Содержание
- 2. Course Topics Topic 1. Cycloalkanes Topic 2. Aromatic Hydrocarbons (Arenes) Topic 3. Halogenated Aromatic Hydrocarbons Topic
- 3. Cycloalkanes Topic 1
- 4. Outline of the lecture Cycloalkanes Naming Cycloalkanes Conformations of Cycloalkanes Chemical Properties of Cycloalkanes
- 5. Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May 2012]. Available from World
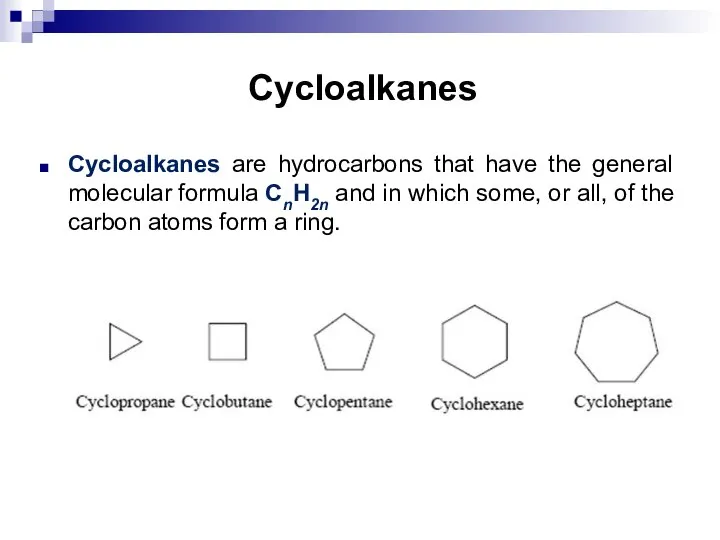
- 6. Cycloalkanes Cycloalkanes are hydrocarbons that have the general molecular formula CnH2n and in which some, or
- 7. Cycloalkanes Cycloalkanes are classified according to the cycle size, the number of cycles, and the way
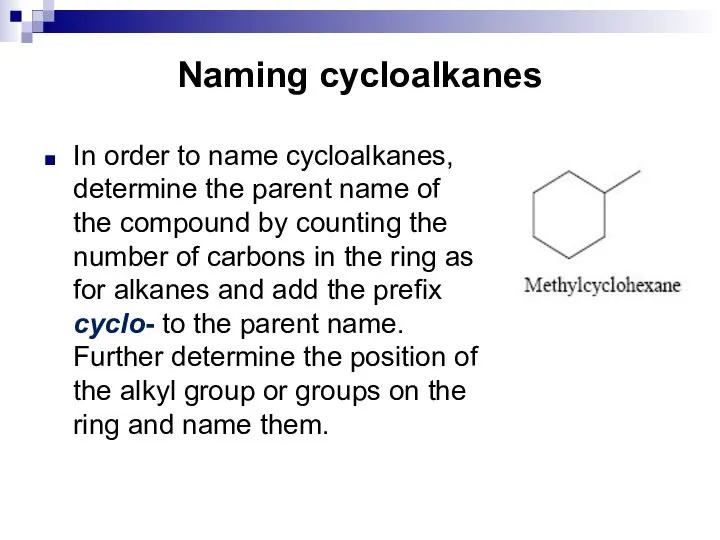
- 8. Naming cycloalkanes In order to name cycloalkanes, determine the parent name of the compound by counting
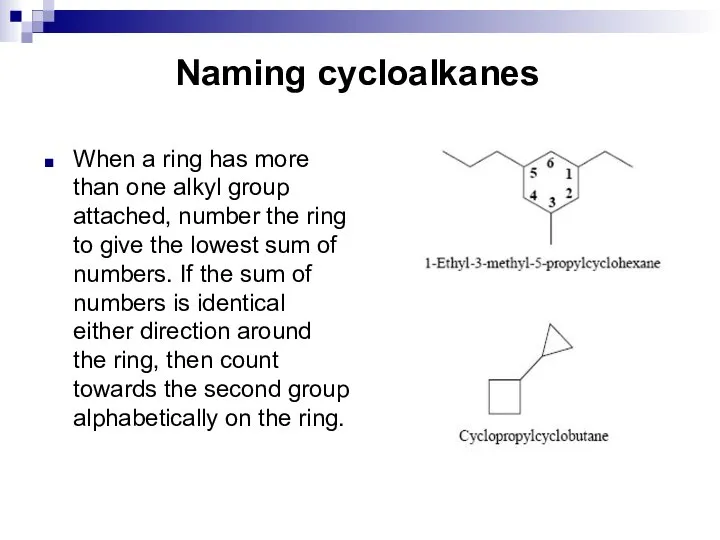
- 9. Naming cycloalkanes When a ring has more than one alkyl group attached, number the ring to
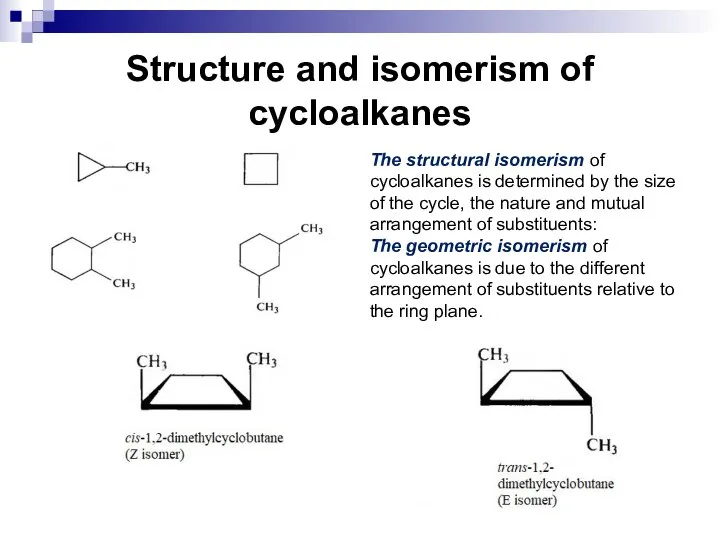
- 10. Structure and isomerism of cycloalkanes The structural isomerism of cycloalkanes is determined by the size of
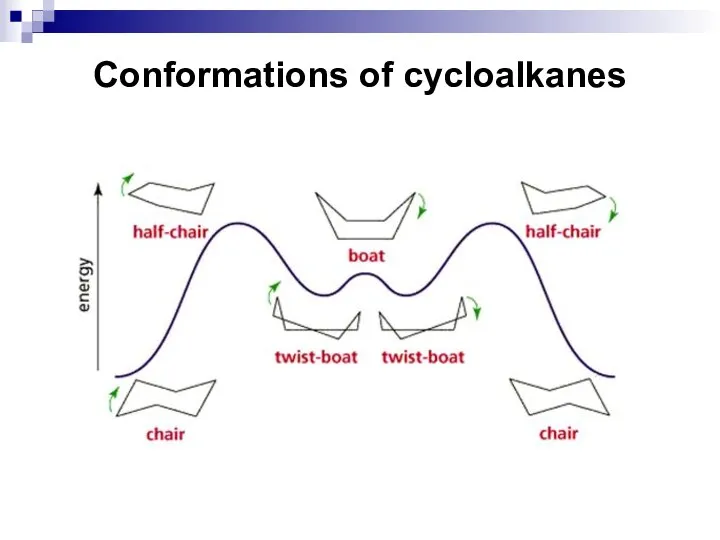
