Содержание
- 2. In physicsIn physics and thermodynamics, an equation of state is a relation between state variables is
- 3. An ideal gas is a theoretical is a theoretical gas is a theoretical gas composed of
- 4. The classical ideal gas law may be written: An isothermal process is a change of a
- 5. An isobaric process is a thermodynamic process in which the pressure stays constant: ΔP = 0.
- 6. Partial pressure In a mixture of gases, each gas has a partial pressure which is the
- 8. Скачать презентацию
Слайд 2 In physicsIn physics and thermodynamics, an equation of state is a relation between state variables is a relation between state variables. More
In physicsIn physics and thermodynamics, an equation of state is a relation between state variables is a relation between state variables. More
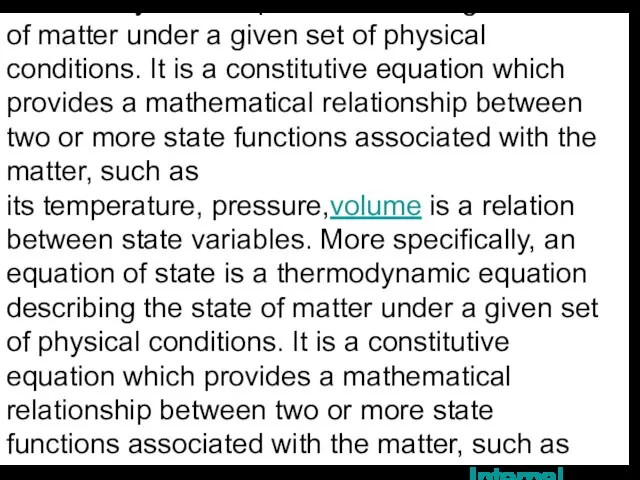
specifically, an equation of state is a thermodynamic equation is a relation between state variables. More specifically, an equation of state is a thermodynamic equation describing the state of matter under a given set of physical conditions. It is a constitutive equation is a relation between state variables. More specifically, an equation of state is a thermodynamic equation describing the state of matter under a given set of physical conditions. It is a constitutive equation which provides a mathematical relationship between two or more state functions is a relation between state variables. More specifically, an equation of state is a thermodynamic equation describing the state of matter under a given set of physical conditions. It is a constitutive equation which provides a mathematical relationship between two or more state functions associated with the matter, such as its temperature is a relation between state variables. More specifically, an equation of state is a thermodynamic equation describing the state of matter under a given set of physical conditions. It is a constitutive equation which provides a mathematical relationship between two or more state functions associated with the matter, such as its temperature, pressure is a relation between state variables. More specifically, an equation of state is a thermodynamic equation describing the state of matter under a given set of physical conditions. It is a constitutive equation which provides a mathematical relationship between two or more state functions associated with the matter, such as its temperature, pressure,volume is a relation between state variables. More specifically, an equation of state is a thermodynamic equation describing the state of matter under a given set of physical conditions. It is a constitutive equation which provides a mathematical relationship between two or more state functions associated with the matter, such as its temperature, pressure,volume, or internal energy is a relation between state variables. More specifically, an equation of state is a thermodynamic equation describing the state of matter under a given set of physical conditions. It is a constitutive equation which provides a mathematical relationship between two or more state functions associated with the matter, such as its temperature, pressure,volume, or internal energy. Equations of state are useful in describing the properties of fluids is a relation between state variables. More specifically, an equation of state is a thermodynamic equation describing the state of matter under a given set of physical conditions. It is a constitutive equation which provides a mathematical relationship between two or more state functions associated with the matter, such as its temperature, pressure,volume, or internal energy. Equations of state are useful in describing the properties of fluids, mixtures of fluids, solids is a relation between state variables. More specifically, an equation of state is a thermodynamic equation describing the state of matter under a given set of physical conditions. It is a constitutive equation which provides a mathematical relationship between two or more state functions associated with the matter, such as its temperature, pressure,volume, or internal energy. Equations of state are useful in describing the properties of fluids, mixtures of fluids, solids, and even the interior of stars.
Слайд 3An ideal gas is a theoretical is a theoretical gas is a theoretical gas composed of many randomly moving point particles is
An ideal gas is a theoretical is a theoretical gas is a theoretical gas composed of many randomly moving point particles is

a theoretical gas composed of many randomly moving point particles that do not interact except when they collide elastically. The ideal gas concept is useful because it obeys the ideal gas law is a theoretical gas composed of many randomly moving point particles that do not interact except when they collide elastically. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state is a theoretical gas composed of many randomly moving point particles that do not interact except when they collide elastically. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics is a theoretical gas composed of many randomly moving point particles that do not interact except when they collide elastically. The ideal gas concept is useful because it obeys the ideal gas law, a simplified equation of state, and is amenable to analysis under statistical mechanics. One mole of an ideal gas has a volume of 22.7 L at STP.
