Содержание
- 2. The first law of thermodynamics may be stated in several ways: 1. The increase in internal
- 4. Principles of the first law of thermodynamics The law of conservation of energy. This states that
- 5. 2. The concept of internal energy and its relationship to temperature. If a system has a
- 6. 3. The flow of heat is a form of energy transfer. Heating is a natural process
- 7. 4. Work is a process of transferring energy to or from a system. Unless otherwise stated,
- 8. 5. When matter is transferred, its associated internal energy is transferred with it. where uexternal denotes
- 9. An isothermal process is a change of a system, in which the temperture remains constant: ΔT
- 10. An isobaric process is a thermodynamic process in which the pressure stays constant: ΔP = 0.
- 12. Скачать презентацию
Слайд 2The first law of thermodynamics may be stated in several ways:
1. The increase in internal
The first law of thermodynamics may be stated in several ways:
1. The increase in internal
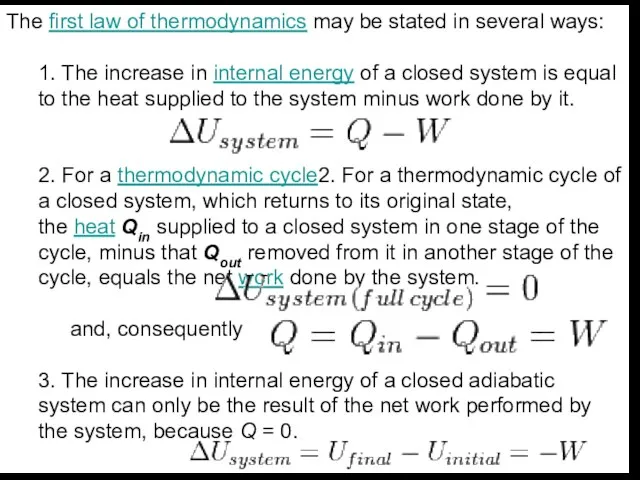
energy of a closed system is equal to the heat supplied to the system minus work done by it.
2. For a thermodynamic cycle2. For a thermodynamic cycle of a closed system, which returns to its original state, the heat Qin supplied to a closed system in one stage of the cycle, minus that Qout removed from it in another stage of the cycle, equals the net work done by the system.
and, consequently
3. The increase in internal energy of a closed adiabatic system can only be the result of the net work performed by the system, because Q = 0.
2. For a thermodynamic cycle2. For a thermodynamic cycle of a closed system, which returns to its original state, the heat Qin supplied to a closed system in one stage of the cycle, minus that Qout removed from it in another stage of the cycle, equals the net work done by the system.
and, consequently
3. The increase in internal energy of a closed adiabatic system can only be the result of the net work performed by the system, because Q = 0.
Слайд 4Principles of the
first law of thermodynamics
The law of conservation of energy.
This states
Principles of the
first law of thermodynamics
The law of conservation of energy.
This states

that energy can be neither created nor destroyed. However, energy can change forms, and energy can flow from one place to another. The total energy of an isolated system does not change.
Слайд 52. The concept of internal energy and its relationship to temperature.
If a
2. The concept of internal energy and its relationship to temperature.
If a

system has a definite temperature, then its total energy has three distinguishable components.
a) If the system is in motion as a whole, it has kinetic energy.
b) If the system as a whole is in an externally imposed force field (e.g. gravity), it has potential energy relative to some reference point.
c) Finally, it has internal energy, which is a fundamental quantity for thermodynamics. Beyond the conceptual frame of macroscopic thermodynamics, it can be explained as the sum of the disorganized kinetic energy of microscopic motions of its constituent atoms, and of the potential energy of interactions between them. Other things being equal, the kinetic energy of microscopic motions of the constituent atoms increases as the system's temperature increases. The establishment of the concept of internal energy is the characteristic distinguishing feature of the first law of thermodynamics.
a) If the system is in motion as a whole, it has kinetic energy.
b) If the system as a whole is in an externally imposed force field (e.g. gravity), it has potential energy relative to some reference point.
c) Finally, it has internal energy, which is a fundamental quantity for thermodynamics. Beyond the conceptual frame of macroscopic thermodynamics, it can be explained as the sum of the disorganized kinetic energy of microscopic motions of its constituent atoms, and of the potential energy of interactions between them. Other things being equal, the kinetic energy of microscopic motions of the constituent atoms increases as the system's temperature increases. The establishment of the concept of internal energy is the characteristic distinguishing feature of the first law of thermodynamics.
