Содержание
- 2. The second law of thermodynamics asserts the irreversibility of natural processes, and the tendency of natural
- 3. The second law refers to a wide variety of processes, reversible and irreversible. All natural processes
- 4. In thermodynamics, entropy is a measure of the number of specific ways in which a thermodynamic
- 5. where T is the absolute temperature of the system, dividing an incremental reversible transfer of heat
- 6. The absolute entropy (S rather than ΔS) was defined later, using either statistical mechanics) was defined
- 7. Carnot cycle The Carnot cycle is a theoretical thermodynamic cycle is a theoretical thermodynamic cycle proposed
- 8. A Carnot cycle illustrated on a PV diagram to illustrate the work done.
- 9. The Carnot cycle when acting as a heat engine consists of the following steps: Reversible isothermal
- 10. 3. Reversible isothermal compression of the gas at the "cold" temperature, T2. (isothermal heat rejection) (3
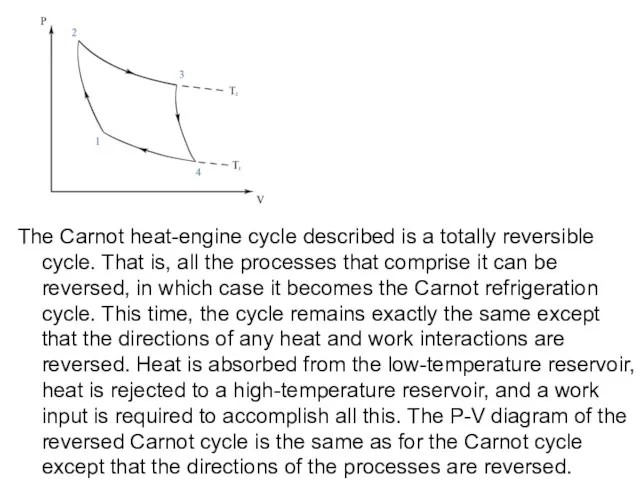
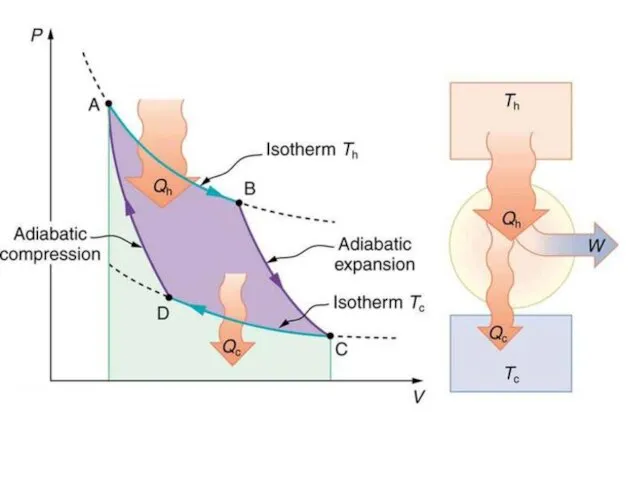
- 11. The Carnot heat-engine cycle described is a totally reversible cycle. That is, all the processes that
- 15. Скачать презентацию
Слайд 2 The second law of thermodynamics asserts the irreversibility of natural processes, and the tendency
The second law of thermodynamics asserts the irreversibility of natural processes, and the tendency

It implies the existence of a quantity called the entropy It implies the existence of a quantity called the entropy of a thermodynamic system. When two initially isolated systems It implies the existence of a quantity called the entropy of a thermodynamic system. When two initially isolated systems in separate but nearby regions of space, each in thermodynamic equilibrium It implies the existence of a quantity called the entropy of a thermodynamic system. When two initially isolated systems in separate but nearby regions of space, each in thermodynamic equilibrium with itself but not necessarily with each other, are then allowed to interact, they will eventually reach a mutual thermodynamic equilibrium. The sum of the entropies of the initially isolated systems is less than or equal to the total entropy of the final combination.
