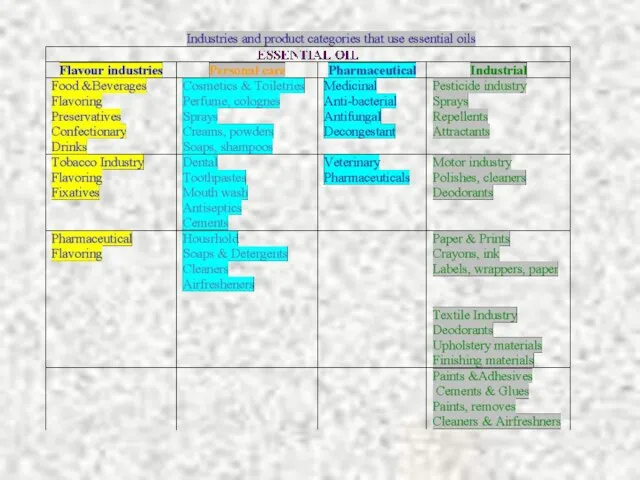
Содержание
- 2. Volatile oils (Aetheroleum) are the odorous principles found in various plant parts. Because they evaporate when
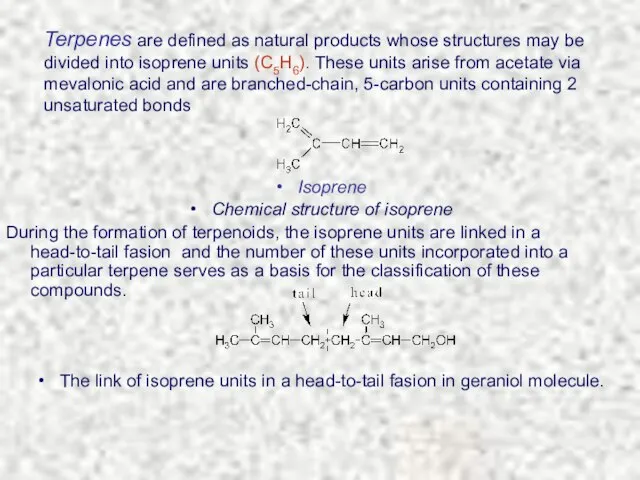
- 4. Terpenes are defined as natural products whose structures may be divided into isoprene units (C5H6). These
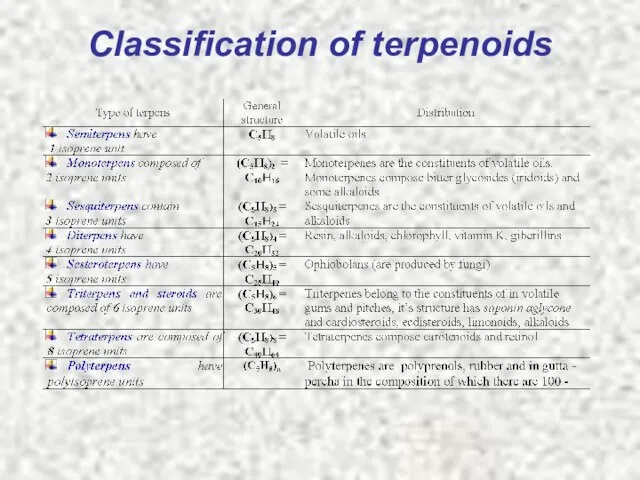
- 5. Classification of terpenoids
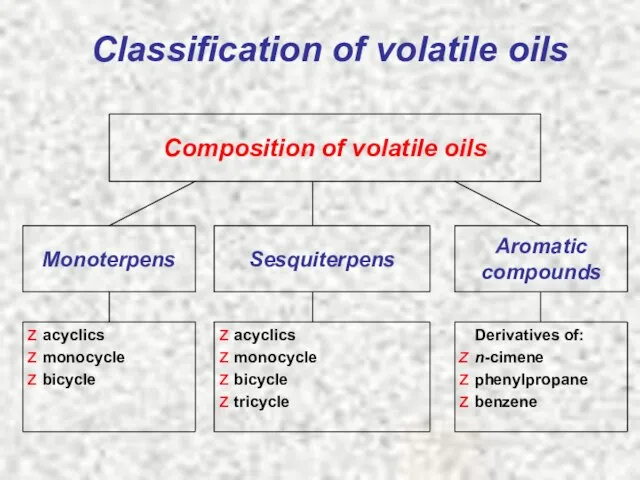
- 6. Classification of volatile oils
- 7. Physical and chemical properties of volatile oils Although volatile oils differ greatly in their chemical constitution,
- 8. Physical and chemical properties of volatile oils As a rule, volatile oils are immiscible with water,
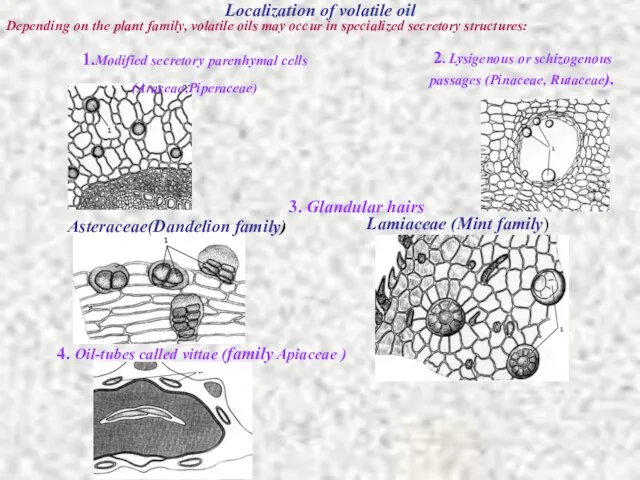
- 9. Localization of volatile oil Depending on the plant family, volatile oils may occur in specialized secretory