- 11. Conformations of cycloalkanes The molecule of any cycloalkane tends to occupy in space such a form
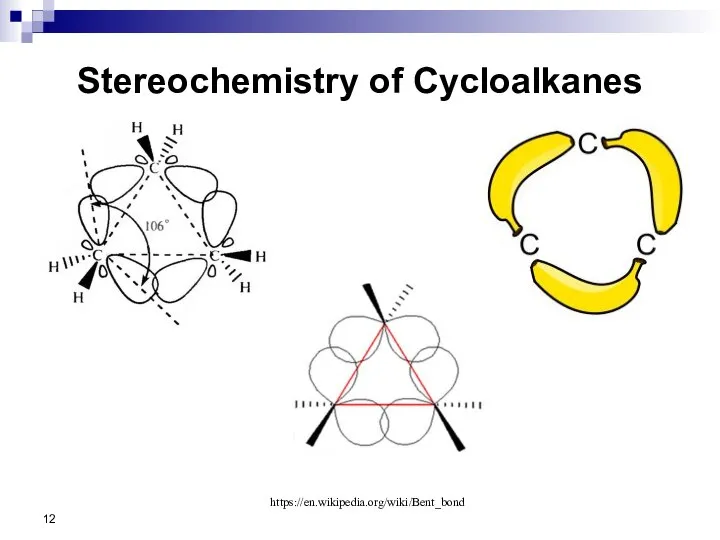
- 12. Stereochemistry of Cycloalkanes https://en.wikipedia.org/wiki/Bent_bond
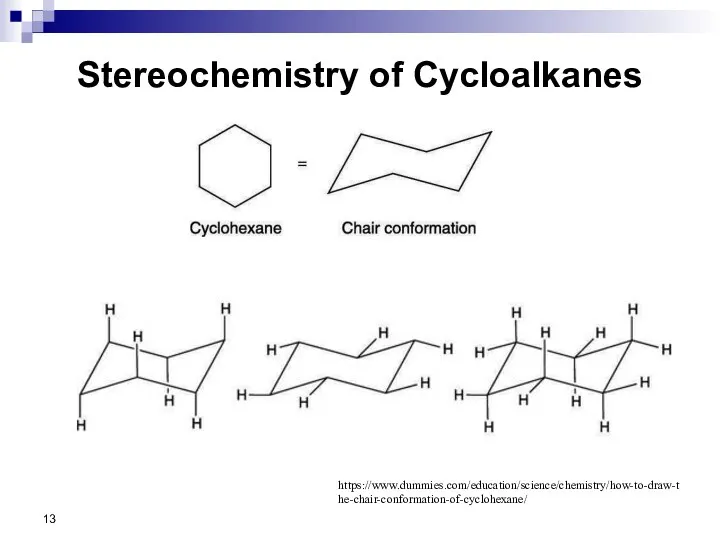
- 13. Stereochemistry of Cycloalkanes https://www.dummies.com/education/science/chemistry/how-to-draw-the-chair-conformation-of-cyclohexane/
- 14. Stereochemistry of Cycloalkanes
- 15. Conformations of cycloalkanes
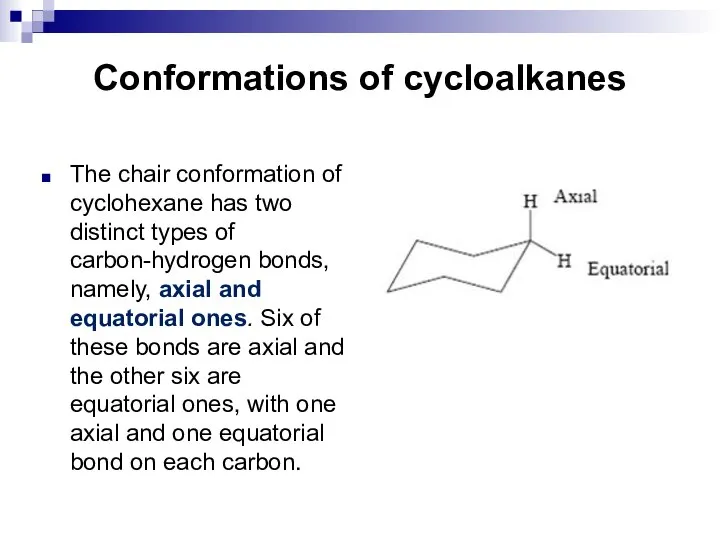
- 16. Conformations of cycloalkanes The chair conformation of cyclohexane has two distinct types of carbon-hydrogen bonds, namely,
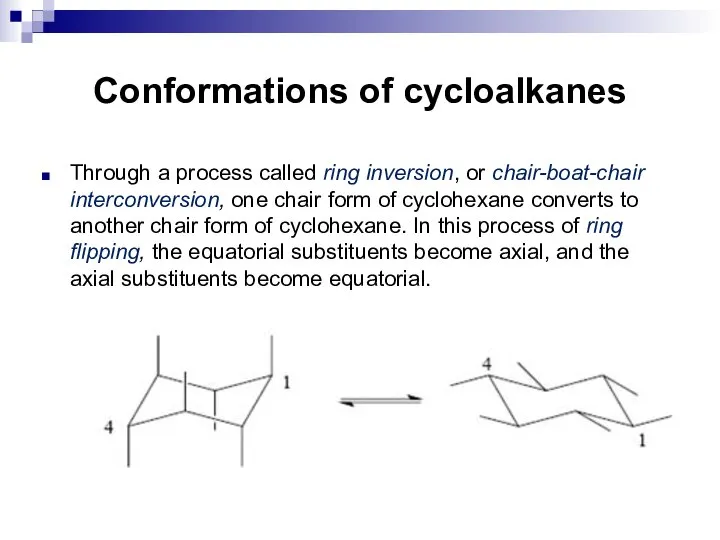
- 17. Conformations of cycloalkanes Through a process called ring inversion, or chair-boat-chair interconversion, one chair form of
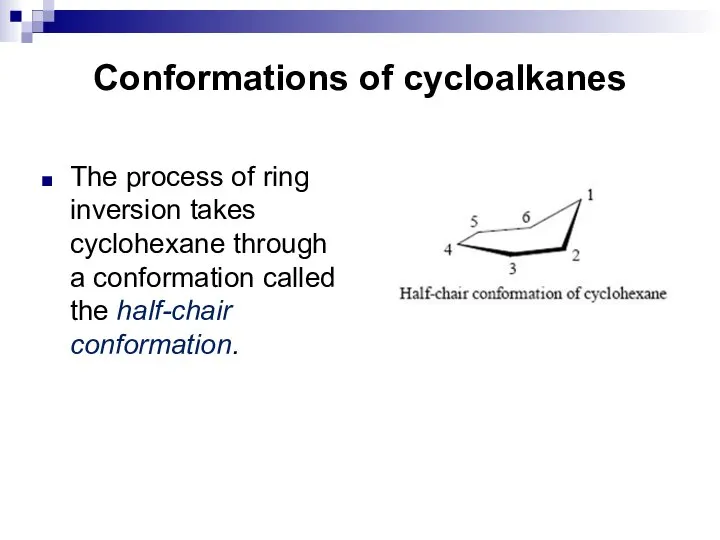
- 18. Conformations of cycloalkanes The process of ring inversion takes cyclohexane through a conformation called the half-chair
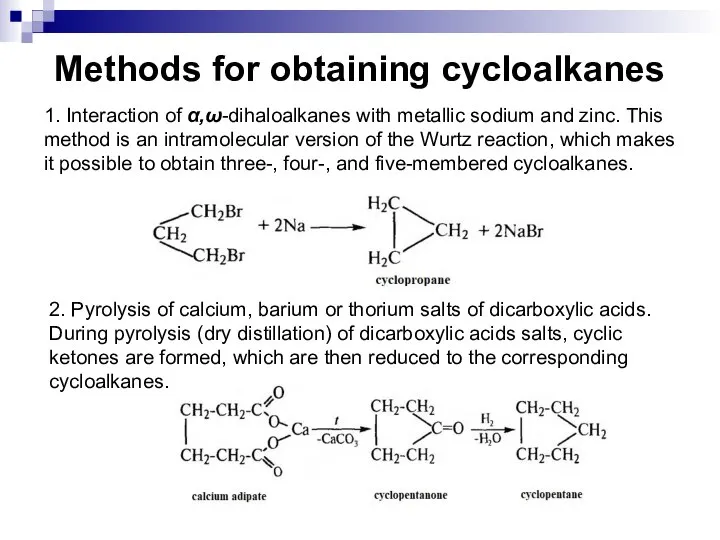
- 19. Methods for obtaining cycloalkanes 1. Interaction of α,ω-dihaloalkanes with metallic sodium and zinc. This method is
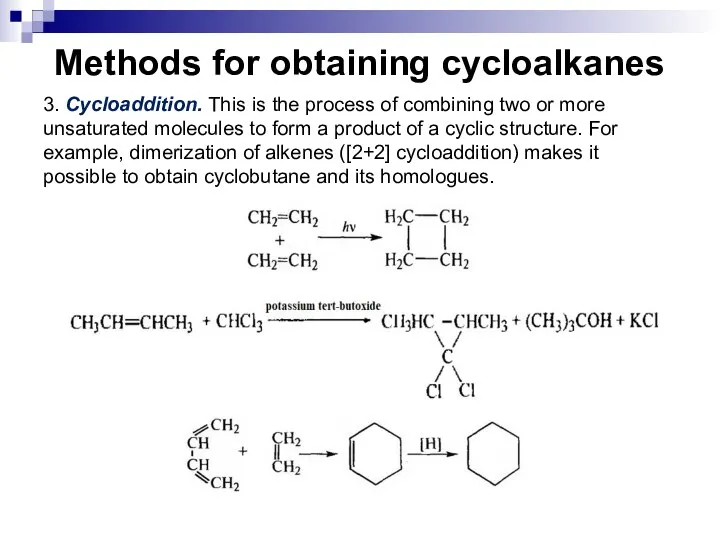