This statement of the law recognizes that in classical thermodynamics, the entropy of a system is defined only when it has reached its own internal thermodynamic equilibrium.
Слайд 3 The second law refers to a wide variety of processes, reversible and
The second law refers to a wide variety of processes, reversible and
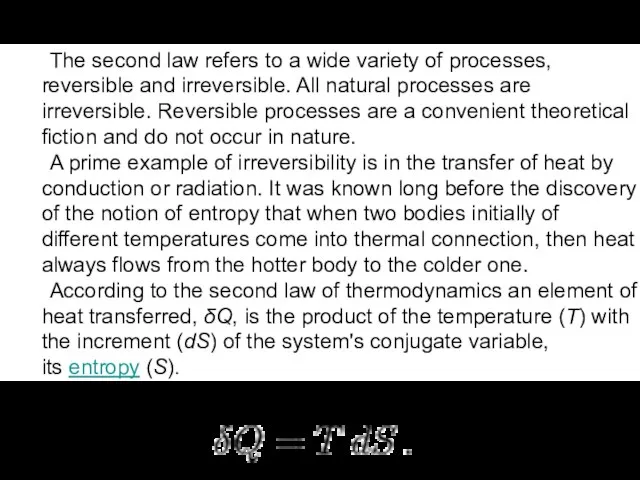
A prime example of irreversibility is in the transfer of heat by conduction or radiation. It was known long before the discovery of the notion of entropy that when two bodies initially of different temperatures come into thermal connection, then heat always flows from the hotter body to the colder one.
According to the second law of thermodynamics an element of heat transferred, δQ, is the product of the temperature (T) with the increment (dS) of the system's conjugate variable, its entropy (S).
Слайд 4 In thermodynamics, entropy is a measure of the number of specific ways in which
In thermodynamics, entropy is a measure of the number of specific ways in which
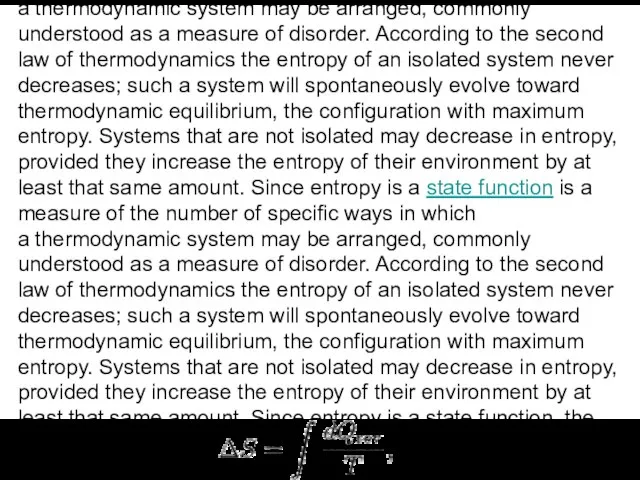
The change in entropy (ΔS) of a system was originally defined for a thermodynamically reversible process as
Слайд 5where T is the absolute temperature of the system, dividing an incremental reversible transfer of heat
where T is the absolute temperature of the system, dividing an incremental reversible transfer of heat
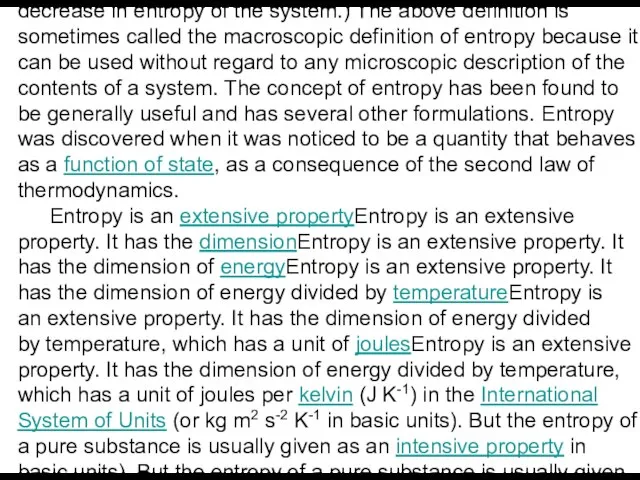
Entropy is an extensive propertyEntropy is an extensive property. It has the dimensionEntropy is an extensive property. It has the dimension of energyEntropy is an extensive property. It has the dimension of energy divided by temperatureEntropy is an extensive property. It has the dimension of energy divided by temperature, which has a unit of joulesEntropy is an extensive property. It has the dimension of energy divided by temperature, which has a unit of joules per kelvin (J K-1) in the International System of Units (or kg m2 s-2 K-1 in basic units). But the entropy of a pure substance is usually given as an intensive property in basic units). But the entropy of a pure substance is usually given as an intensive property — either entropy per unit mass (SI unit: J K-1 kg-1) or entropy per unit amount of substance (SI unit: J K-1 mol-1).