Слайд 63. The flow of heat is a form of energy transfer.
Heating is a natural
3. The flow of heat is a form of energy transfer.
Heating is a natural

process of moving energy to or from a system other than by work or the transfer of matter. The heat passes only from a hotter to a colder system.
If the system has rigid walls impermeable to matter, and no external long-range force field affects it, and consequently energy cannot be transferred as work into or out from the system then:
where Q denotes the amount of energy transferred into the system as heat.
If the system has rigid walls impermeable to matter, and no external long-range force field affects it, and consequently energy cannot be transferred as work into or out from the system then:
where Q denotes the amount of energy transferred into the system as heat.
Слайд 74. Work is a process of transferring energy to or from a system.
4. Work is a process of transferring energy to or from a system.
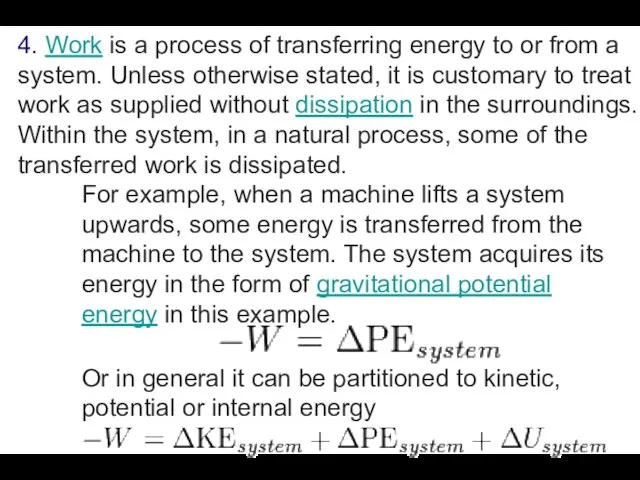
Unless otherwise stated, it is customary to treat work as supplied without dissipation in the surroundings. Within the system, in a natural process, some of the transferred work is dissipated.
For example, when a machine lifts a system upwards, some energy is transferred from the machine to the system. The system acquires its energy in the form of gravitational potential energy in this example.
Or in general it can be partitioned to kinetic, potential or internal energy
For example, when a machine lifts a system upwards, some energy is transferred from the machine to the system. The system acquires its energy in the form of gravitational potential energy in this example.
Or in general it can be partitioned to kinetic, potential or internal energy
Слайд 85. When matter is transferred, its associated internal energy is transferred with
5. When matter is transferred, its associated internal energy is transferred with
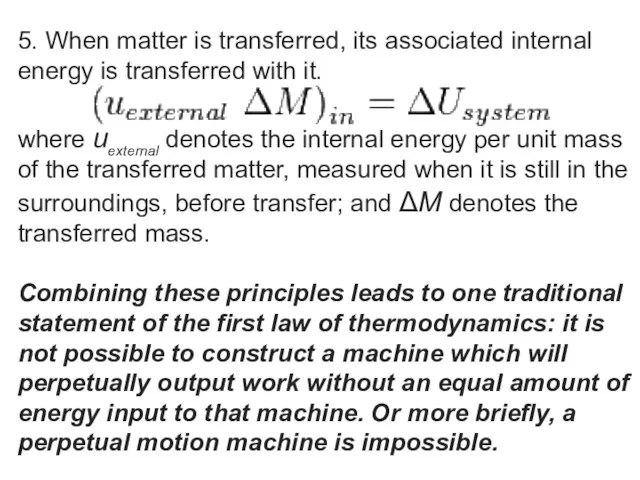
it.
where uexternal denotes the internal energy per unit mass of the transferred matter, measured when it is still in the surroundings, before transfer; and ΔM denotes the transferred mass.