- 20. Methods for obtaining cycloalkanes 3. Cycloaddition. This is the process of combining two or more unsaturated
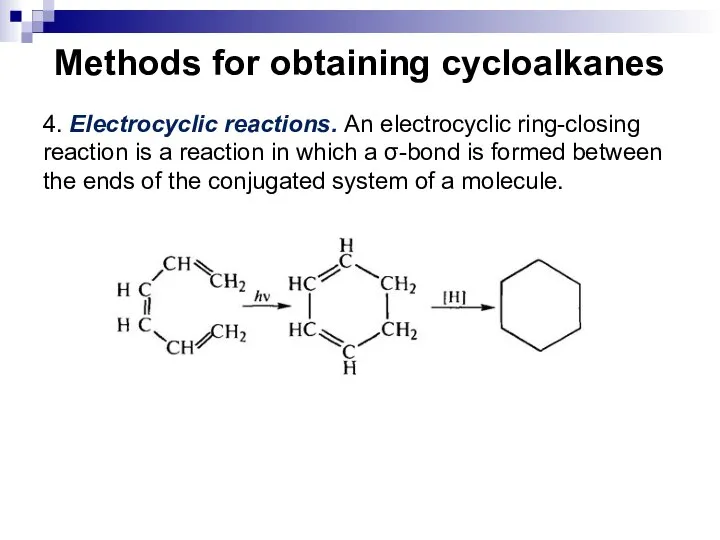
- 21. Methods for obtaining cycloalkanes 4. Electrocyclic reactions. An electrocyclic ring-closing reaction is a reaction in which
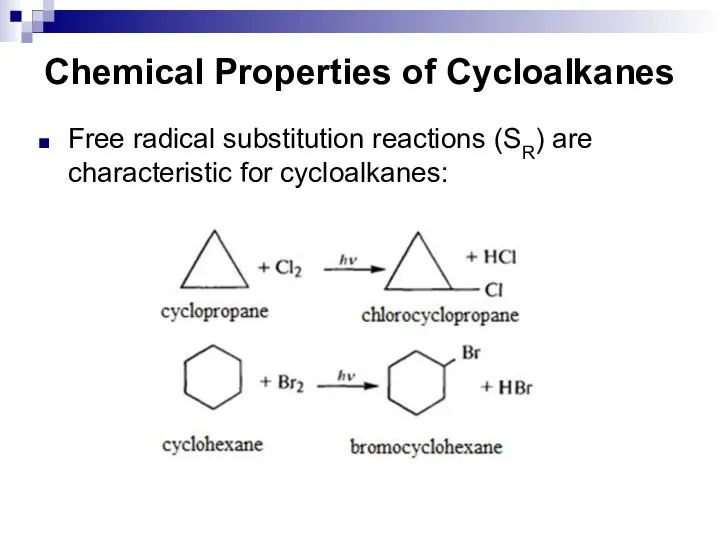
- 22. Chemical Properties of Cycloalkanes Free radical substitution reactions (SR) are characteristic for cycloalkanes:
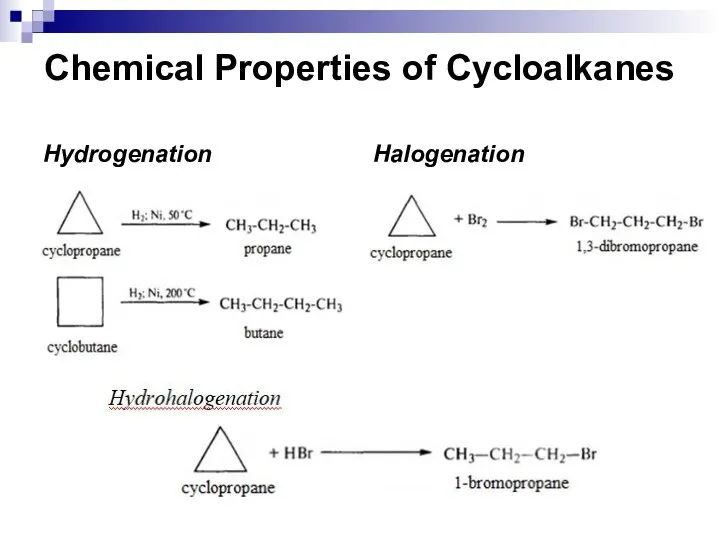
- 23. Chemical Properties of Cycloalkanes Hydrogenation Halogenation
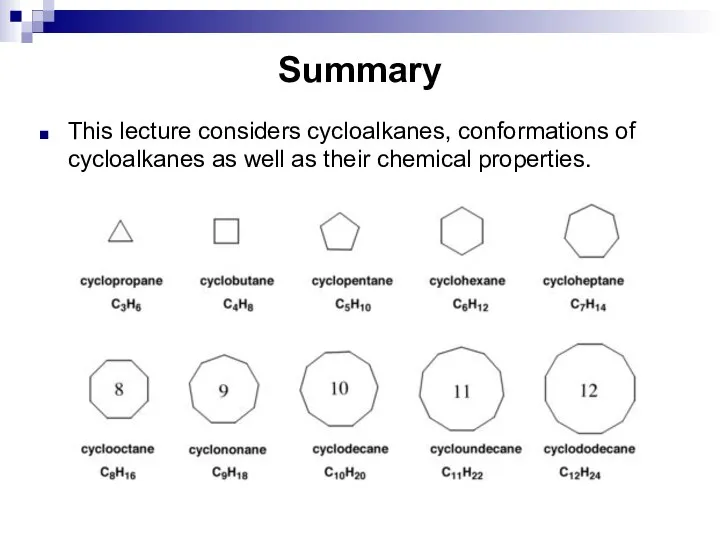
- 24. Summary This lecture considers cycloalkanes, conformations of cycloalkanes as well as their chemical properties.
- 25. Questions and Assignments What are cycloalkanes? Give examples. Name conformations of cycloalkanes. What is ring inversion?
- 26. Aromatic Hydrocarbons (Arenes) Topic 2
- 27. Outline of the lecture Aromaticity. Hückel's Rule Benzene and its Derivatives Nomenclature and isomerism of benzene
- 28. Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May 2012]. Available from World
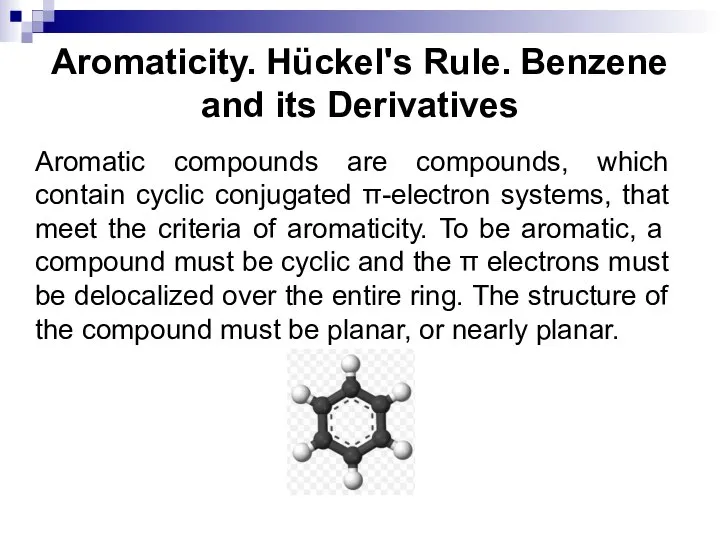
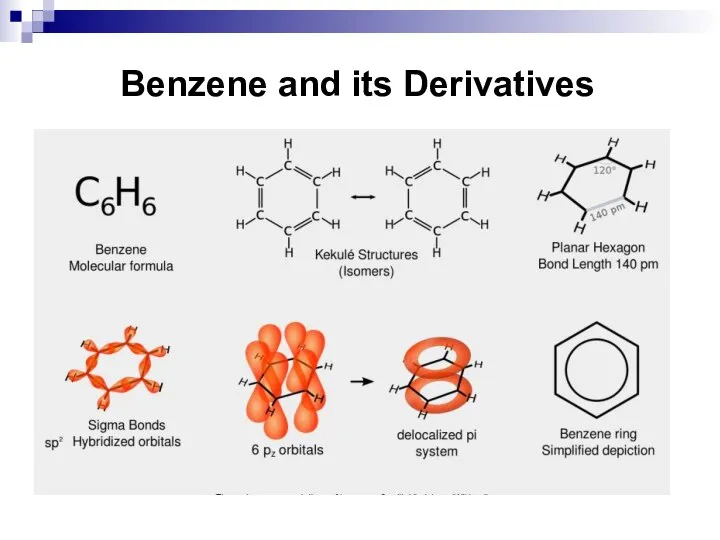