At normal conditions such as standard temperature and pressureAt normal conditions such as standard temperature and pressure, most real gasesAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogenAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygenAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygen, hydrogenAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygen, hydrogen, noble gasesAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxideAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxide can be treated like ideal gases within reasonable tolerances. Generally, a gas behaves more like an ideal gas at higher temperatureAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxide can be treated like ideal gases within reasonable tolerances. Generally, a gas behaves more like an ideal gas at higher temperature and lower pressureAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxide can be treated like ideal gases within reasonable tolerances. Generally, a gas behaves more like an ideal gas at higher temperature and lower pressure, as the workAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxide can be treated like ideal gases within reasonable tolerances. Generally, a gas behaves more like an ideal gas at higher temperature and lower pressure, as the work which is against intermolecular forces becomes less significant compared with the particles' kinetic energy, and the size of the molecules becomes less significant compared to the empty space between them.
The ideal gas model tends to fail at lower temperatures or higher pressures, when intermolecular forces and molecular size become important. At some point of low temperature and high pressure, real gases undergo a phase transitionThe ideal gas model tends to fail at lower temperatures or higher pressures, when intermolecular forces and molecular size become important. At some point of low temperature and high pressure, real gases undergo a phase transition, such as to a liquidThe ideal gas model tends to fail at lower temperatures or higher pressures, when intermolecular forces and molecular size become important. At some point of low temperature and high pressure, real gases undergo a phase transition, such as to a liquid or a solidThe ideal gas model tends to fail at lower temperatures or higher pressures, when intermolecular forces and molecular size become important. At some point of low temperature and high pressure, real gases undergo a phase transition, such as to a liquid or a solid. The model of an ideal gas, however, does not describe or allow phase transitions. These must be modeled by more complex equations of state.
At normal conditions such as standard temperature and pressureAt normal conditions such as standard temperature and pressure, most real gasesAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogenAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygenAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygen, hydrogenAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygen, hydrogen, noble gasesAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxideAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxide can be treated like ideal gases within reasonable tolerances. Generally, a gas behaves more like an ideal gas at higher temperatureAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxide can be treated like ideal gases within reasonable tolerances. Generally, a gas behaves more like an ideal gas at higher temperature and lower pressureAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxide can be treated like ideal gases within reasonable tolerances. Generally, a gas behaves more like an ideal gas at higher temperature and lower pressure, as the workAt normal conditions such as standard temperature and pressure, most real gases behave qualitatively like an ideal gas. Many such as nitrogen, oxygen, hydrogen, noble gases, and some heavier gases like carbon dioxide can be treated like ideal gases within reasonable tolerances. Generally, a gas behaves more like an ideal gas at higher temperature and lower pressure, as the work which is against intermolecular forces becomes less significant compared with the particles' kinetic energy, and the size of the molecules becomes less significant compared to the empty space between them.
The ideal gas model tends to fail at lower temperatures or higher pressures, when intermolecular forces and molecular size become important. At some point of low temperature and high pressure, real gases undergo a phase transitionThe ideal gas model tends to fail at lower temperatures or higher pressures, when intermolecular forces and molecular size become important. At some point of low temperature and high pressure, real gases undergo a phase transition, such as to a liquidThe ideal gas model tends to fail at lower temperatures or higher pressures, when intermolecular forces and molecular size become important. At some point of low temperature and high pressure, real gases undergo a phase transition, such as to a liquid or a solidThe ideal gas model tends to fail at lower temperatures or higher pressures, when intermolecular forces and molecular size become important. At some point of low temperature and high pressure, real gases undergo a phase transition, such as to a liquid or a solid. The model of an ideal gas, however, does not describe or allow phase transitions. These must be modeled by more complex equations of state.
Слайд 4The classical ideal gas law may be written:
An isothermal process is a change of a system, in which the
The classical ideal gas law may be written:
An isothermal process is a change of a system, in which the

temperature remains constant: ΔT = 0. This typically occurs when a system is in contact with an outside thermal reservoir (heat bath = 0. This typically occurs when a system is in contact with an outside thermal reservoir (heat bath), and the change occurs slowly enough to allow the system to continually adjust to the temperature of the reservoir through heat exchange. In contrast, an adiabatic process is where a system exchanges no heat with its surroundings (Q = 0). In other words, in an isothermal process, the value ΔT = 0 and therefore ΔU = 0 (only for an ideal gas) but Q ≠ 0, while in an adiabatic process, ΔT ≠ 0 but Q = 0.
