Слайд 2Application of Physics in Anesthesiology
Basic knowledge of physics necessary for a

full understanding of the functioning of many anesthetic apparatus.
Слайд 3main topics of the lectures
pressure and flow of gases and liquids.
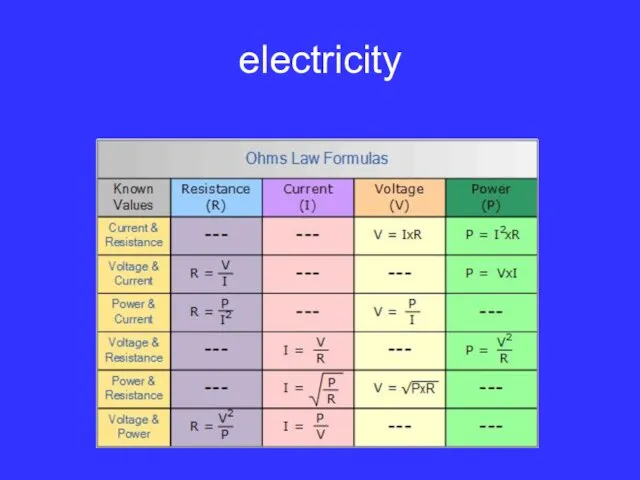
electricity and

electrical safety.
Слайд 4UNITS OF MEASUREMENT
Base SI units
- length (meter)
- mass (kilogram)
-

time (second)
- current (ampere)
- temp (kelvin)
- luminous intensity (candela)
- amount of substance (mole)
Слайд 5DERIVED UNITS
- temp in degrees celcius
- force (newton)
- pressure

(pascal)
- pressure (bar)
- energy (electron volt)
- power (watt)
- frequency (hertz)
- volume ( liter)
UNITS OF MEASUREMENT
Слайд 6UNITS OF MEASUREMENT
UNITS NOT IN THE SI SYSTEM
- pressure (mmHg)
-

pressure (cmh2o)
- pressure (std atmosphere)
- energy (calorie)
- force (kilogram weight)
Слайд 7UNITS OF MEASUREMENT
- 1 kilopascal = 7.5mmHg.
- 1 Bar =

750mmHg
- 1 kilopascal = 10.2cmH2O
- 1 std atmosphere = 101.325kPa
- 1 calorie = 4.18 J
- 1 kilogram weight = 9.81N
- Pounds / in2(PSI) -Atmospheric Pressure PATM=14.7 PSI)
Слайд 8BASIC DEFINITIONS
Fundamental values in the physics of mass, length, and are time.
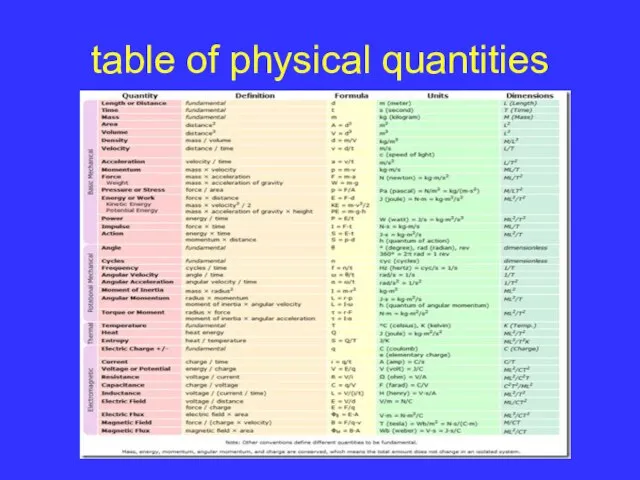
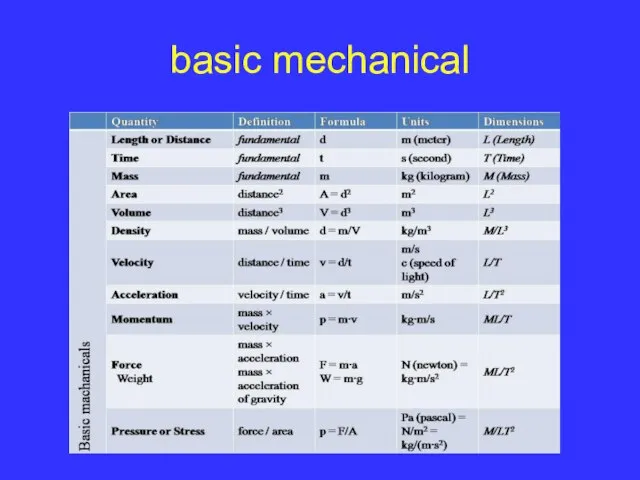
Слайд 9table of physical quantities
Слайд 12FLUIDS
Substances may exist in solid, liquid or gaseous form. In solids, molecules