- 29. Aromaticity. Hückel's Rule. Benzene and its Derivatives Aromatic compounds are compounds, which contain cyclic conjugated π-electron
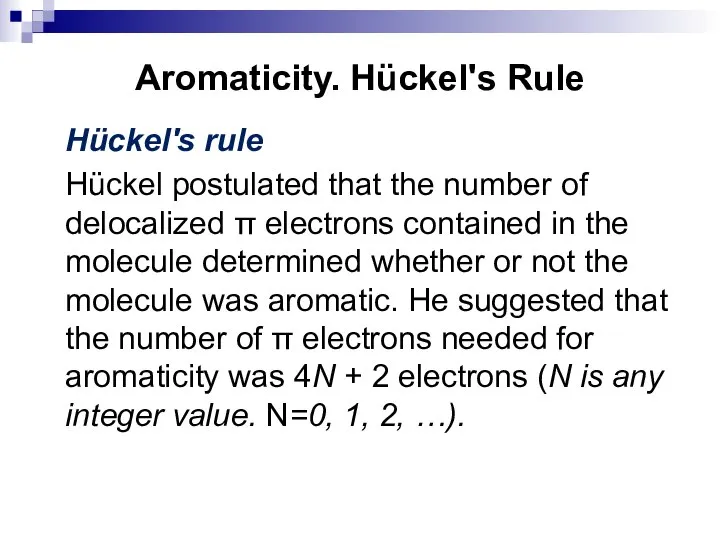
- 30. Aromaticity. Hückel's Rule Hückel's rule Hückel postulated that the number of delocalized π electrons contained in
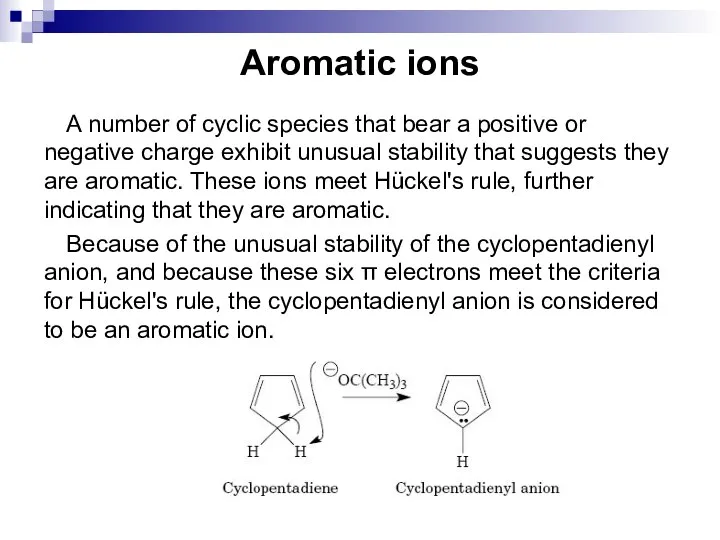
- 31. Aromatic ions A number of cyclic species that bear a positive or negative charge exhibit unusual
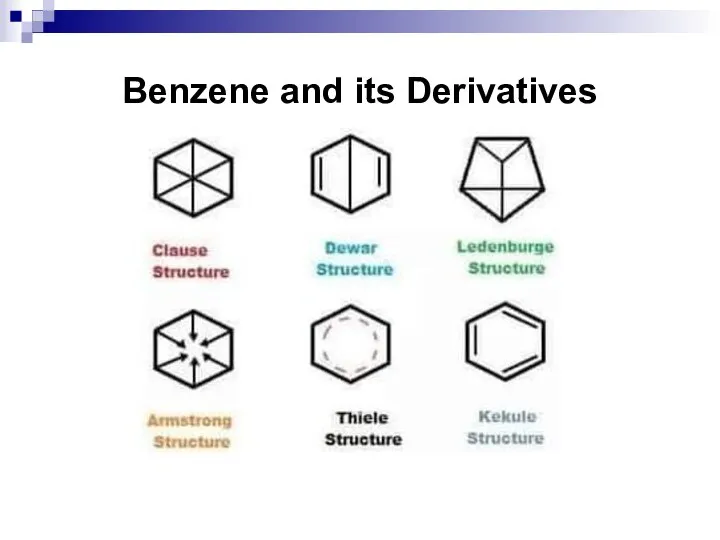
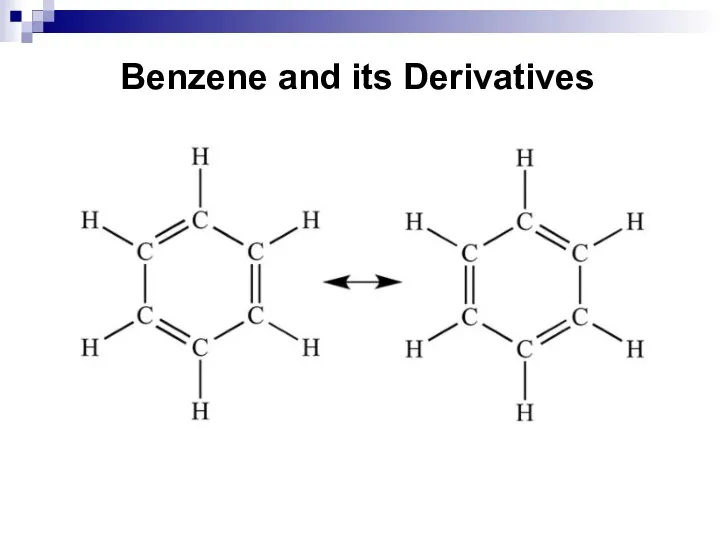
- 32. Benzene and its Derivatives
- 33. Benzene and its Derivatives
- 34. Benzene and its Derivatives
- 35. Benzene and its Derivatives
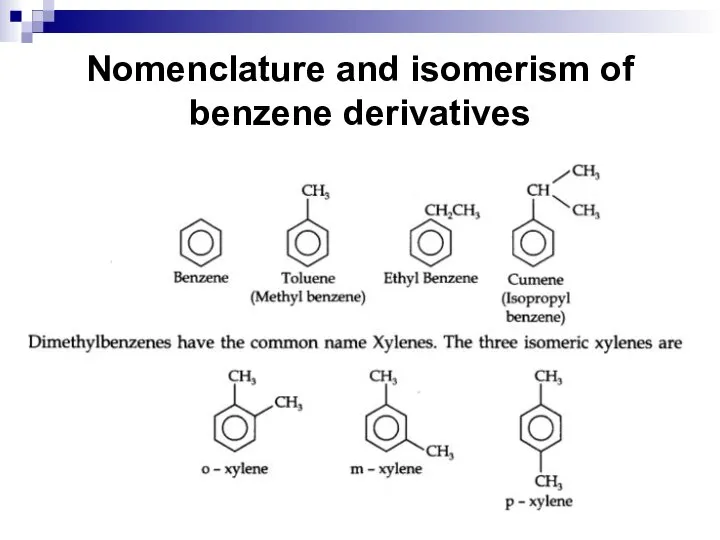
- 36. Nomenclature and isomerism of benzene derivatives According to the IUPAC nomenclature arenes are considered as derivatives
- 37. Nomenclature and isomerism of benzene derivatives
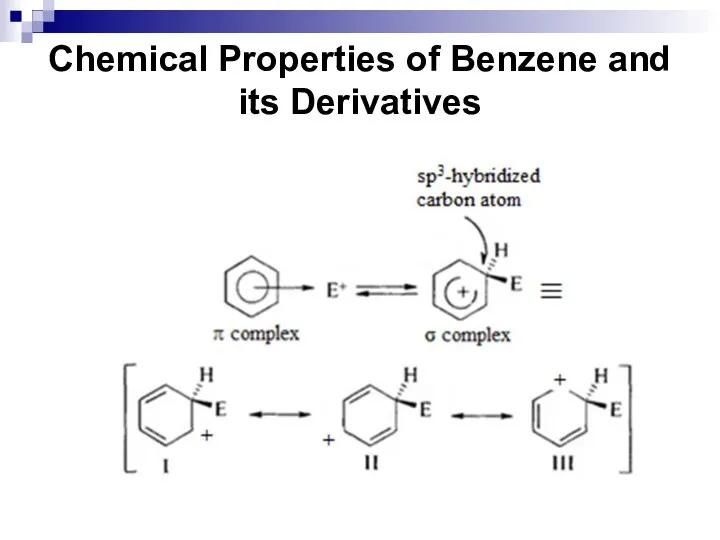
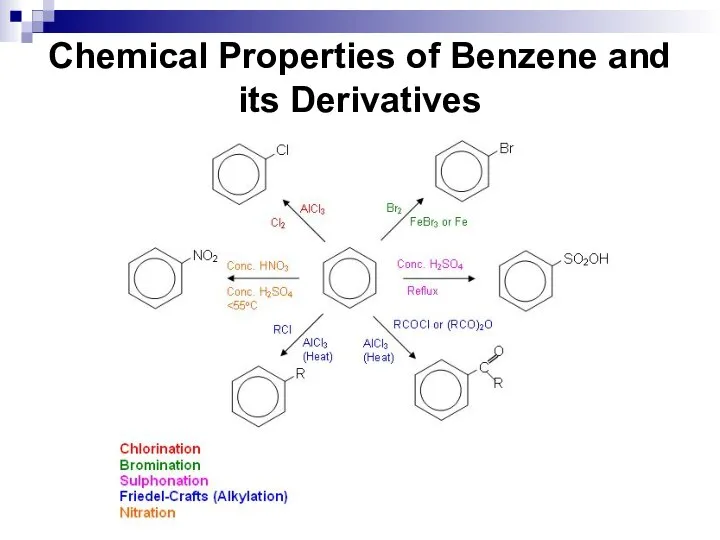
- 38. Chemical Properties of Benzene and its Derivatives
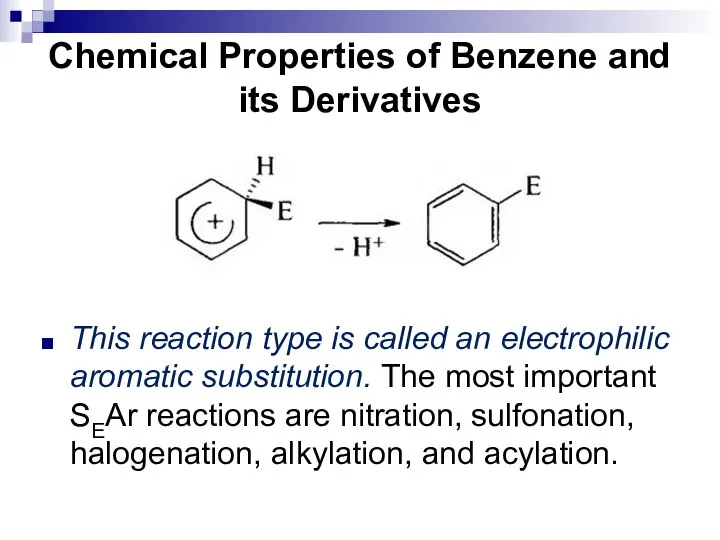
- 39. Chemical Properties of Benzene and its Derivatives This reaction type is called an electrophilic aromatic substitution.
