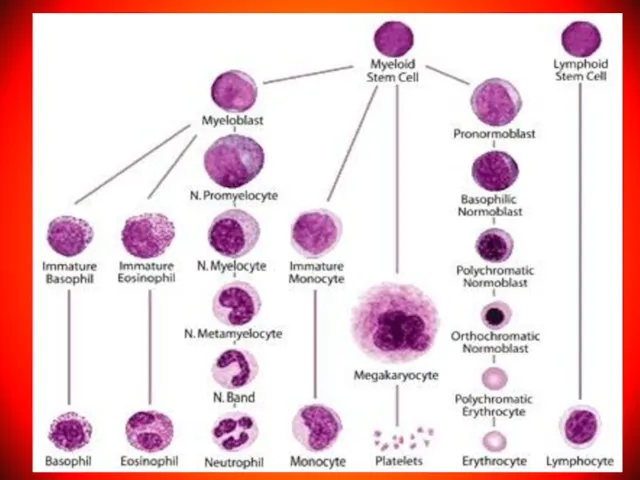
Содержание
- 3. Аnemia – pathologic state, accompanied by decrease in the level of hemoglobin and the quantity of
- 4. Anemia is defined as hematocrit (Hct), hemoglobin (Hb), red blood cells (RBC) concentration > 2 SD
- 5. Anemia is a condition in which a person’s blood has a lower number of red blood
- 6. Normal red blood cells live about 120 days in the bloodstream and then die. Their main
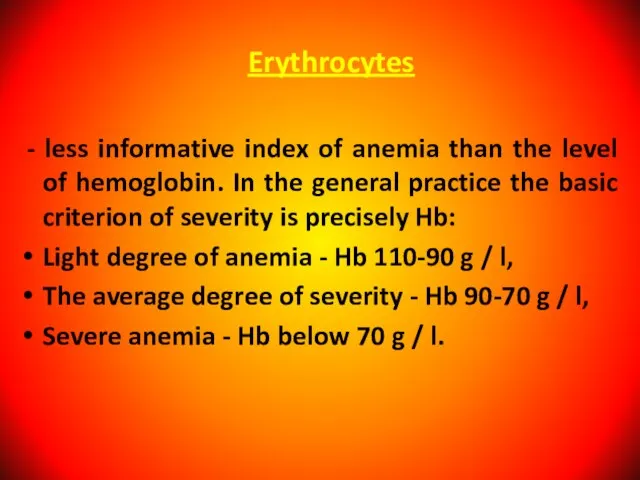
- 7. Erythrocytes - less informative index of anemia than the level of hemoglobin. In the general practice
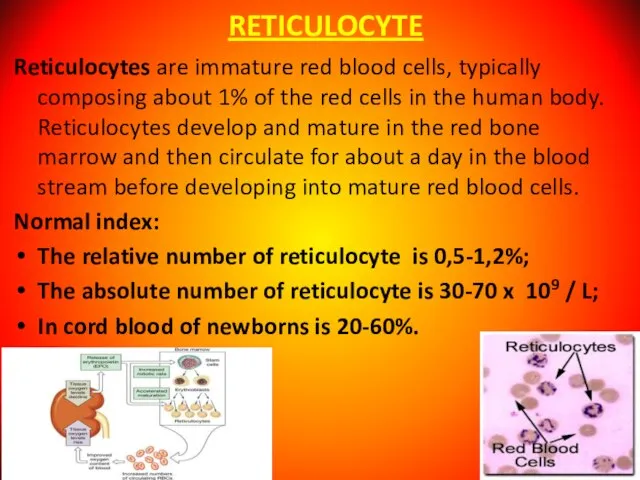
- 8. RETICULOCYTE Reticulocytes are immature red blood cells, typically composing about 1% of the red cells in
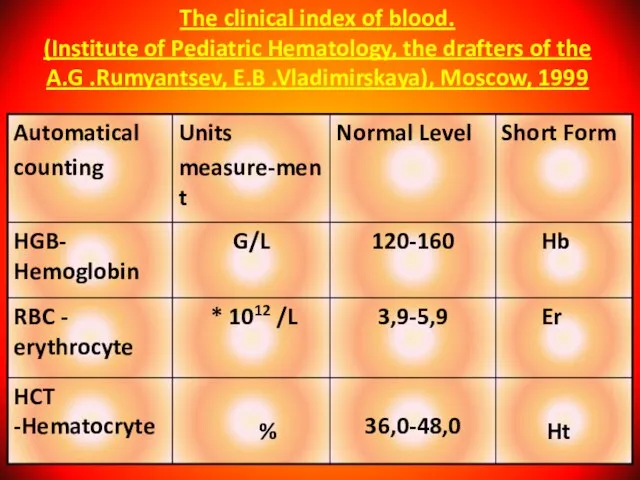
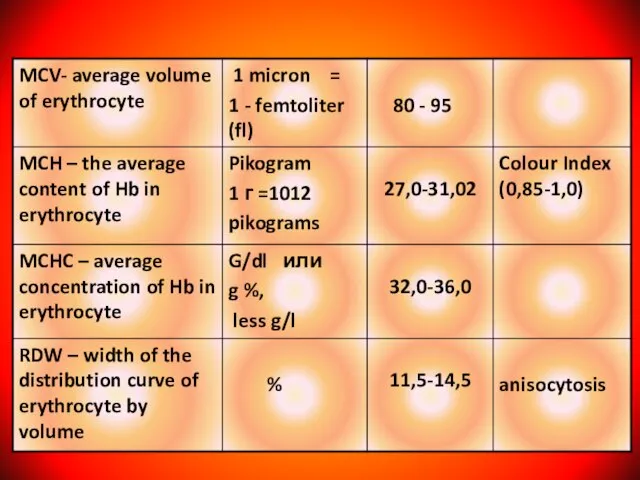
- 9. The clinical index of blood. (Institute of Pediatric Hematology, the drafters of the A.G .Rumyantsev, E.B
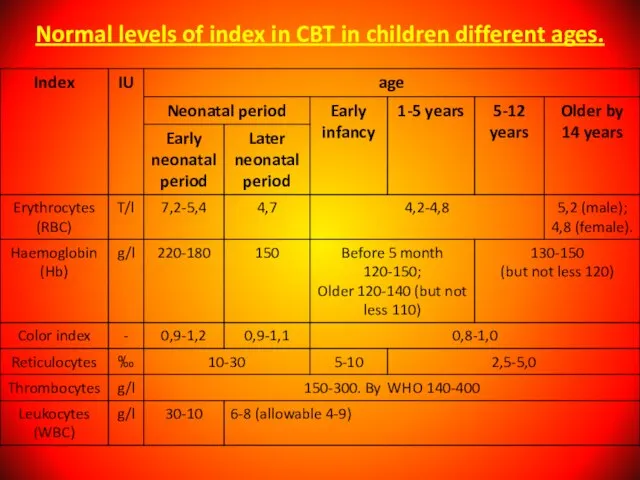
- 11. Normal levels of index in CBT in children different ages.