Слайд 6 The absolute entropy (S rather than ΔS) was defined later, using either statistical mechanics) was defined
The absolute entropy (S rather than ΔS) was defined later, using either statistical mechanics) was defined
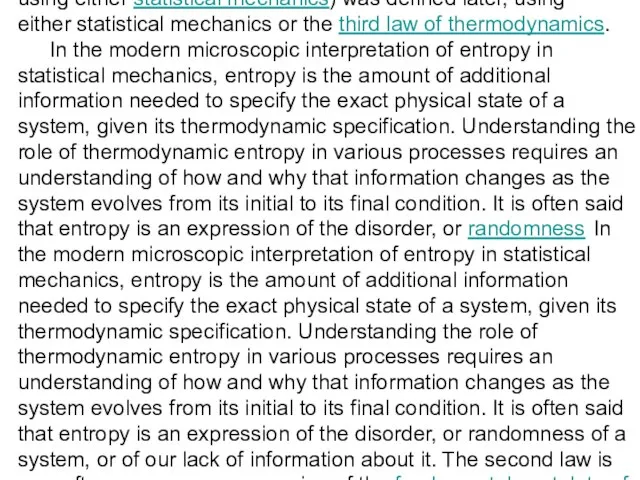
In the modern microscopic interpretation of entropy in statistical mechanics, entropy is the amount of additional information needed to specify the exact physical state of a system, given its thermodynamic specification. Understanding the role of thermodynamic entropy in various processes requires an understanding of how and why that information changes as the system evolves from its initial to its final condition. It is often said that entropy is an expression of the disorder, or randomness In the modern microscopic interpretation of entropy in statistical mechanics, entropy is the amount of additional information needed to specify the exact physical state of a system, given its thermodynamic specification. Understanding the role of thermodynamic entropy in various processes requires an understanding of how and why that information changes as the system evolves from its initial to its final condition. It is often said that entropy is an expression of the disorder, or randomness of a system, or of our lack of information about it. The second law is now often seen as an expression of the fundamental postulate of statistical mechanics through the modern definition of entropy.
Слайд 7 Carnot cycle
The Carnot cycle is a theoretical thermodynamic cycle is a theoretical thermodynamic cycle proposed by Nicolas
Carnot cycle
The Carnot cycle is a theoretical thermodynamic cycle is a theoretical thermodynamic cycle proposed by Nicolas

Every single thermodynamic system exists in a particular state. When a system is taken through a series of different states and finally returned to its initial state, a thermodynamic cycle is said to have occurred. In the process of going through this cycle, the system may perform work on its surroundings, thereby acting as a heat engine Every single thermodynamic system exists in a particular state. When a system is taken through a series of different states and finally returned to its initial state, a thermodynamic cycle is said to have occurred. In the process of going through this cycle, the system may perform work on its surroundings, thereby acting as a heat engine. A system undergoing a Carnot cycle is called a Carnot heat engine, although such a "perfect" engine is only a theoretical limit and cannot be built in practice.