oscillate about a fixed point, whereas in liquids the molecules possess higher velocities and move more freely and thus do not bear a constant relationship in space to other molecules.
Слайд 13FLUIDS
The molecules of gases also move freely, but to an even greater

extent. Both gases and liquids are termed fluids. Liquids are incompressible and at constant temperature occupy a fixed volume, conforming to the shape o f a container; gases have no fixed volume but expand to occupy the total space o f a container.
but expand to occupy the total space o f a container.
Слайд 15GAS PRESSURES
There are three important laws which determine the behaviour of gases

and which are important to anaesthetists.
Слайд 16GAS PRESSURES
Boyle’s law states that, at constant temperature, the volume ( V)

of a given mass of gas varies inversely with its absolute pressure (P):
P1*V1 = P2*V2
Слайд 17GAS PRESSURES
Charles’ law states that, at constant pressure, the volume of a

given mass o f gas varies directly with its absolute temperature ( T):
V1/T1=V2/T2
T1 = Initial Temperature (Kelvin - K)
V1 = Initial Volume (L or mL)
T2 = Final Temperature (Kelvin - K)
V2 = Final Volume (L or mL)
Слайд 18GAS PRESSURES
The third gas law indicates that at constant volume the absolute

pressure on the gas varies directly with the absolute temperature or P / T = constant. Therefore at constant volume a doubling of temperature results in a doubling of pressure.
Слайд 19GAS PRESSURES
Combining these three gas laws: P1*V1/T1=P2*V2/T2

Слайд 20GAS PRESSURES
The behaviour of a mixture of gases in a container is

described by Dalton’s law of partial pressures.
This states that, in a mixture of gases, the pressure exerted by each gas is the same as that which it would exert if it alone occupied the container.
Thus, in a cylinder o f compressed air at a pressure of 100 bar, the pressure exerted by nitrogen is equal to 79 bar (as the fractional concentration o f nitrogen is 0.79).
Слайд 21Avogadro’s hypothesis
Avogadro’s hypothesis states that equal volumes of gases at the same

temperature and pressure contain equal numbers of molecules.
Слайд 25Pressure notation in anaesthesia

Слайд 26Pressure notation in anaesthesia
























 Машиностроительный комплекс
Машиностроительный комплекс Уважаемые аудиторы!Компания «Эксперт-Эко» представляет первый в Республике Беларусь продукт в области технологии аудита – прог
Уважаемые аудиторы!Компания «Эксперт-Эко» представляет первый в Республике Беларусь продукт в области технологии аудита – прог Картины бытового жанра
Картины бытового жанра Искусство зримых образов. Изображение в театре и кино
Искусство зримых образов. Изображение в театре и кино презентация
презентация С А У Н А
С А У Н А Создание изображения для фона презентации
Создание изображения для фона презентации Sales forecast
Sales forecast Презентация на тему Великие художники второй половины 19 века
Презентация на тему Великие художники второй половины 19 века  Русский модернизм
Русский модернизм Презентация на тему Древние эпосы Индии
Презентация на тему Древние эпосы Индии Презентация на тему Василий Андреевич Жуковский
Презентация на тему Василий Андреевич Жуковский  Исследование процесса получения порошков магнитных сплавов и лигатур
Исследование процесса получения порошков магнитных сплавов и лигатур Процессы и потоки в ОС Windows
Процессы и потоки в ОС Windows Истощение природных ресурсов
Истощение природных ресурсов ФИС_обзор
ФИС_обзор Сядем рядком да потолкуем ладком
Сядем рядком да потолкуем ладком «НАРОДНАЯ ИПОТЕКА» Порядок предоставления бюджетных субсидий гражданам, открывающим вклады в кредитных организациях с целью на
«НАРОДНАЯ ИПОТЕКА» Порядок предоставления бюджетных субсидий гражданам, открывающим вклады в кредитных организациях с целью на Презентация на тему Рождение феодального общества у древних славян
Презентация на тему Рождение феодального общества у древних славян  Интернет - сервисы как инструмент контроля знаний в начальной школе.
Интернет - сервисы как инструмент контроля знаний в начальной школе. Первое знакомство с вероятностью
Первое знакомство с вероятностью Конкурс как способ организации досугового мероприятия
Конкурс как способ организации досугового мероприятия Компании РООС, Новый Диск и Просвещение-МЕДИА представляют Net Школа 3.0 информационное пространство образовательного учреждения © 2
Компании РООС, Новый Диск и Просвещение-МЕДИА представляют Net Школа 3.0 информационное пространство образовательного учреждения © 2 Синквейн – средство творческого выражения
Синквейн – средство творческого выражения Об опыте аккредитации глобальных компаний в национальных органах по аккредитации
Об опыте аккредитации глобальных компаний в национальных органах по аккредитации КАК РАБОТАТЬ С ВЕСАМИ!!!
КАК РАБОТАТЬ С ВЕСАМИ!!! О
О Дневник достижений капоэйра
Дневник достижений капоэйра