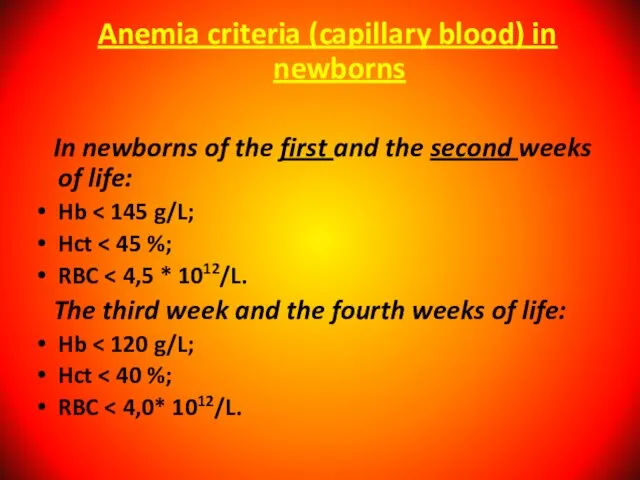
- 13. Anemia criteria (capillary blood) in newborns In newborns of the first and the second weeks of
- 14. Normal index of Hb according WHO criteria (capillary blood) : In infants from 2 to 6
- 15. CAUSES OF NEONATAL ANEMIA: BLOOD LOSS (POSTHEMORRHAGIC) the commonest cause of anemia, including: A. Obstetrical causes:
- 16. C. Feto-placental transfusion Occurs only with monochorionic (i.e., monozygotic) twins and if placental vessels allow shunting
- 17. Blood loss Gastrointestinal: gastro-oesophageal reflux, Meckel’s diverticulum, cow’s milk protein intolerance; Parasites – hookworm; Menstruation in
- 18. 2. INCREASED RBC DESTRUCTION (HAEMOLYTIC): Endogenic causes: Hereditary RBC disorders (rare), including: •RBC Enzyme defects (e.g.,
- 19. B. Exogenic causes: • Immune hemolysis: -Rh incompatibility, - ABO incompitability, - Minor blood group incompatibility
- 20. 3. DECREASED RBC PRODUCTION (HYPOPLASTIC, DEFICIENCY): A. Anemia of prematurity due to transient deficiency of erythropoietin;
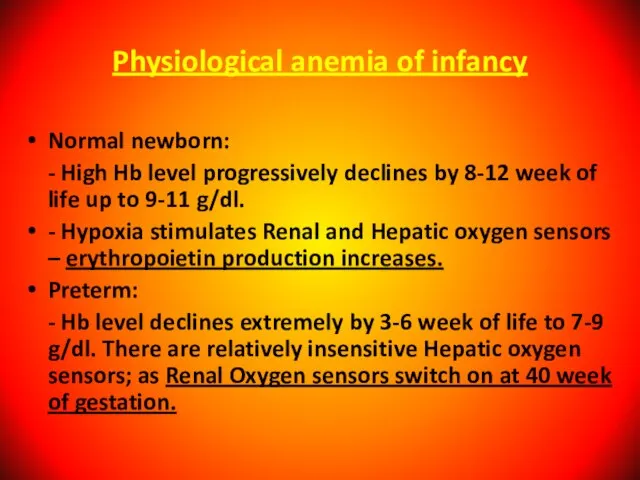
- 21. Physiological anemia of infancy Normal newborn: - High Hb level progressively declines by 8-12 week of
- 22. I. Anemia resulting from blood loss: A) Acute; B) Chronical. II. Anemia resulting from a insufficiency
- 23. B) Acquired aplastic anemia (atrophic anemia, anemia gravis): a) With Pancytopenia (acute, subacute, chronic); b) With
- 24. b) microcytic anemia: - iron deficiency (asiderotic anemia); copper deficiency; Lead poisoning; Thalassaemia. F) physiologic anemia
- 25. A) Aquired: A. Immunopathologic; Б. Infectious; B. Vitamin deficiency; Г. Toxic; Д. Marchiafava-Micheli anemia; Е. Disseminated
- 26. IV. Mixed genesis anemia: A. In acute infections, sepsis. Б. In burns. B. In tumors and
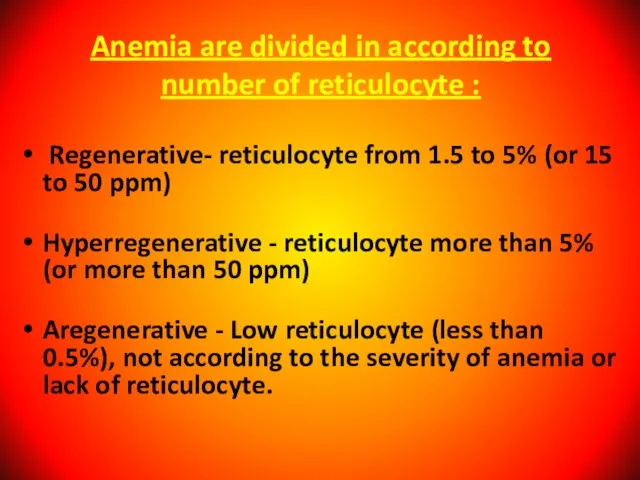
- 27. Anemia are divided in according to number of reticulocyte : Regenerative- reticulocyte from 1.5 to 5%
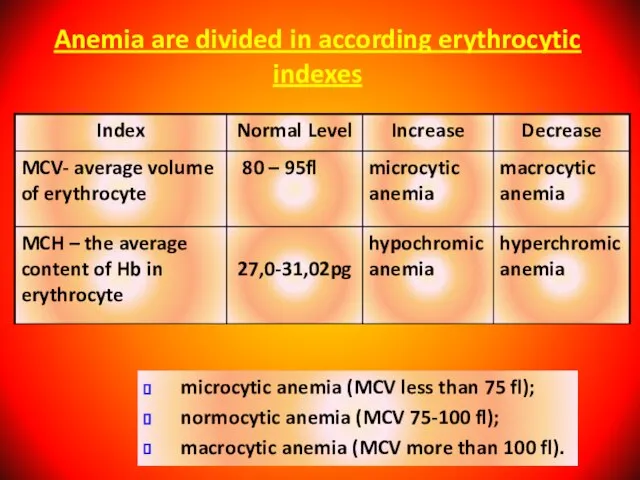