Combining these principles leads to one traditional statement of the first law of thermodynamics: it is not possible to construct a machine which will perpetually output work without an equal amount of energy input to that machine. Or more briefly, a perpetual motion machine is impossible.
where uexternal denotes the internal energy per unit mass of the transferred matter, measured when it is still in the surroundings, before transfer; and ΔM denotes the transferred mass.
Combining these principles leads to one traditional statement of the first law of thermodynamics: it is not possible to construct a machine which will perpetually output work without an equal amount of energy input to that machine. Or more briefly, a perpetual motion machine is impossible.
Слайд 9An isothermal process is a change of a system, in which the temperture remains constant: ΔT = 0. This
An isothermal process is a change of a system, in which the temperture remains constant: ΔT = 0. This
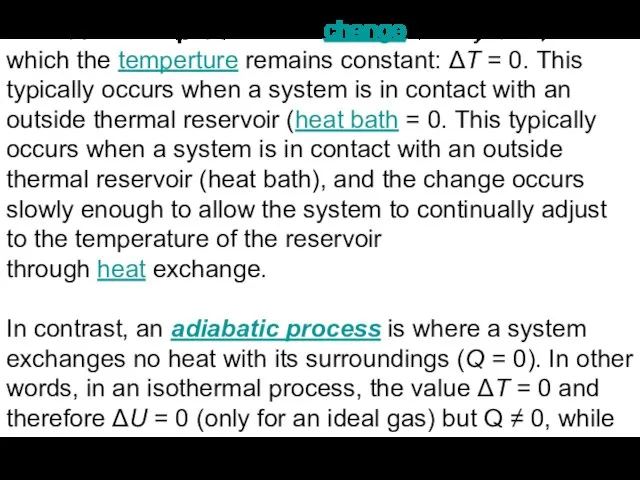
typically occurs when a system is in contact with an outside thermal reservoir (heat bath = 0. This typically occurs when a system is in contact with an outside thermal reservoir (heat bath), and the change occurs slowly enough to allow the system to continually adjust to the temperature of the reservoir through heat exchange.
In contrast, an adiabatic process is where a system exchanges no heat with its surroundings (Q = 0). In other words, in an isothermal process, the value ΔT = 0 and therefore ΔU = 0 (only for an ideal gas) but Q ≠ 0, while in an adiabatic process, ΔT ≠ 0 but Q = 0.
In contrast, an adiabatic process is where a system exchanges no heat with its surroundings (Q = 0). In other words, in an isothermal process, the value ΔT = 0 and therefore ΔU = 0 (only for an ideal gas) but Q ≠ 0, while in an adiabatic process, ΔT ≠ 0 but Q = 0.
Слайд 10An isobaric process is a thermodynamic process in which the pressure stays constant: ΔP = 0. The
An isobaric process is a thermodynamic process in which the pressure stays constant: ΔP = 0. The
term derives from the Greek iso- (equal) and baros (weight). The heat transferred to the system does work, but also changes the internal energy of the system:
An isochoric process, also called a constant-volume process, is a thermodynamic process, is a thermodynamic process during which the volume, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant. An isochoric process is exemplified by the heating or the cooling of the contents of a sealed, inelastic container.
An isochoric process, also called a constant-volume process, is a thermodynamic process, is a thermodynamic process during which the volume, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant. An isochoric process is exemplified by the heating or the cooling of the contents of a sealed, inelastic container.