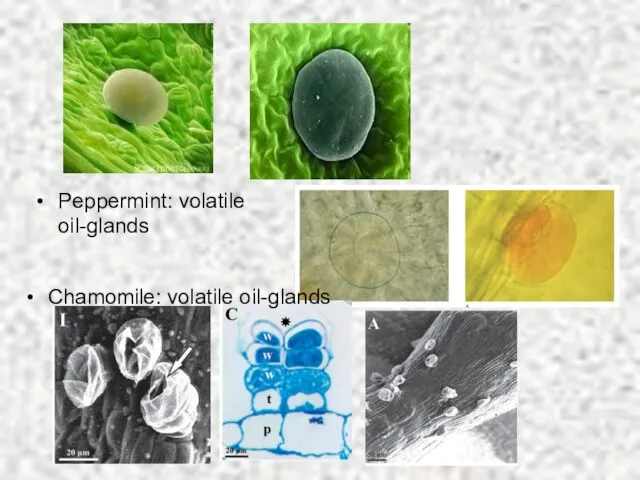
- 10. Peppermint: volatile oil-glands Chamomile: volatile oil-glands
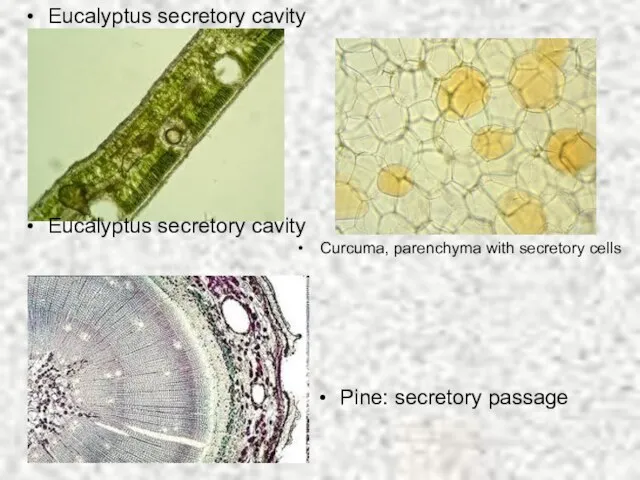
- 11. Eucalyptus secretory cavity Eucalyptus secretory cavity Curcuma, parenchyma with secretory cells Pine: secretory passage
- 12. Methods of obtaining of volatile oils Distillation: Three types of distillation are used by industrial firms:
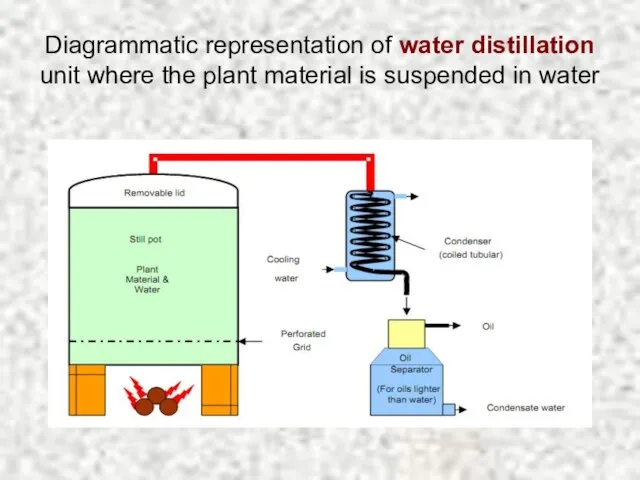
- 13. Diagrammatic representation of water distillation unit where the plant material is suspended in water
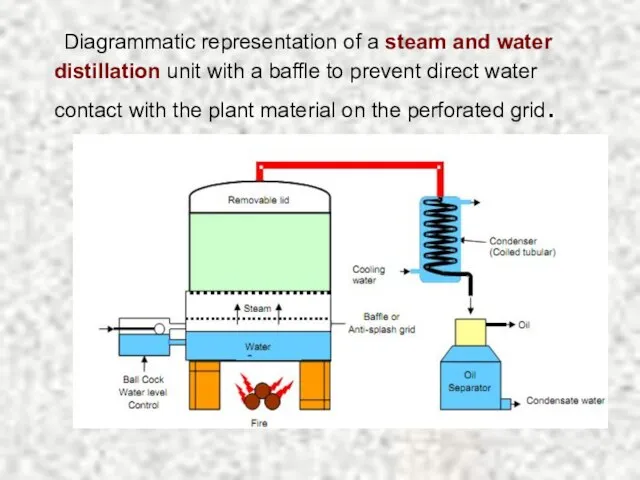
- 14. Diagrammatic representation of a steam and water distillation unit with a baffle to prevent direct water
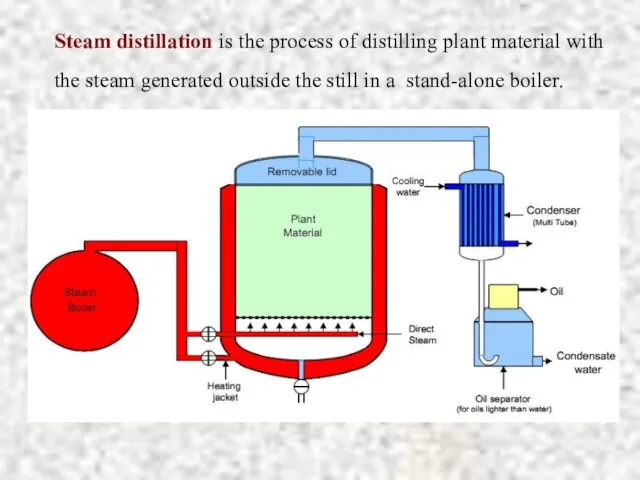
- 15. Steam distillation is the process of distilling plant material with the steam generated outside the still