- 28. Anemia are divided in according erythrocytic indexes microcytic anemia (MCV less than 75 fl); normocytic anemia
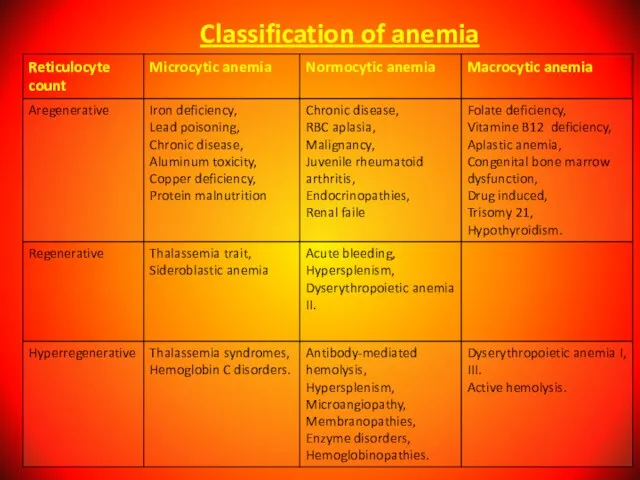
- 29. Classification of anemia
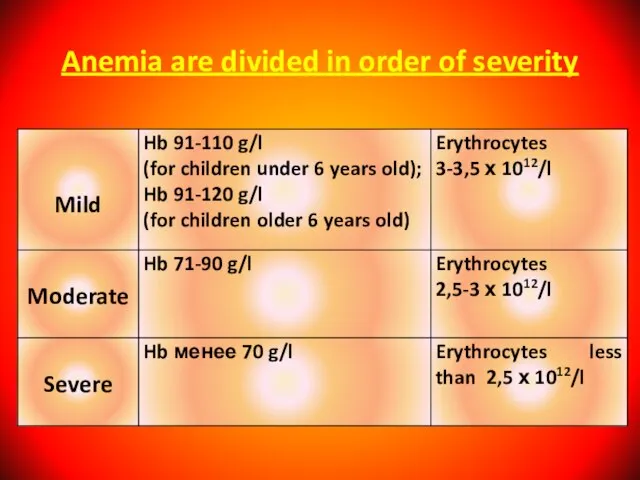
- 30. Anemia are divided in order of severity
- 31. Morphology Nomenclature of Erythrocytes Acanthocyte Dacrocyte Drepanocyte Echinocyte Elliptocyte Megalocyte Schizocyte Spherocyte Stomatocyte Spicule: Liver disease
- 32. Anemia are divided in according Colour Index Normochromal anemia – colour index = 0,85 - 1,05;
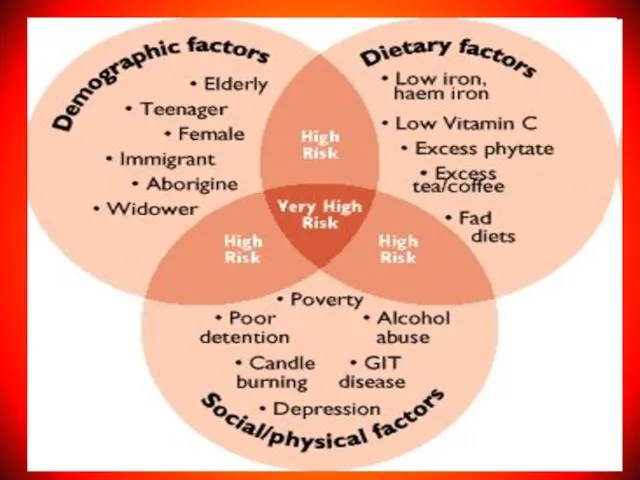
- 33. IRON DEFICIENCY ANEMIA (IDA) IDА is recorded in 20% of the world's population. 83-90% of all
- 34. CAUSES FOR DEVELOPMENT of IDA Alimentary iron deficiency as a consequence of an unbalanced diet; Increasing
- 35. They are: - 75-80% belongs to the hemoglobin; - 20 - 25% reserve; - 5-10% part
- 36. Anemic Syndrome - Decrease amount of Hemoglobin Complaints: General weakness, reduction in the appetite, physical and
- 37. Sideropenic Syndrome (Deficit of Iron) dystrophic changes in the skin and its appendages (shedding of hair,
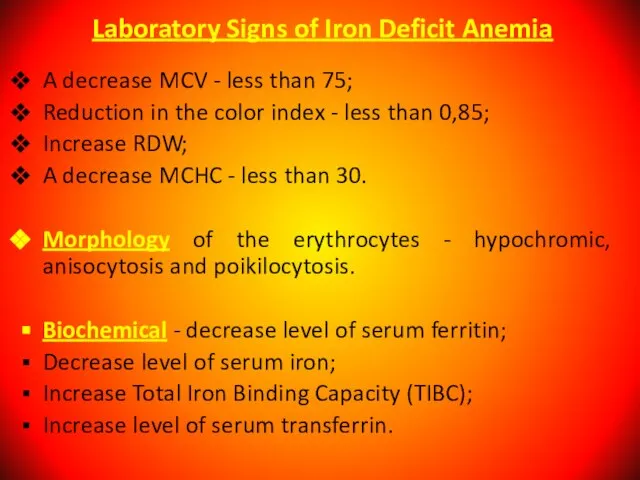
- 38. Laboratory Signs of Iron Deficit Anemia A decrease MCV - less than 75; Reduction in the
- 39. Developmental Stages of Iron Deficiency Anemia (WHO, 1977) Pre-latent (exhaustion of iron reserve in tissues; index
- 40. Differential Diagnosis of Iron Deficiency Anemia it is carried out with other forms of the hypochromic
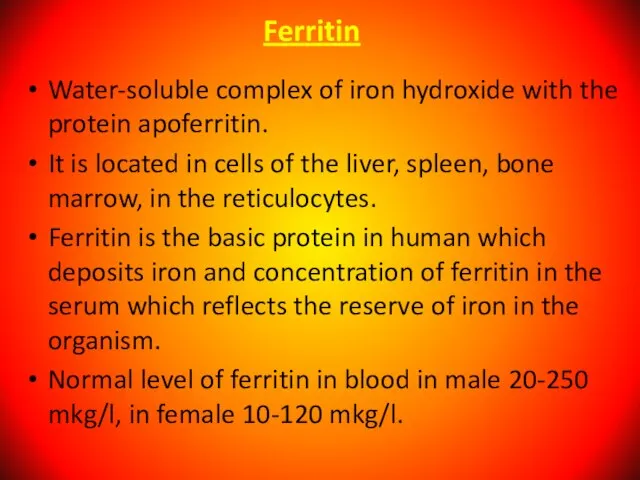
- 41. Ferritin Water-soluble complex of iron hydroxide with the protein apoferritin. It is located in cells of
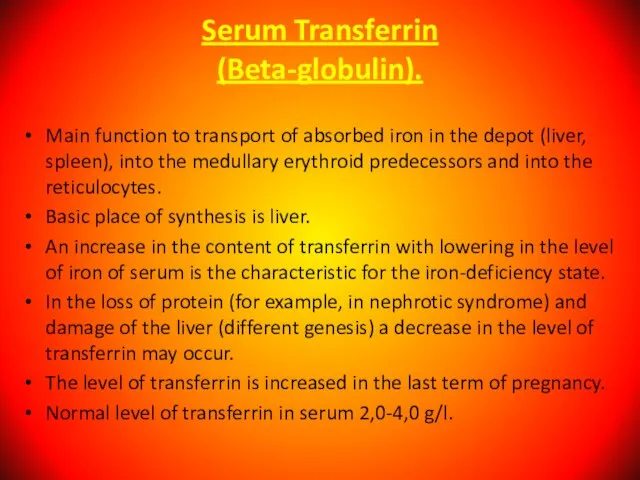
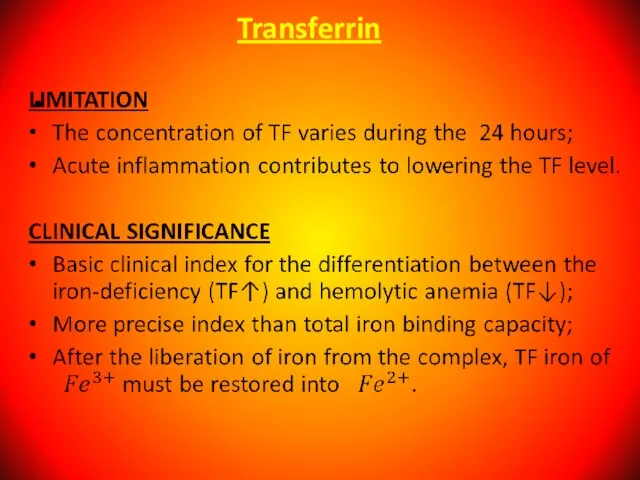
- 42. Serum Transferrin (Beta-globulin). Main function to transport of absorbed iron in the depot (liver, spleen), into
- 43. Transferrin
- 44. – We take in 10-30mg Fe/day; – We absorb 0.5-1.0mg/day (5.5-10%); – Absorption may increase to
- 45. Treatment of Iron Deficiency Anemia Diet: meat, liver, yeast, fish. Oral preparations: the rate of absorbtion