- Предыдущая
Ideal gas law Equation of stateСледующая -
The second law of thermodynamics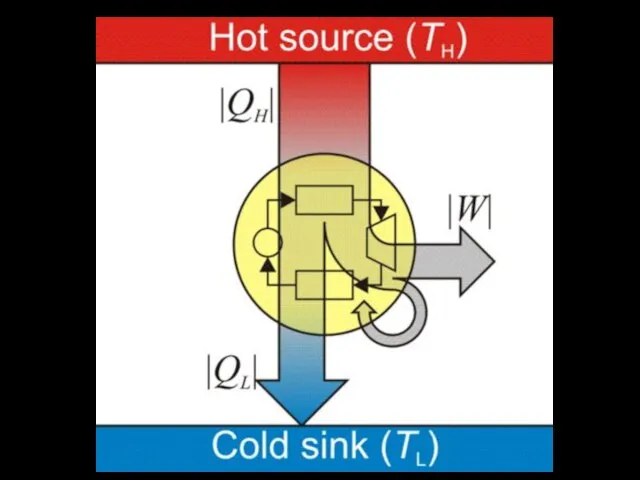
 Знакомство с миром профессионального труда как одна из составляющих программы развития личности дошкольника
Знакомство с миром профессионального труда как одна из составляющих программы развития личности дошкольника Вера моя крепка
Вера моя крепка Место физкультуры и спорта в моей жизни. Введение
Место физкультуры и спорта в моей жизни. Введение Техническое регулирование деятельности предприятий и организаций нефтегазового комплекса
Техническое регулирование деятельности предприятий и организаций нефтегазового комплекса Азбука в загадках-добавлялках
Азбука в загадках-добавлялках Предпосылки создания Древнерусского Государства
Предпосылки создания Древнерусского Государства Сельский туризм. Западный опыт. Общий обзор.
Сельский туризм. Западный опыт. Общий обзор. Reflection
Reflection Презентация на тему Начало создания колониальных империй
Презентация на тему Начало создания колониальных империй  Загрязнение воздуха
Загрязнение воздуха Летняя математическая школа
Летняя математическая школа Моря, омывающие берега России (8 класс)
Моря, омывающие берега России (8 класс) Практика для студентов в отделе кадров
Практика для студентов в отделе кадров Родительское собрание: «Итоговая аттестация учащихся 11-х классов в 2011-2012 учебном году» 22 ноября 2011 г
Родительское собрание: «Итоговая аттестация учащихся 11-х классов в 2011-2012 учебном году» 22 ноября 2011 г О методических и организационно-технологических аспектах проведения ГИА-9 в новой форме в 2011-2012 г.г.Станченко С.В., заместитель д
О методических и организационно-технологических аспектах проведения ГИА-9 в новой форме в 2011-2012 г.г.Станченко С.В., заместитель д Антикафе Чтец
Антикафе Чтец Бизнес-проект по открытию предприятия по производству и реализации корпусной мебели
Бизнес-проект по открытию предприятия по производству и реализации корпусной мебели Нейтронные звезды
Нейтронные звезды ОРГАНИЗАЦИОННАЯ СТРУКТУРА ПРЕДПРИЯТИЯ (ОРГАНИЗАЦИИ)
ОРГАНИЗАЦИОННАЯ СТРУКТУРА ПРЕДПРИЯТИЯ (ОРГАНИЗАЦИИ) Презентация на тему Владимиро-Суздальское княжество (6 класс)
Презентация на тему Владимиро-Суздальское княжество (6 класс) РЕЗУЛЬТАТЫ ПРОЕКТА«Настройка образовательных программ в российских вузах»(TUNING Educational Programmes in Russian HEIs)
РЕЗУЛЬТАТЫ ПРОЕКТА«Настройка образовательных программ в российских вузах»(TUNING Educational Programmes in Russian HEIs) Лето ягодное Часть 4
Лето ягодное Часть 4 Хозяйство России
Хозяйство России 20170201_baykal_8_klass
20170201_baykal_8_klass HISTORICAL BACKGROUND OF THE UK (Ancient Britain
HISTORICAL BACKGROUND OF THE UK (Ancient Britain Учебно-методический комплект «ИСТОРИЯ РОССИИ 1945-2008 гг.»(Концептуальные основы курса.Авторский коллектив.)
Учебно-методический комплект «ИСТОРИЯ РОССИИ 1945-2008 гг.»(Концептуальные основы курса.Авторский коллектив.) Шенген визы: открой себе Европу
Шенген визы: открой себе Европу Языковой портфолио как инструмент рефлексивной самооценки учащихся
Языковой портфолио как инструмент рефлексивной самооценки учащихся