- 40. Chemical Properties of Benzene and its Derivatives
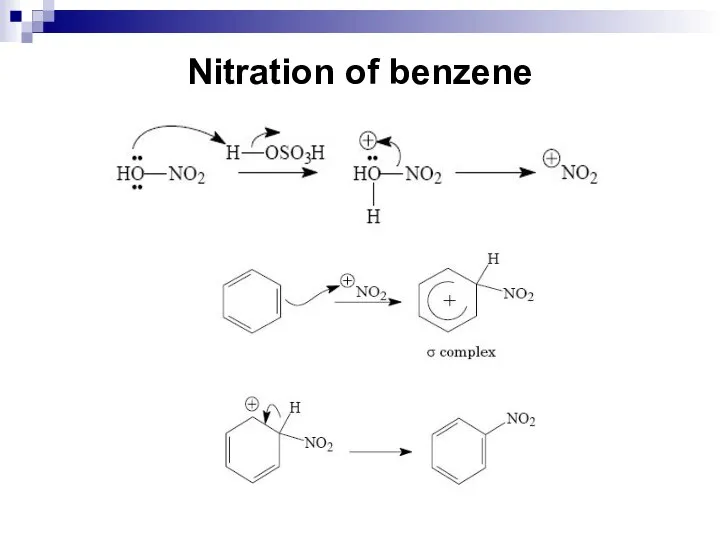
- 41. Nitration of benzene Concentrated nitric acid or a mixture of concentrated nitric and sulfuric acid (nitrating
- 42. Nitration of benzene
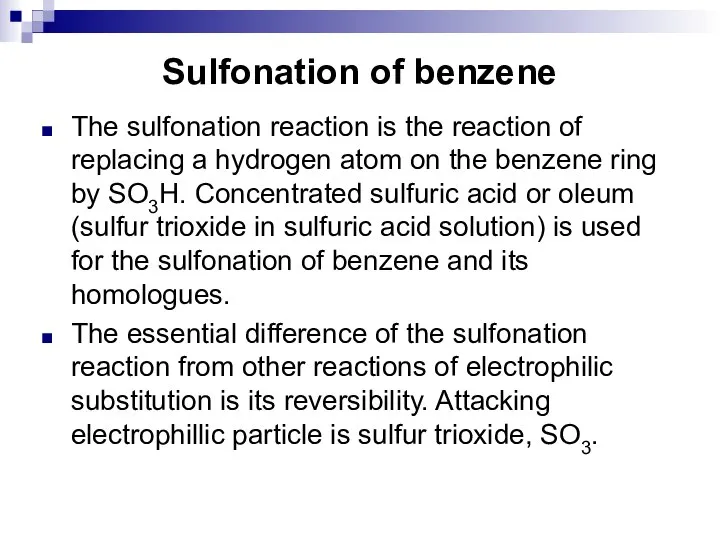
- 43. Sulfonation of benzene The sulfonation reaction is the reaction of replacing a hydrogen atom on the
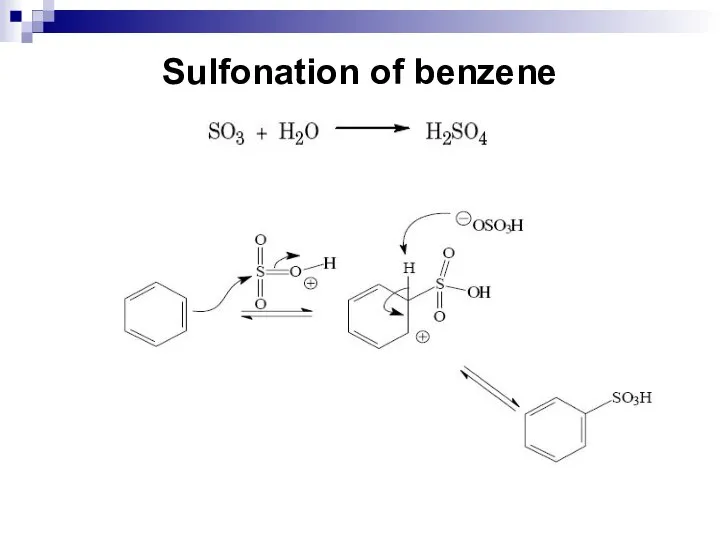
- 44. Sulfonation of benzene
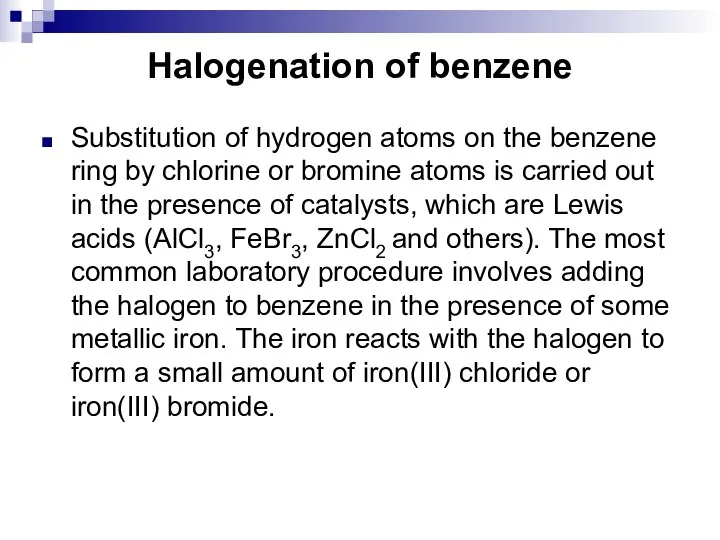
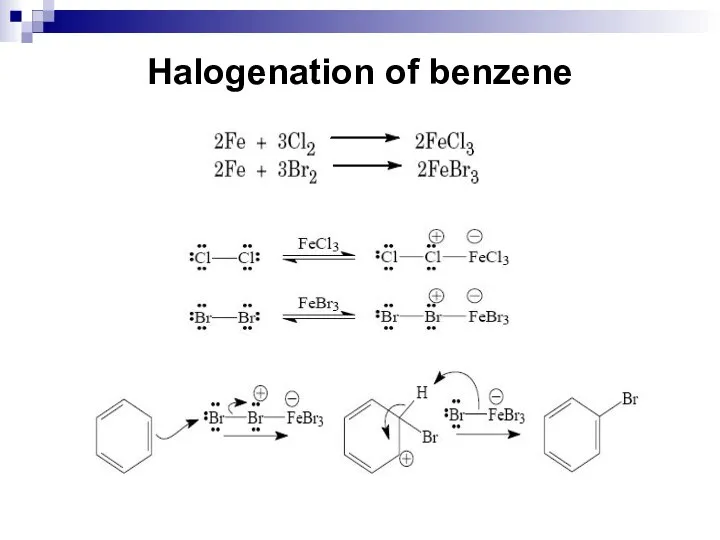
- 45. Halogenation of benzene Substitution of hydrogen atoms on the benzene ring by chlorine or bromine atoms
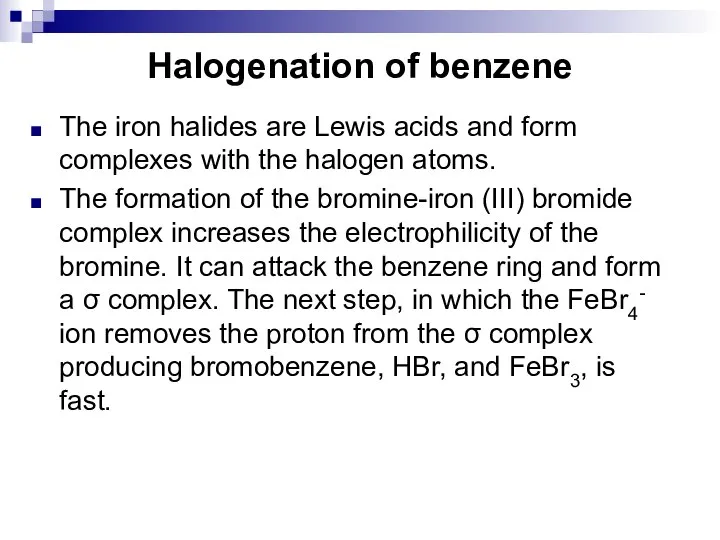
- 46. Halogenation of benzene The iron halides are Lewis acids and form complexes with the halogen atoms.