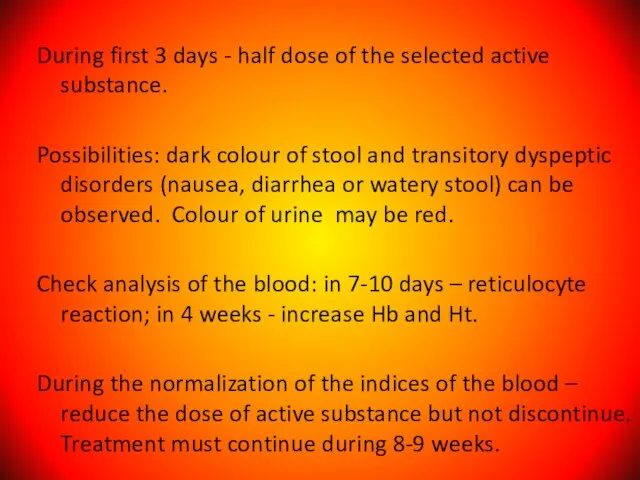
- 46. During first 3 days - half dose of the selected active substance. Possibilities: dark colour of
- 47. Therapeutic dose of Iron drug 0 – 3 years – 5 – 8 mg/kg/day; 3 –
- 48. Indication for Parenteral Introduction of Iron in exceptional cases; in severe iron deficiency anemia; intolerance of
- 49. Complications of Parenteral Introduction Local reactions (pains, phlebitis); General reactions (anaphylaxis, fever, head and articulate pains,
- 50. Signs of overdose of Iron In the first 6-8 hours - epigastral pains, nausea, vomiting (including
- 51. Iron Overload Syndrome ! Human does not have special mechanism of the excretion of iron! Its
- 52. Megaloblastic anemia it is inheritable and acquired anemia when in bone marrow the megaloblasts are present.
- 53. Causes of B12 Deficiency Anemia Decreased ingestion (e.g., poor dietary intake); Atrophy of the mucous membrane
- 54. Causes of Folic Acid Deficiency Alimentary; Increased need (prematurity birth, rapid growth rate, pregnancy); Feeding by
- 55. Clinical Anemic syndrome; Skin is pale with the lemon shade; Slight jaundice of the scleras; Glossitis,
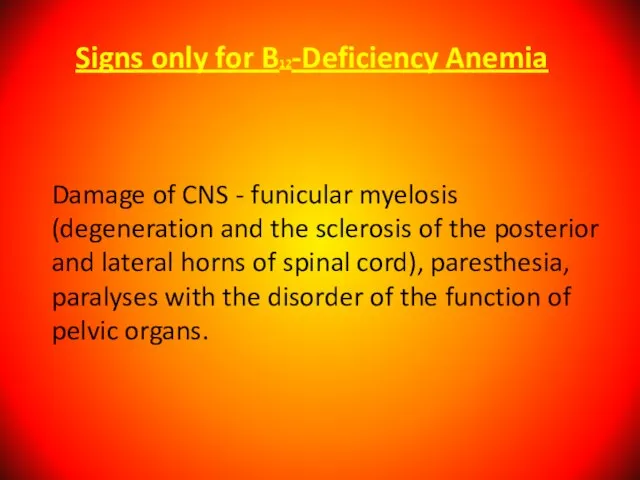
- 56. Signs only for B12-Deficiency Anemia Damage of CNS - funicular myelosis (degeneration and the sclerosis of
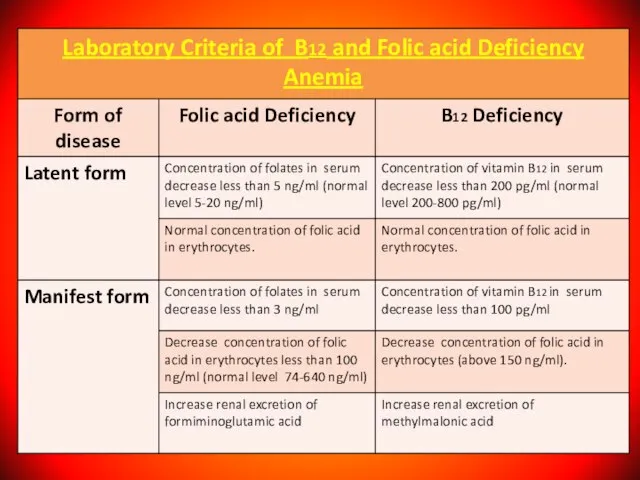
- 57. Diagnosis of B12 and Folic acid Deficiency Anemia
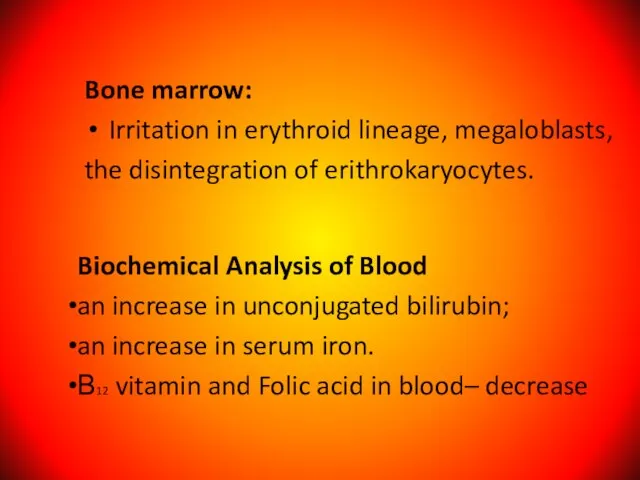
- 58. Bone marrow: Irritation in erythroid lineage, megaloblasts, the disintegration of erithrokaryocytes. Biochemical Analysis of Blood an
- 60. Treatment of megaloblastic anemia Treatment of megaloblastic anemia depends on the underlying cause. Folate deficiency due
- 61. Oral B-12 supplementation is as effective as parenteral supplementation in patients with nutritional deficiency. Even in
- 62. Criteria of Effective Treatment Subjective improvement of patient condition during the first days of treatment; Reticulocytosis,