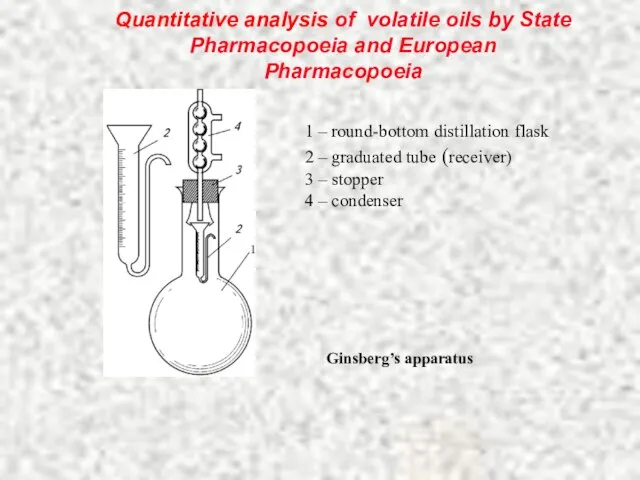
- 16. Quantitative analysis of volatile oils by State Pharmacopoeia and European Pharmacopoeia 1 – round-bottom distillation flask
- 17. Enfleurage 1.Construct enfleurage chassis: wooden frame filled with glass that fit tight together. 2. Solid fat
- 18. Assay of volatile oils (European Pharmacopoeia) European Pharmacopoeia demands to carry out the analysis: Organoleptic analysis:
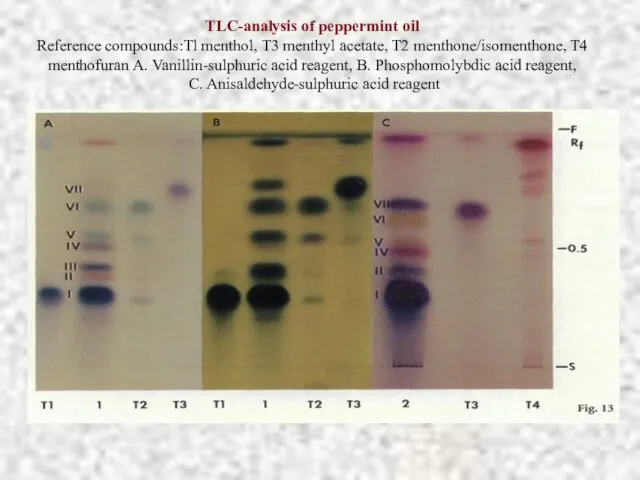
- 19. TLC-analysis of peppermint oil Reference compounds:Tl menthol, T3 menthyl acetate, T2 menthone/isomenthone, T4 menthofuran A. Vanillin-sulphuric
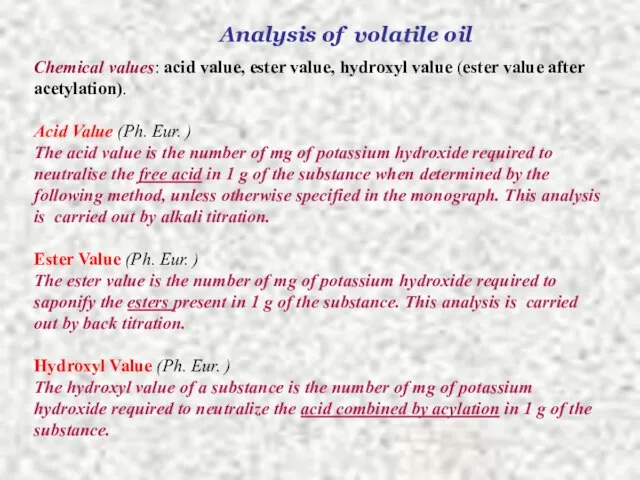
- 20. Analysis of volatile oil Chemical values: acid value, ester value, hydroxyl value (ester value after acetylation).