- 47. Halogenation of benzene
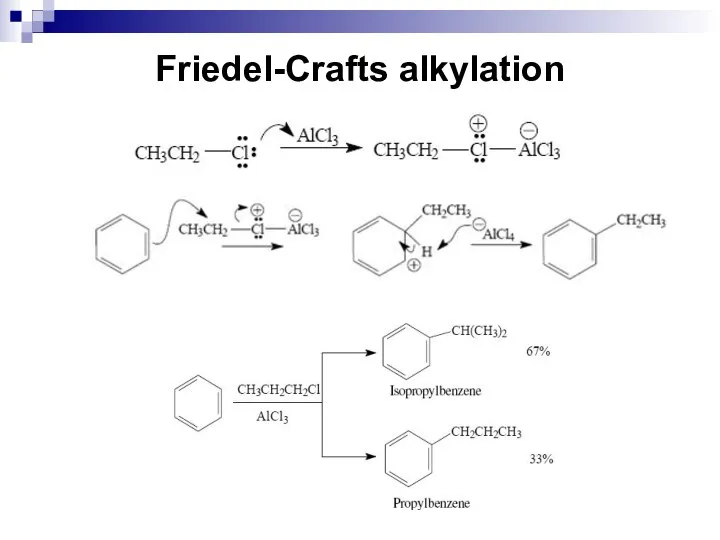
- 48. Friedel-Crafts alkylation The mechanism for an alkylation reaction begins as the Lewis acid, in this case
- 49. Friedel-Crafts alkylation
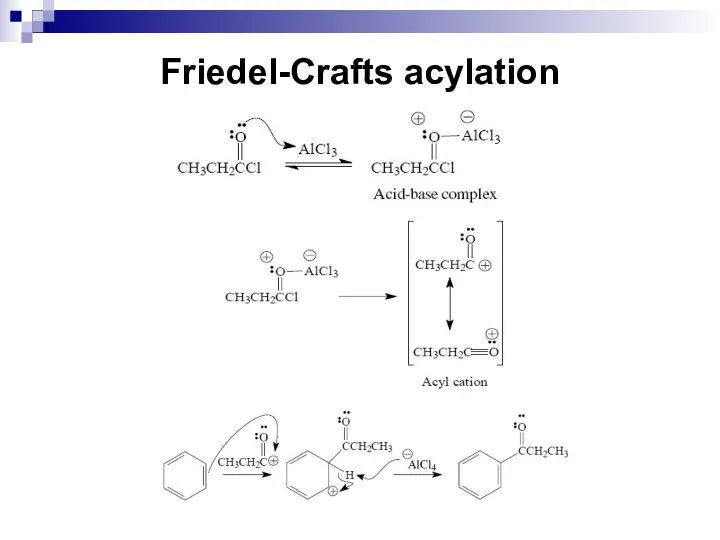
- 50. Friedel-Crafts acylation A variation of the Friedel-Crafts reaction is the acylation reaction. A Friedel-Crafts acylation reaction
- 51. Friedel-Crafts acylation
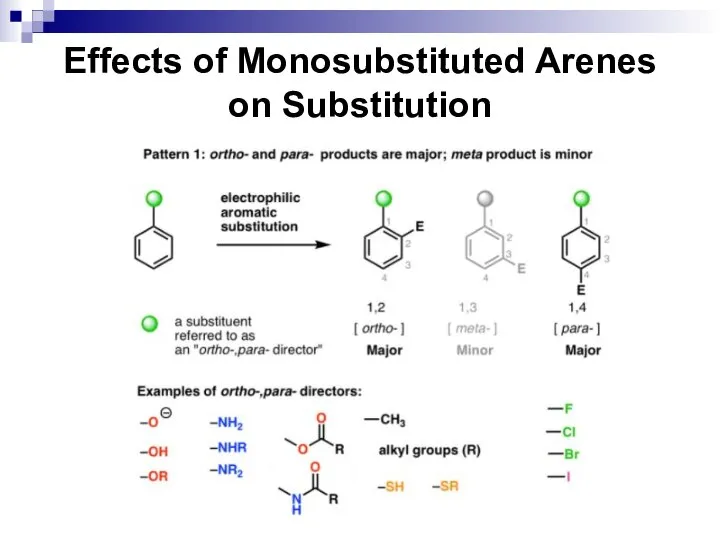
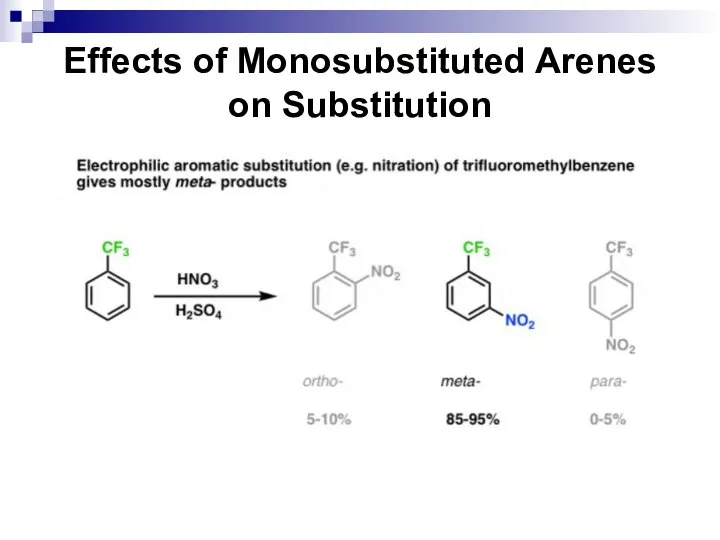
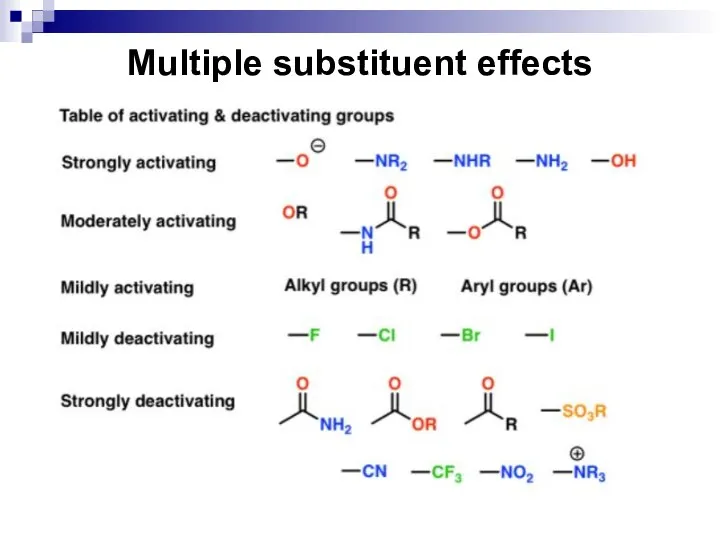
- 52. Effects of Monosubstituted Arenes on Substitution All benzene ring substituent groups are either ortho, para directors
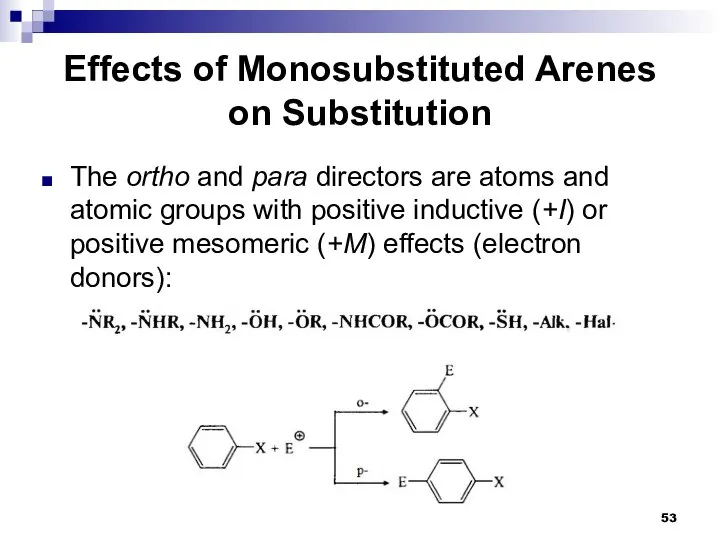
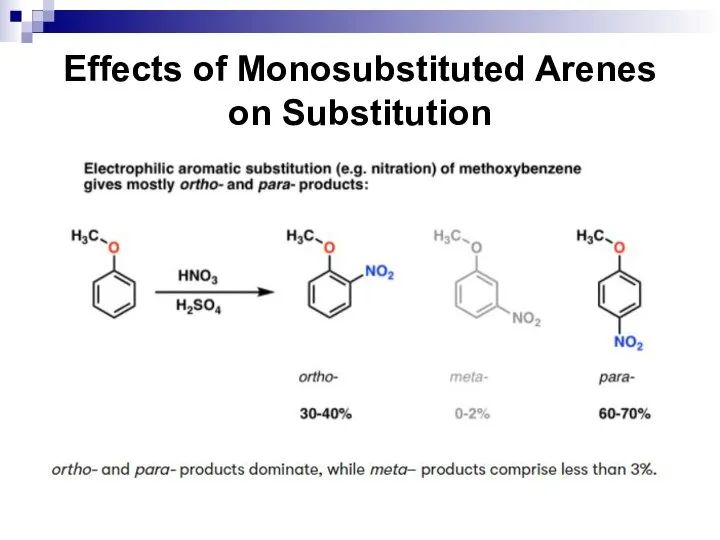
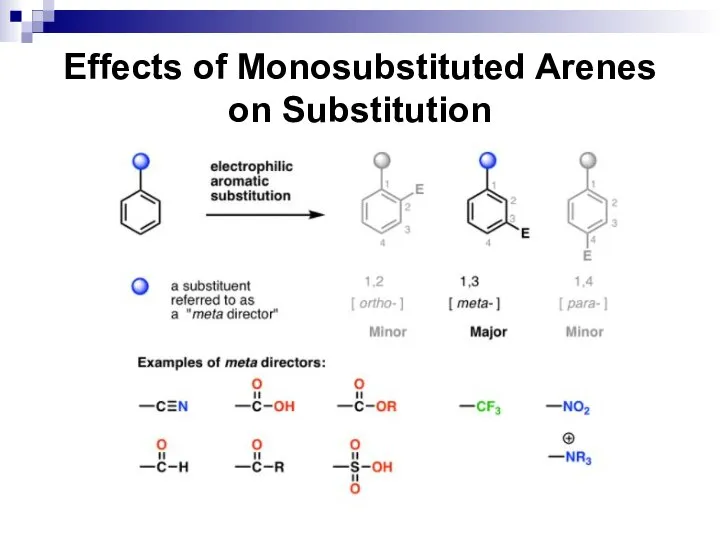
- 53. Effects of Monosubstituted Arenes on Substitution The ortho and para directors are atoms and atomic groups
- 54. Effects of Monosubstituted Arenes on Substitution