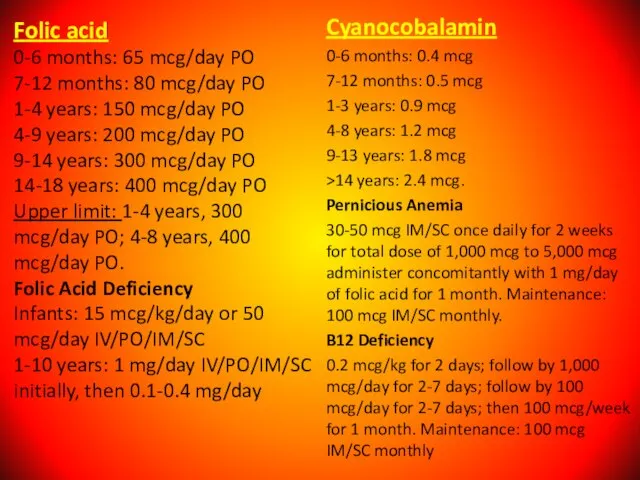
- 63. Folic acid 0-6 months: 65 mcg/day PO 7-12 months: 80 mcg/day PO 1-4 years: 150 mcg/day
- 64. Hemolytic anemia = reduced red-cell life span. Hemolysis is the premature destruction of erythrocytes. A hemolytic
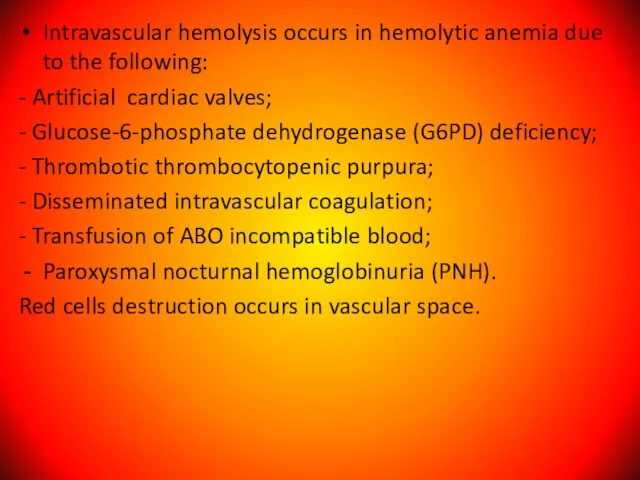
- 65. Intravascular hemolysis occurs in hemolytic anemia due to the following: - Artificial cardiac valves; - Glucose-6-phosphate
- 66. Laboratory sings of Intravascular hemolysis: Indirect hyperbilirubinemia; Erythroid hyperplasia; Hemoglobinemia; Reticulocytosis; Hemoglobinuria; Absence or reduced of
- 67. Extravascular hemolysis: Red cells destruction occurs in reticuloendothelial system; Clinical states associated with extravascular hemolysis: autoimmune
- 68. Laboratory sings of Extravascular hemolysis: Indirect hyperbilirubinemia; Erythroid hyperplasia; Inareased excretion of bilirubin by bile; Hemosiderosis.
- 69. Etiology Hereditary disorders may cause hemolysis as a result of erythrocyte membrane abnormalities, enzymatic defects, and
- 70. Clinical features of hemolytic anemia: Patients with minimal or long-standing hemolytic anemia may be asymptomatic, and
- 71. Bronze skin color and diabetes occur in hematosiderosis; iron overload may occur in patients who have
- 72. Laboratory sings of hemolytic anemia: normocytic/macrocytic, hyperchromatic anemia; reticulocytosis; Increased serum iron; Antiglobin Coomb’ test is
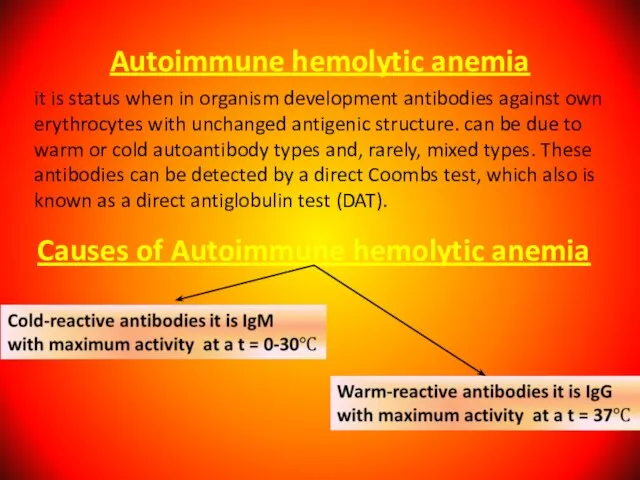
- 73. Autoimmune hemolytic anemia it is status when in organism development antibodies against own erythrocytes with unchanged
- 74. Autoimmune hemolytic anemia caused by warm-reactive antibodies: Primary. Secondary: 1. acute - viral infections; - drugs
- 75. Autoimmune hemolytic anemia caused by cold-reactive antibodies: Primary. Secondary: - mycoplasma infections; - viral infections; -
- 76. Autoimmune hemolytic anemia - diagnosis - Possitive direct Coombs’ test. Treatment: Steroids; Splenectomy; Immunosupressive agents; Transfusion.