- 21. bactericidal, anticeptical, fungicidal, antiinflammatory (antiphlogistic), antispasmodic, expectoration, carminative,
- 23. Скачать презентацию







 Презентация на тему Аппликация из ткани
Презентация на тему Аппликация из ткани План этажа
План этажа современные решения для автобизнеса
современные решения для автобизнеса День открытых дверей. Приемная кампания на 2021-2022 учебный год
День открытых дверей. Приемная кампания на 2021-2022 учебный год Размножение и развитие птиц. Годовой жизненный цикл. Сезонные явления
Размножение и развитие птиц. Годовой жизненный цикл. Сезонные явления «Оптимизация межкультурного взаимодействия как стратегическое условие противодействия ксенофобии и этнокультурному экстремизм
«Оптимизация межкультурного взаимодействия как стратегическое условие противодействия ксенофобии и этнокультурному экстремизм Игра – путешествие по сказкам А.С.Пушкина
Игра – путешествие по сказкам А.С.Пушкина Интеграция ДО в единую информационную среду Лицея
Интеграция ДО в единую информационную среду Лицея Вибрационные методы диагностики
Вибрационные методы диагностики Всемирный день моря
Всемирный день моря Атомно-абсорбционный анализ
Атомно-абсорбционный анализ Цель проекта: 1.Показать, что понимание человечеством функциональных связей и взаимосвязей между отдельными качествами жизни(добр
Цель проекта: 1.Показать, что понимание человечеством функциональных связей и взаимосвязей между отдельными качествами жизни(добр Видеопрезентация учителя французского языка Тутаевой Елены Николаевны
Видеопрезентация учителя французского языка Тутаевой Елены Николаевны Презентация на тему Склонение имен существительных
Презентация на тему Склонение имен существительных  Публичный информационно-аналитический доклад о состоянии и результатах деятельности муниципального общеобразовательного уч
Публичный информационно-аналитический доклад о состоянии и результатах деятельности муниципального общеобразовательного уч Портрет в русской живописи начала ХІХ века
Портрет в русской живописи начала ХІХ века Кинотеатр имени А.С. Пушкина
Кинотеатр имени А.С. Пушкина Антимонопольный контроль на рынке автомобильного топлива
Антимонопольный контроль на рынке автомобильного топлива План проведения недели математики
План проведения недели математики Загадки трудных слов
Загадки трудных слов  Святки
Святки MEDICAL SYMBOLS: FACTS, ERRORS AND CONFUSION Moreover
MEDICAL SYMBOLS: FACTS, ERRORS AND CONFUSION Moreover Презентация на тему Мультимедиа технологии
Презентация на тему Мультимедиа технологии  Презентация на тему Презентация к уроку окружающего мира 4 класс
Презентация на тему Презентация к уроку окружающего мира 4 класс  Батырлык дәресе “Кайнар йөрәк”
Батырлык дәресе “Кайнар йөрәк” Корпоративная культура
Корпоративная культура Высшая школа менеджмента Санкт-Петербургский государственный университет Библиотека современной школы бизнеса: формирование к
Высшая школа менеджмента Санкт-Петербургский государственный университет Библиотека современной школы бизнеса: формирование к Программа Непрерывного ОбразованияGRUNDTVIG Многосторонний проект INTEGRA- Объединение Мигрантов - Элементарные Языкоые знания для Ко
Программа Непрерывного ОбразованияGRUNDTVIG Многосторонний проект INTEGRA- Объединение Мигрантов - Элементарные Языкоые знания для Ко