in high doses
Driving and other tasks requiring thinking are difficult
Psychological dependence is possible
Слайд 69LSD, mescaline, and psilocybin all contain a benzene ring (6 carbon); LSD

and psilocybin contain an indole ring (6 carbon benzene ring fused to a 5-membered ring containing a secondary nitrogen)
LSD is fat-soluble and easily diffuses into the brain
Psilocybin mimics the structure of the brain hormone serotonin






























































 Вплив соціально-економічних умов на формування особистості
Вплив соціально-економічних умов на формування особистості Банки в посткризисной экономике:модернизация ради прибыли
Банки в посткризисной экономике:модернизация ради прибыли Внедрение здоровьесберегающих технологий В.Ф. Базарного в практику работы учителя-дефектолога
Внедрение здоровьесберегающих технологий В.Ф. Базарного в практику работы учителя-дефектолога Задача линейного программирования и транспортная задача
Задача линейного программирования и транспортная задача  Авиационная травма
Авиационная травма Прощаемся с тёплым летом
Прощаемся с тёплым летом Поддержка программы Microsoft IT Academy
Поддержка программы Microsoft IT Academy Дополнительная общеразвивающая программа технической направленности самоделкины
Дополнительная общеразвивающая программа технической направленности самоделкины Взаимосвязь животных в природе
Взаимосвязь животных в природе Добывающие предприятия ОАО "Атомредметзолото" как естественные минерально-сырьевые центры по урану на территории России и предло
Добывающие предприятия ОАО "Атомредметзолото" как естественные минерально-сырьевые центры по урану на территории России и предло Мобильная фотография
Мобильная фотография История профсоюзного движения. Знаковые события
История профсоюзного движения. Знаковые события Трудовые правоотношения и заключение трудового договора
Трудовые правоотношения и заключение трудового договора Архитектурный облик Древней Руси
Архитектурный облик Древней Руси Презентация на тему Ввод информации с бумажных носителей
Презентация на тему Ввод информации с бумажных носителей Основное свойство дроби
Основное свойство дроби Бегун сегодня и завтра
Бегун сегодня и завтра Белый камень
Белый камень Гражданское право. Понятие и предмет гражданского права
Гражданское право. Понятие и предмет гражданского права business letter
business letter  Драматические образы в музыке
Драматические образы в музыке Кавказская война
Кавказская война Поворот
Поворот Семья в историческом интерьере
Семья в историческом интерьере Занимательная орфография
Занимательная орфография Понятие и виды социального предпринимательства
Понятие и виды социального предпринимательства Презентация на тему Породы древесины
Презентация на тему Породы древесины Общая характеристика производства по делам об административных правонарушениях. Доказательства и процесс доказывания. Тема №2
Общая характеристика производства по делам об административных правонарушениях. Доказательства и процесс доказывания. Тема №2