- 77. Hereditary microspherocytosis 1. Pathophysiology: red cell membrane protein defects (spectrin deficiency) resulting cytoskeleton instability. 2. Family
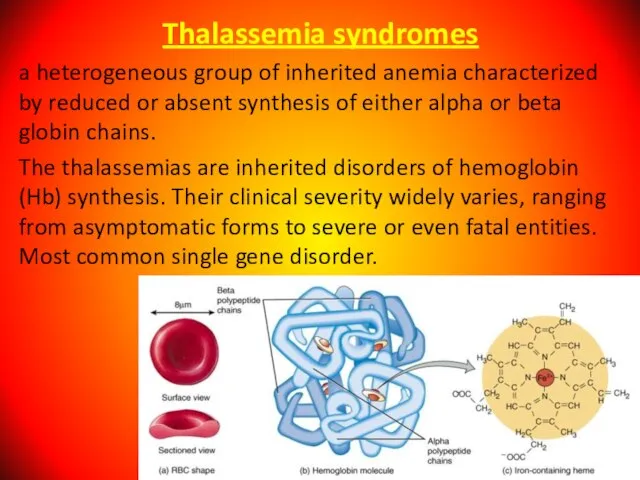
- 78. Thalassemia syndromes a heterogeneous group of inherited anemia characterized by reduced or absent synthesis of either
- 79. Alpha Thalassemia The alpha thalassemia patient's hemoglobin does not produce enough alpha protein. This type is
- 80. One faulty (mutated) gene - there are either no symptoms at all, or they are very
- 81. Three mutated genes - the patient will have hemoglobin H disease, i.e. chronic anemia. A person
- 82. Mortality/Morbidity α thalassemia major is a mortal disease, and virtually all affected fetuses are born with
- 83. Beta Thalassemia We need two globin genes to make beta globin chains. We get one from
- 84. Severity of beta thalassemia also depends on how many genes are mutated: If one globin gene
- 85. Signs And Symptoms Of Beta Thalassemia The majority of infants with beta thalassemia will not have
- 86. In patients with various types of β thalassemia, mortality and morbidity vary according to the severity
- 87. Age Despite thalassemia's inherited nature, age at onset of symptoms varies significantly. In α thalassemia, clinical
- 88. People with thalassemia mainly have anemia-like symptoms. Jaundice; Fatigue; Pale skin; Cold hands and feet; Dyspnea;
- 89. Signs And Symptoms Of Alpha Thalassemia The majority of children with hemoglobin H are generally healthy.
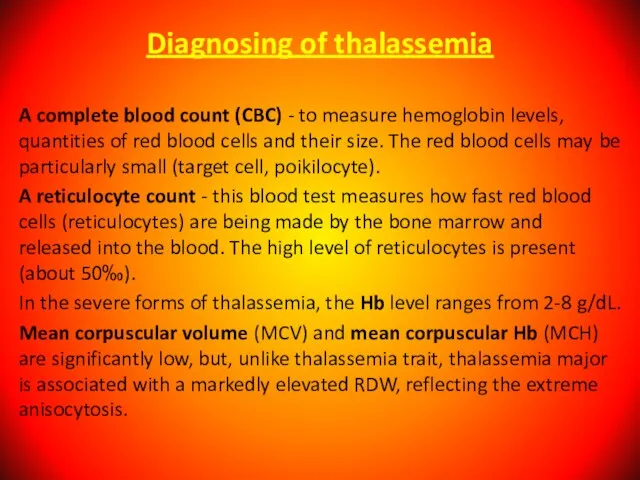
- 90. Diagnosing of thalassemia A complete blood count (CBC) - to measure hemoglobin levels, quantities of red
- 91. The WBC count is usually elevated in β thalassemia major; this is due, in part, to
- 92. Treatment of thalassemia Blood transfusions - this is done to replenish hemoglobin and red blood cell
- 93. Complications of thalassemia Iron overload; Enlarged spleen (splenomegaly); Infection; Bone deformities.
- 94. Congenital sideroblastic anemias Congenital sideroblastic anemias generally present with lower hemoglobin and more microcytosis than myelodysplastic
- 95. Etiology Congenital causes of sideroblastic anemia include the following: δ-ALAS mutation; ABC7 mutation; PSU1 mutation; Pearson
- 96. Major causes of death in cases of sideroblastic anemia are secondary hemochromatosis from transfusions and leukemia.
- 97. Clinical Presentation Incoordination (cerebellar symptoms); Failure of growth; Diarrhea (malabsorption); Polyuria, blindness, deafness (associated with DIDMOAD
- 98. Medication history of antibiotics, antituberculous agents, chelators, or chemotherapy; Ingestion of supplements, especially zinc; Prolonged dependence
- 99. Physical Examination General - Growth retardation in children; Vital signs – Hypothermia; Oral - Lead line
- 100. Complete Blood Cell Count and Peripheral Smear CBC count usually reveals anemia. The mean corpuscular volume
- 101. Sideroblastic anemia also seen in combined vitamin B-12 deficiency with iron deficiency. But iron studies may