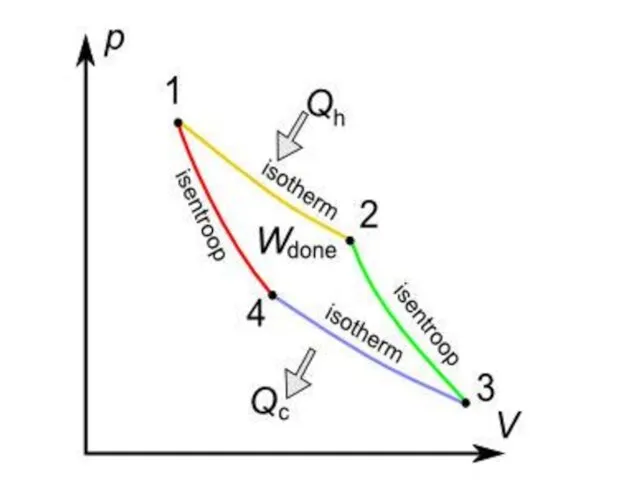
Слайд 8A Carnot cycle illustrated on a PV diagram to illustrate the work done.
A Carnot cycle illustrated on a PV diagram to illustrate the work done.
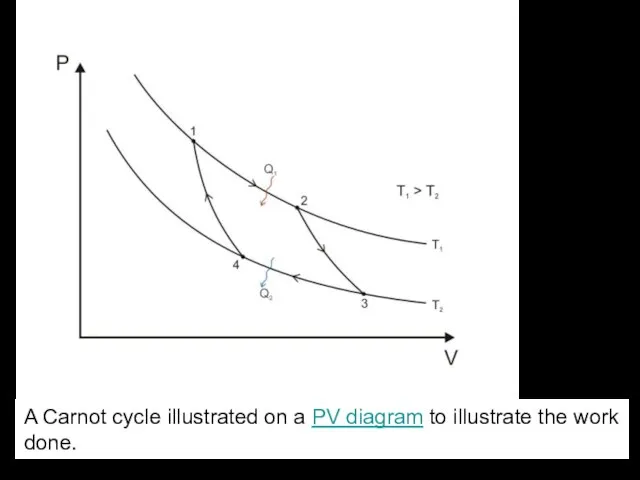
Слайд 9 The Carnot cycle when acting as a heat engine consists of the
The Carnot cycle when acting as a heat engine consists of the

Reversible isothermal expansion of the gas at the "hot" temperature, T1 (isothermal heat addition or absorption).During this step (1 to 2 on Figure) the gas is allowed to expand and it does work on the surroundings. The temperature of the gas does not change during the process, and thus the expansion is isothermal. The gas expansion is propelled by absorption of heat energy Q1 and of entropy
from the high temperature reservoir.
Reversible adiabatic Reversible adiabatic expansion of the gas (adiabatic work output). For this step (2 to 3 on Figure) the mechanisms of the engine are assumed to be thermally insulated, thus they neither gain nor lose heat. The gas continues to expand, doing work on the surroundings, and losing an equivalent amount of internal energy. The gas expansion causes it to cool to the "cold" temperature, T2. The entropy remains unchanged.
Слайд 103. Reversible isothermal compression of the gas at the "cold" temperature, T2. (isothermal
3. Reversible isothermal compression of the gas at the "cold" temperature, T2. (isothermal
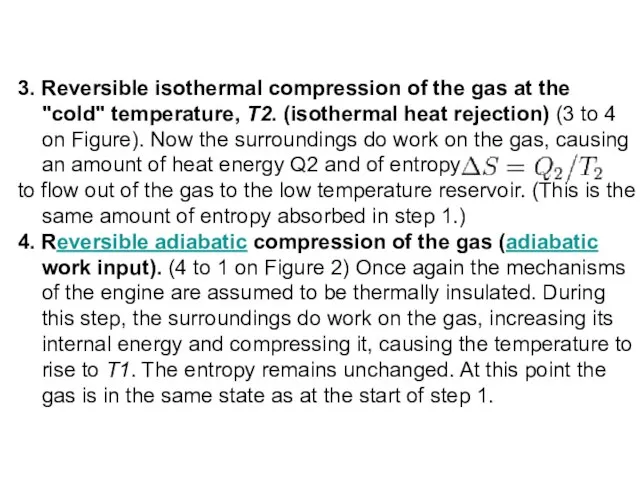
to flow out of the gas to the low temperature reservoir. (This is the same amount of entropy absorbed in step 1.)