Слайд 5An isobaric process is a thermodynamic process in which the pressure stays constant: ΔP = 0. The
An isobaric process is a thermodynamic process in which the pressure stays constant: ΔP = 0. The

term derives from the Greek iso- (equal) and baros (weight). The heat transferred to the system does work, but also changes the internal energy of the system:
An isochoric process, also called a constant-volume process, is a thermodynamic process, is a thermodynamic process during which the volume, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant. An isochoric process is exemplified by the heating or the cooling of the contents of a sealed, inelastic container:
An isochoric process, also called a constant-volume process, is a thermodynamic process, is a thermodynamic process during which the volume, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant. An isochoric process is exemplified by the heating or the cooling of the contents of a sealed, inelastic container:
Слайд 6Partial pressure
In a mixture of gases, each gas has a partial pressure which is the
Partial pressure
In a mixture of gases, each gas has a partial pressure which is the

hypothetical pressure is the hypothetical pressure of that gas if it alone occupied the volume is the hypothetical pressure of that gas if it alone occupied the volume of the mixture at the same temperature is the hypothetical pressure of that gas if it alone occupied the volume of the mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of each individual gas in the mixture as stated by Dalton's law.
- Предыдущая
Hearts Anatomy and PhysiologyСледующая -
The first law of thermodynamics Денежные переводы физических лиц: состояние и тенденции развития (на примере ПАО Сбербанк)
Денежные переводы физических лиц: состояние и тенденции развития (на примере ПАО Сбербанк) Особенности истории информатики
Особенности истории информатики Мотивирование топ-менеджеров в банковской сфере: подходы и инструменты
Мотивирование топ-менеджеров в банковской сфере: подходы и инструменты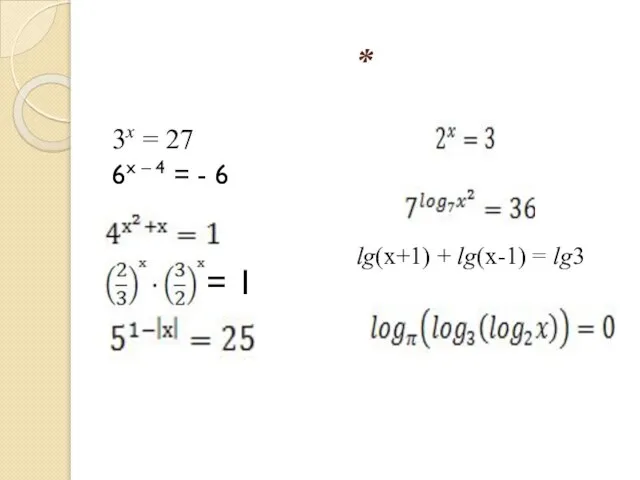 = 1
= 1 Изустная среда мордовского села
Изустная среда мордовского села Показатели рыночной активности
Показатели рыночной активности Выхухоль. Красная книга
Выхухоль. Красная книга Обработка текстовой и графической информации. Создаём комбинированный документ
Обработка текстовой и графической информации. Создаём комбинированный документ Телевидение: я выбираю или меня выбирают. Автор и руководитель проекта: Симонова Ольга Владимировна 2004 год
Телевидение: я выбираю или меня выбирают. Автор и руководитель проекта: Симонова Ольга Владимировна 2004 год Что я сделаю, чтобы доверенный мне магазин стал лучшим на рынке
Что я сделаю, чтобы доверенный мне магазин стал лучшим на рынке Туалетная вода для мужчин Faberlic Intense
Туалетная вода для мужчин Faberlic Intense Межпроцедурные анализы и оптимизации
Межпроцедурные анализы и оптимизации Внешняя политика СССР в 1930-е гг. и накануне Великой Отечественной войны
Внешняя политика СССР в 1930-е гг. и накануне Великой Отечественной войны Урок 3 Человек и Бог в православии-повторение
Урок 3 Человек и Бог в православии-повторение 17 век
17 век ПЕРЕГОВОРЫ
ПЕРЕГОВОРЫ Изготовление вечернего платья
Изготовление вечернего платья Теорiя iгор
Теорiя iгор  Презентация на тему Роль гормонов в обмене веществ, росте и развитии организма биология 8 класс
Презентация на тему Роль гормонов в обмене веществ, росте и развитии организма биология 8 класс аня хочет санчо панчо
аня хочет санчо панчо Создание таблиц в Microsoft Word
Создание таблиц в Microsoft Word Teenagers’ society problems
Teenagers’ society problems Воля познавать
Воля познавать Любимое блюдо моей семьи
Любимое блюдо моей семьи Ладья. Урок №8
Ладья. Урок №8 Описательные характеристики распределения тестовых результатов
Описательные характеристики распределения тестовых результатов Презентация на тему Одиночество
Презентация на тему Одиночество Ich gehe mit meine Laterne
Ich gehe mit meine Laterne