- 102. Treatment Treatment of sideroblastic anemia may include removal of toxic agents; administration of pyridoxine, thiamine, or
- 103. SICKLE CELL ANEMIA A serious condition in which red blood cells can become sickle-shaped. In sickle
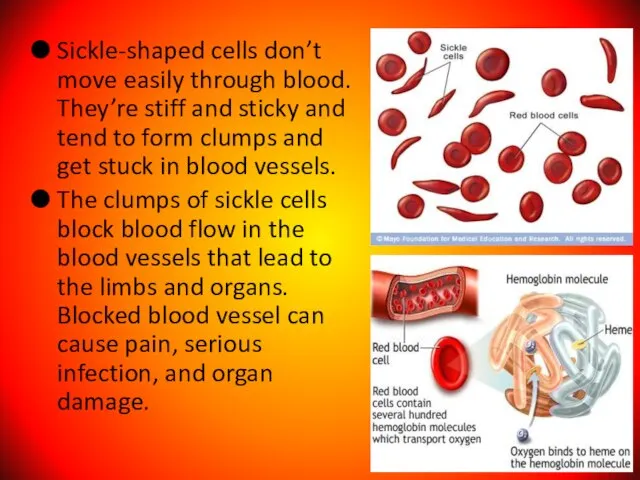
- 104. Sickle-shaped cells don’t move easily through blood. They’re stiff and sticky and tend to form clumps
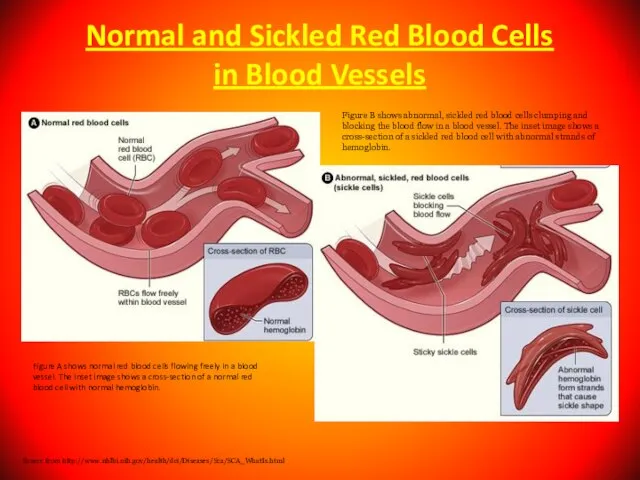
- 105. Normal and Sickled Red Blood Cells in Blood Vessels Figure A shows normal red blood cells
- 106. People who have sickle cell anemia are born with it; means inherited, lifelong condition. They inherit
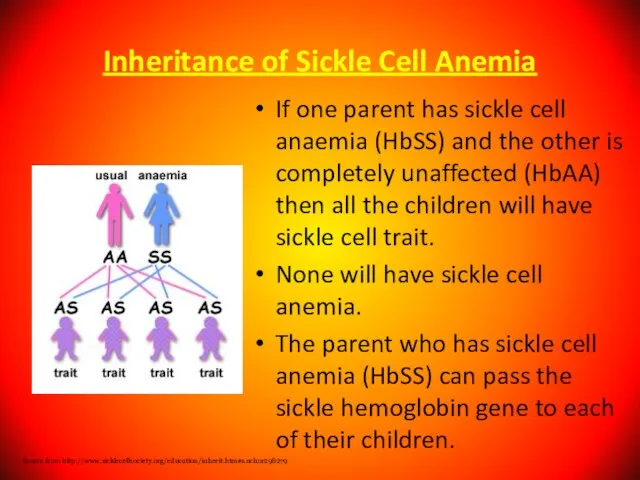
- 107. Inheritance of Sickle Cell Anemia If one parent has sickle cell anaemia (HbSS) and the other
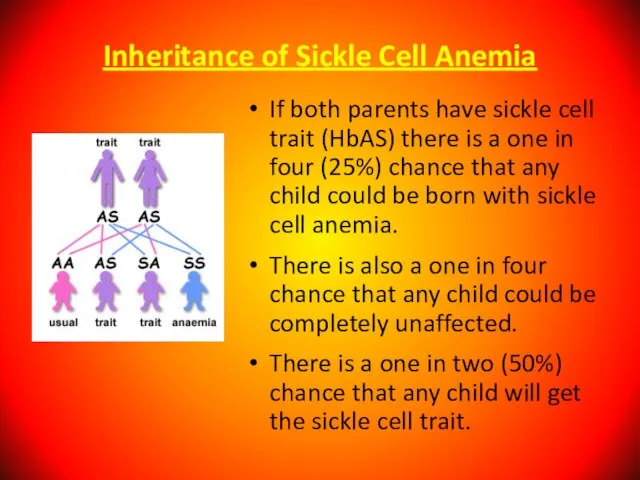
- 108. Inheritance of Sickle Cell Anemia If both parents have sickle cell trait (HbAS) there is a
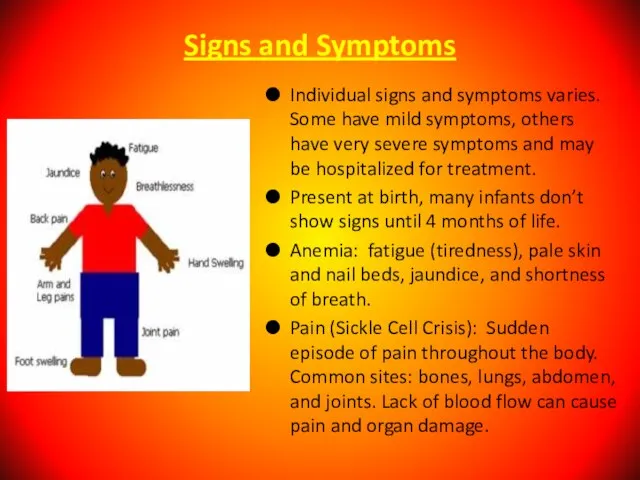
- 110. Signs and Symptoms Individual signs and symptoms varies. Some have mild symptoms, others have very severe
- 111. Differential Diagnoses Acute Anemia; Carotid Cavernous Fistula; Hands Rheumatoid Arthritis; Hemoglobin C Disease; Hemolytic Anemia; Legg-Calve-Perthes
- 112. Complication of Sickle Cell Anemia Splenic Crisis Infections Acute Chest Syndrome Delayed growth and puberty in
- 113. Treatment Effective treatment is available to help relieve the symptoms and complications of sickle cell anemia.no
- 114. Prevention Identify what can trigger the “Crisis” such as stress, avoid extremes of heat and cold
- 115. Aplastic Anemia is a syndrome of bone marrow failure characterized by peripheral pancytopenia and marrow hypoplasia.
- 116. Fanconi Anemia Fanconi anemia is the most frequently reported of the rare inherited bone marrow failure
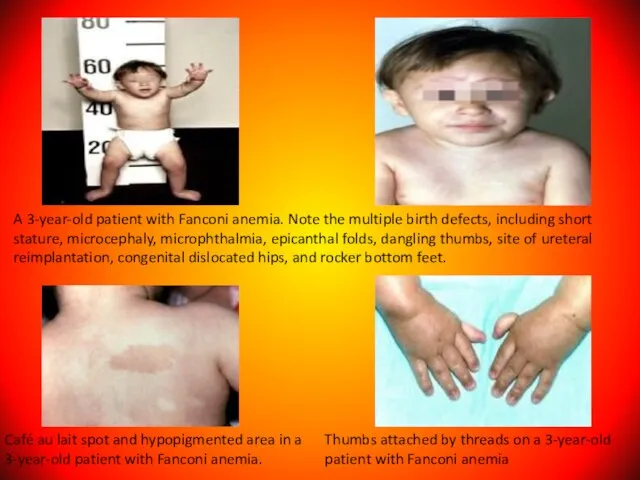
- 117. Multiple congenital anomalies (60-75%): Short stature, petechiae and bruises, abnormal skin pigmentation, café au lait spots,
- 118. A 3-year-old patient with Fanconi anemia. Note the multiple birth defects, including short stature, microcephaly, microphthalmia,
- 119. Bone marrow failure: Thrombocytopenia, leukopenia, or aplastic anemia; most patients with Fanconi anemia have bone marrow
- 120. Gonads may display the following abnormalities: Males - Hypogenitalia, undescended testes, hypospadias, abnormal or absent testis,
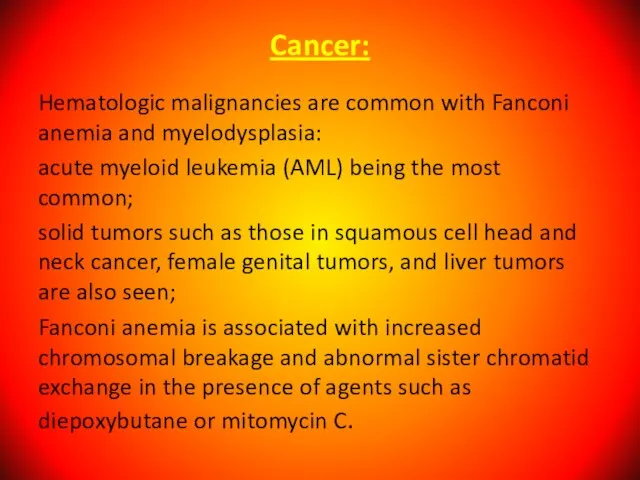
- 121. Cancer: Hematologic malignancies are common with Fanconi anemia and myelodysplasia: acute myeloid leukemia (AML) being the
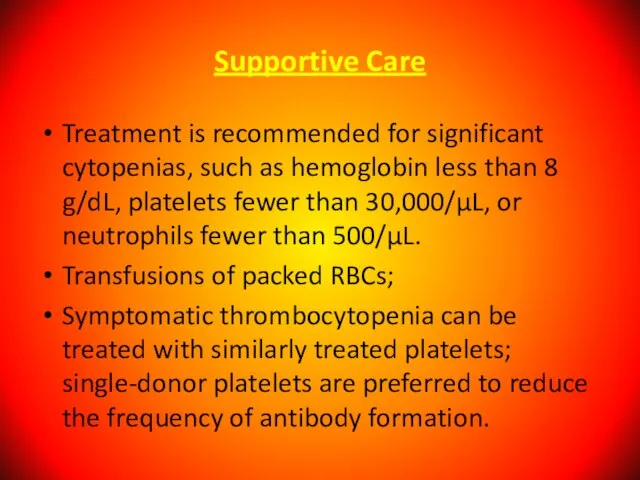
- 122. Supportive Care Treatment is recommended for significant cytopenias, such as hemoglobin less than 8 g/dL, platelets
- 124. Скачать презентацию















































































 Лабораторна оцінка характеристик біологічно активних препаратів з протизапальними властивостями на прикладі бетаїну
Лабораторна оцінка характеристик біологічно активних препаратів з протизапальними властивостями на прикладі бетаїну раздел совместного доклада М.Носковой и Т.Минаевой
раздел совместного доклада М.Носковой и Т.Минаевой Единицы силы