4. Reversible adiabatic compression of the gas (adiabatic work input). (4 to 1 on Figure 2) Once again the mechanisms of the engine are assumed to be thermally insulated. During this step, the surroundings do work on the gas, increasing its internal energy and compressing it, causing the temperature to rise to T1. The entropy remains unchanged. At this point the gas is in the same state as at the start of step 1.
Слайд 11The Carnot heat-engine cycle described is a totally reversible cycle. That is,
The Carnot heat-engine cycle described is a totally reversible cycle. That is,
 Проектирование полуботинок с настрочными берцами
Проектирование полуботинок с настрочными берцами Бюджет для граждан на 2022 год
Бюджет для граждан на 2022 год Ластоногие и Китообразные, Парнокопытные и Непарнокопытные, Хоботные
Ластоногие и Китообразные, Парнокопытные и Непарнокопытные, Хоботные РЕЗУЛЬТАТЫ РАБОТЫПОДСИСТЕМЫ «ВЕДЕНИЯ»
РЕЗУЛЬТАТЫ РАБОТЫПОДСИСТЕМЫ «ВЕДЕНИЯ» Переход к предоставлению услуги «Социальная поддержка ветеранов труда, лиц, проработавших в тылу в период Великой Отечественной в
Переход к предоставлению услуги «Социальная поддержка ветеранов труда, лиц, проработавших в тылу в период Великой Отечественной в Ивановское сельское поселение. Исполнение бюджета
Ивановское сельское поселение. Исполнение бюджета О компании Jura Elektroapparate AG
О компании Jura Elektroapparate AG The flag of the uk
The flag of the uk Желаем Вам приятного просмотра! Для смены слайдов нажимайте клавишу ПРОБЕЛ.
Желаем Вам приятного просмотра! Для смены слайдов нажимайте клавишу ПРОБЕЛ. На пути к Библиотеке 2.0: освоение перспективных интернет-технологий
На пути к Библиотеке 2.0: освоение перспективных интернет-технологий Расчёт на прочность при изгибе
Расчёт на прочность при изгибе Видеонаблюдение при проведении выборов депутатов Государственной Думы
Видеонаблюдение при проведении выборов депутатов Государственной Думы В царстве грибов
В царстве грибов Бизнес планирование предприятий
Бизнес планирование предприятий План мероприятий на каникулы
План мероприятий на каникулы Н. В. Гоголь в разделе «Что такое слово и словесность» пишет: "Говорится все, записывается немногое, и только то, что нужно. Отсюда зн
Н. В. Гоголь в разделе «Что такое слово и словесность» пишет: "Говорится все, записывается немногое, и только то, что нужно. Отсюда зн Значение природных ресурсов
Значение природных ресурсов Презентация 6-7 СРО Шевченко Д.В
Презентация 6-7 СРО Шевченко Д.В Как выполняли арифметические действия в Древнем Риме?
Как выполняли арифметические действия в Древнем Риме? Мастер-класс
Мастер-класс Презентация на тему: Проблемы подросткового возраста и его особенности
Презентация на тему: Проблемы подросткового возраста и его особенности Телекоммуникации
Телекоммуникации Сварные соединения и швы
Сварные соединения и швы Цапина Елена Михайловна Классный руководитель6 «а» класса Школа №9 г.Можга
Цапина Елена Михайловна Классный руководитель6 «а» класса Школа №9 г.Можга Презентация на тему Обучение грамоте и развитие речи
Презентация на тему Обучение грамоте и развитие речи Les meilleures montres dans le monde
Les meilleures montres dans le monde Презентация на тему Открытия Ломоносова в области физики
Презентация на тему Открытия Ломоносова в области физики  Маньяки… кто есть кто
Маньяки… кто есть кто