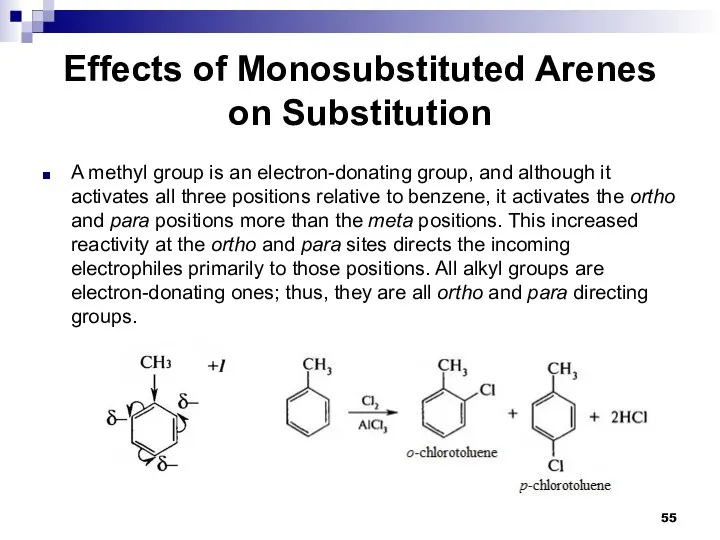
- 55. Effects of Monosubstituted Arenes on Substitution A methyl group is an electron-donating group, and although it
- 56. Effects of Monosubstituted Arenes on Substitution
- 57. Effects of Monosubstituted Arenes on Substitution
- 58. Effects of Monosubstituted Arenes on Substitution
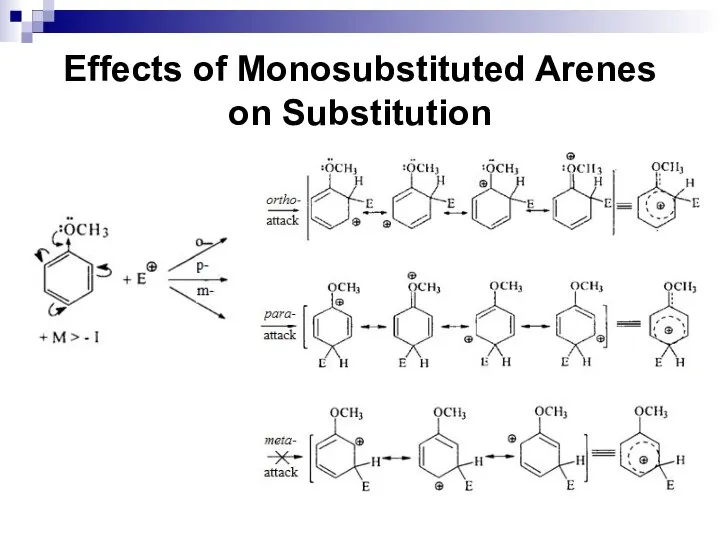
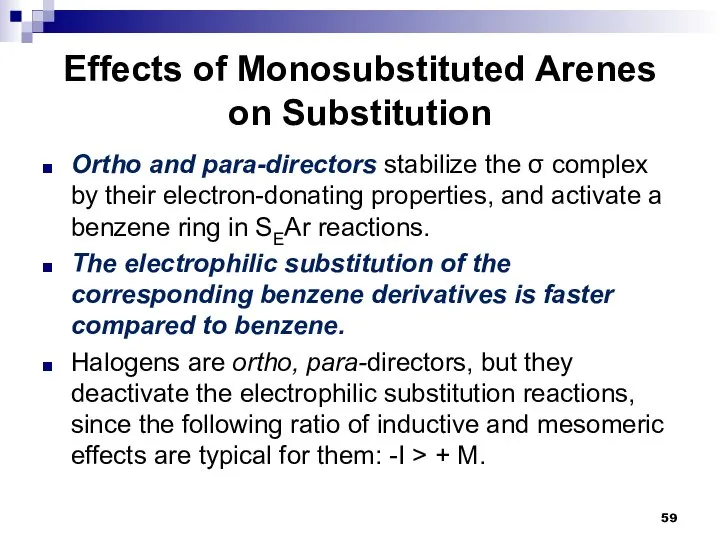
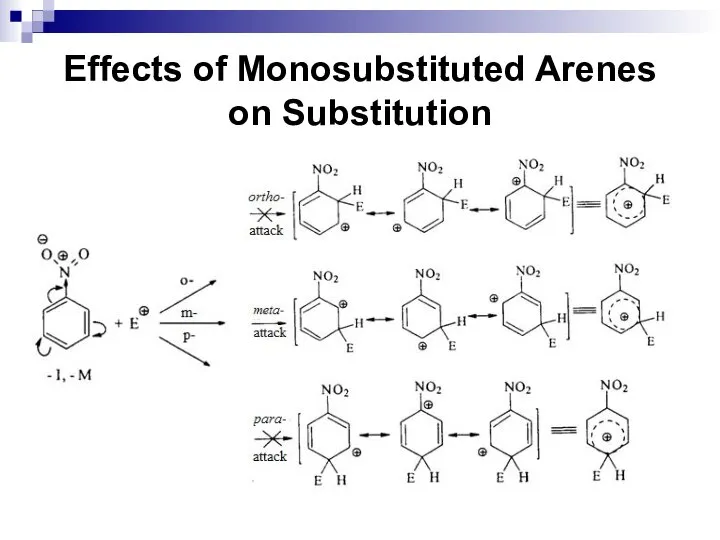
- 59. Effects of Monosubstituted Arenes on Substitution Ortho and para-directors stabilize the σ complex by their electron-donating
- 60. Effects of Monosubstituted Arenes on Substitution
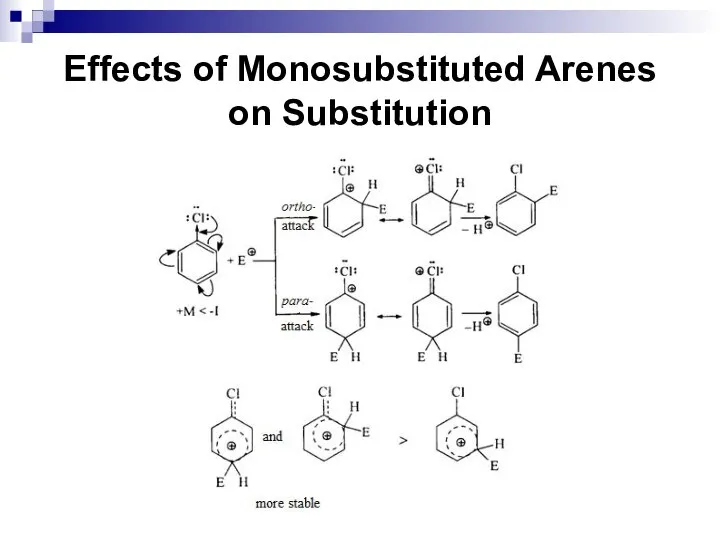
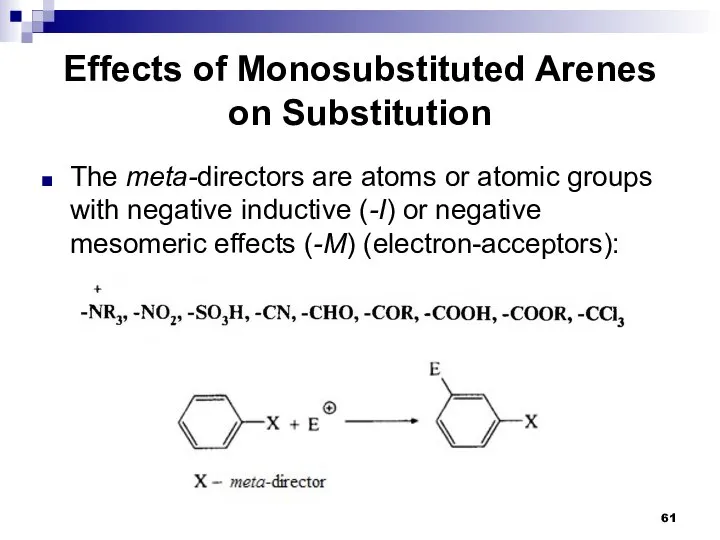
- 61. Effects of Monosubstituted Arenes on Substitution The meta-directors are atoms or atomic groups with negative inductive
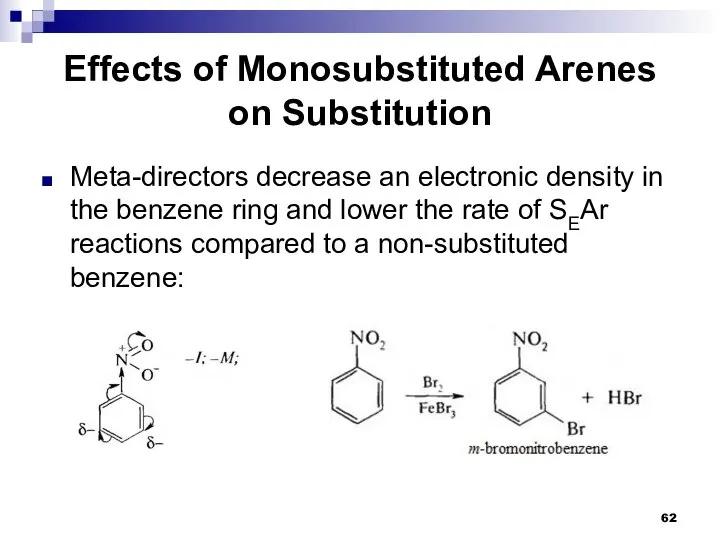