Единицы силы Окружающий мир (1-4)Система Д.Б.Эльконина-В.В.Давыдова
Окружающий мир (1-4)Система Д.Б.Эльконина-В.В.Давыдова Особенности делового общения с собеседниками различных психотипов
Особенности делового общения с собеседниками различных психотипов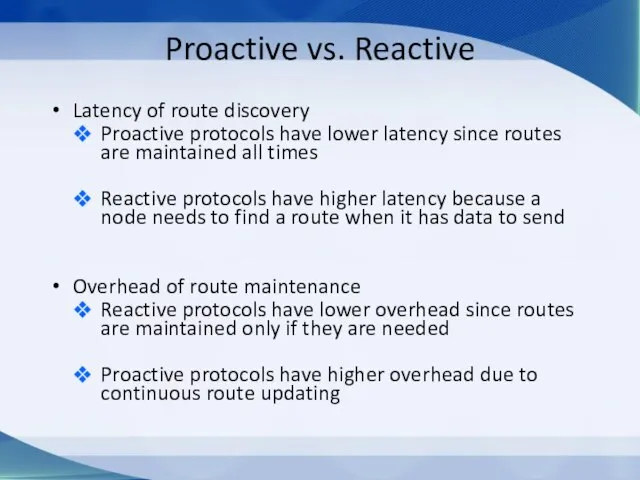 Proactive vs. Reactive
Proactive vs. Reactive  Оценка рекреационного воздействия на природу Троицких Альп
Оценка рекреационного воздействия на природу Троицких Альп Некоторые направления деятельности АПКИТ по поддержке ИТ-разработки в России, стимулирования информатизации и развития инфрастр
Некоторые направления деятельности АПКИТ по поддержке ИТ-разработки в России, стимулирования информатизации и развития инфрастр Кафедра культурно-дозвіллєвої діяльності Інституту культури ім. О. Корнійчука
Кафедра культурно-дозвіллєвої діяльності Інституту культури ім. О. Корнійчука Деяния Святых Апостолов Автор: Лука Аудитория: Феофил Дата: ранние 60-ые года I века Тема: Церковь Структура Деяний:Петр (1-12) Павел (13-28
Деяния Святых Апостолов Автор: Лука Аудитория: Феофил Дата: ранние 60-ые года I века Тема: Церковь Структура Деяний:Петр (1-12) Павел (13-28 Нестеров М. В. Труды Сергия Радонежского
Нестеров М. В. Труды Сергия Радонежского Двери в г.Самара и Самарская область RoomDoors
Двери в г.Самара и Самарская область RoomDoors Управляющий Совет
Управляющий Совет Моделирование в экологическом образовании детей дошкольного возраста
Моделирование в экологическом образовании детей дошкольного возраста Местное самоуправление
Местное самоуправление Подготовка участников школьного и муниципального этапов Всероссийской олимпиады школьников по технологии
Подготовка участников школьного и муниципального этапов Всероссийской олимпиады школьников по технологии Презентация на тему Человек и природа. Влияние человека на окружающую среду
Презентация на тему Человек и природа. Влияние человека на окружающую среду Русский пейзаж в поэзии и живописи
Русский пейзаж в поэзии и живописи Презентация на тему Групповые приёмы работы в начальной школе
Презентация на тему Групповые приёмы работы в начальной школе ГИА 9
ГИА 9 Стажировка учащихся Академии акварели и изящных искусств
Стажировка учащихся Академии акварели и изящных искусств Ремонт в квартире
Ремонт в квартире Плавление и отвердевание кристаллических тел в условиях абсолютного вакуума
Плавление и отвердевание кристаллических тел в условиях абсолютного вакуума 1954Открытие школыПервый выпуск1959Е.В. БедняковаБ.П. Жижиков. - презентация
1954Открытие школыПервый выпуск1959Е.В. БедняковаБ.П. Жижиков. - презентация Арифметические основы организации компьютера
Арифметические основы организации компьютера  Биография Льва Николаевича Толстого
Биография Льва Николаевича Толстого Microsoft Office Business Scorecards Manager 2005 и SQL Server 2005 Business Intelligence – платформа для управления корпоративной эффективностью
Microsoft Office Business Scorecards Manager 2005 и SQL Server 2005 Business Intelligence – платформа для управления корпоративной эффективностью Е-здравоохранение Республики Казахстан
Е-здравоохранение Республики Казахстан