- 62. Effects of Monosubstituted Arenes on Substitution Meta-directors decrease an electronic density in the benzene ring and
- 63. Effects of Monosubstituted Arenes on Substitution
- 64. Effects of Monosubstituted Arenes on Substitution
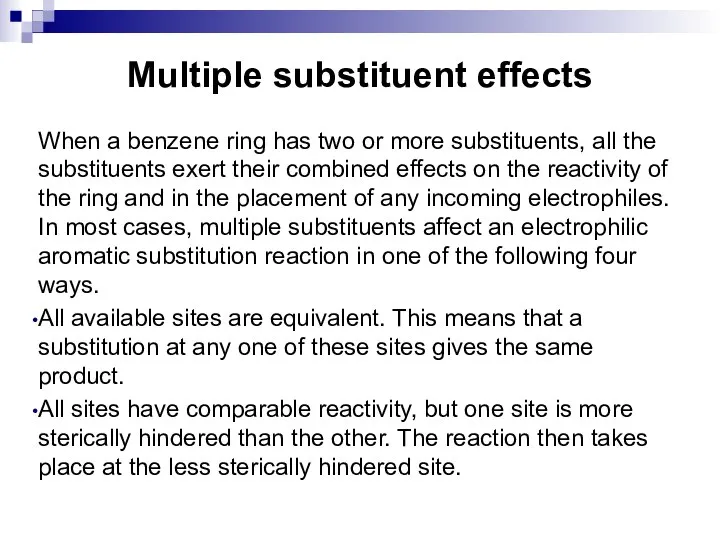
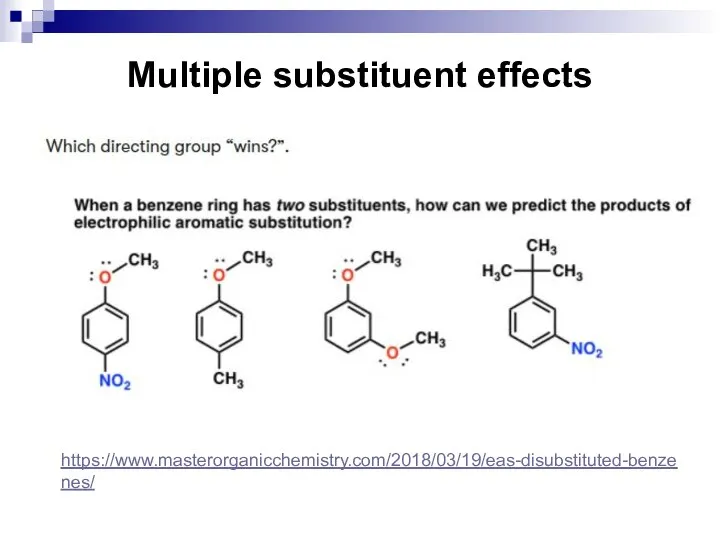
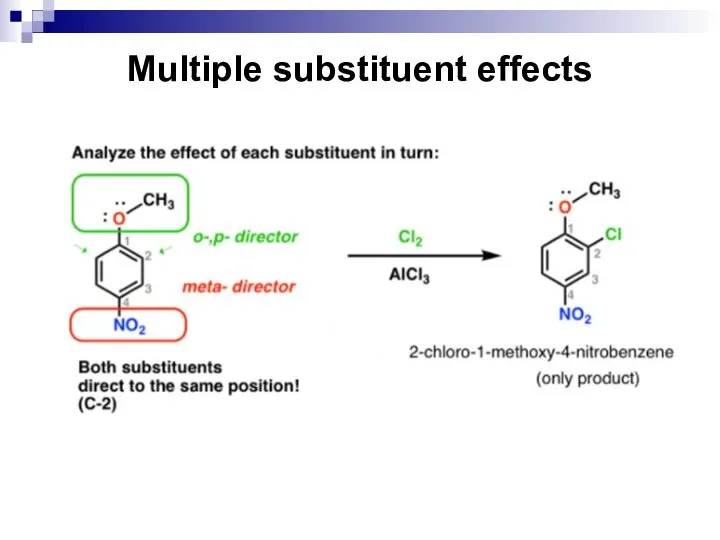
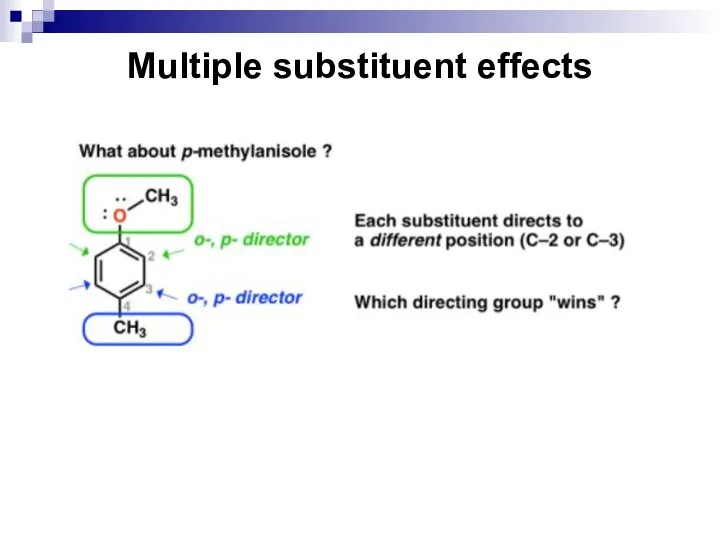
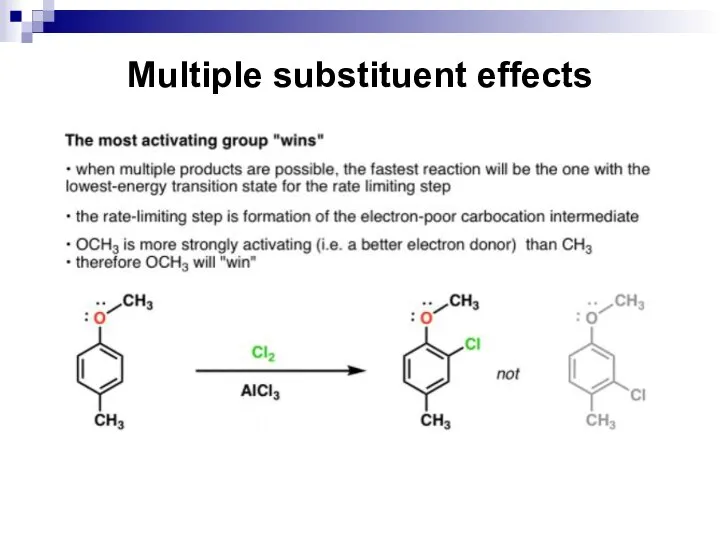
- 65. Multiple substituent effects When a benzene ring has two or more substituents, all the substituents exert
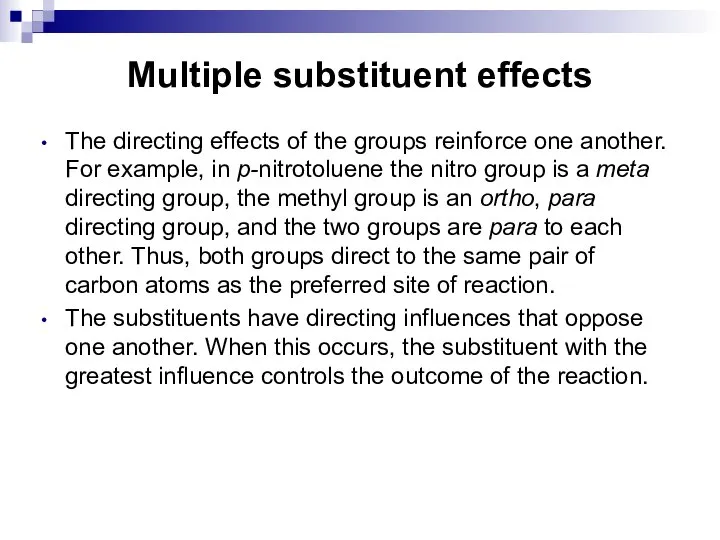
- 66. Multiple substituent effects The directing effects of the groups reinforce one another. For example, in p-nitrotoluene
- 67. Multiple substituent effects https://www.masterorganicchemistry.com/2018/03/19/eas-disubstituted-benzenes/
- 68. Multiple substituent effects
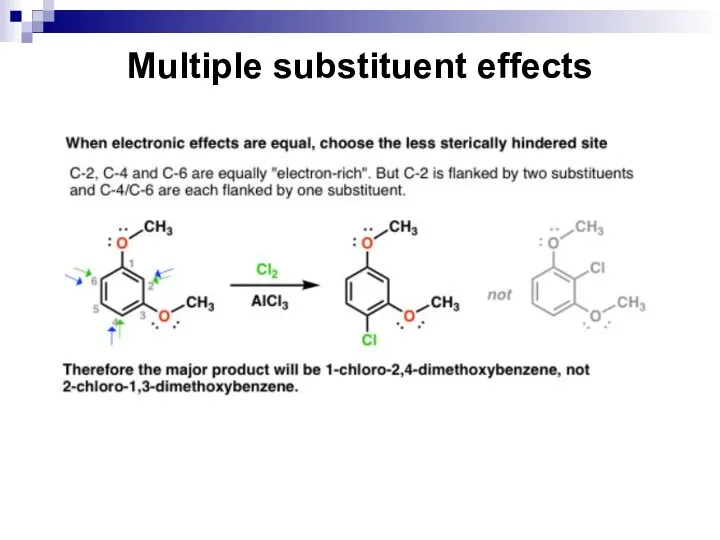
- 69. Multiple substituent effects
- 70. Multiple substituent effects
- 71. Multiple substituent effects
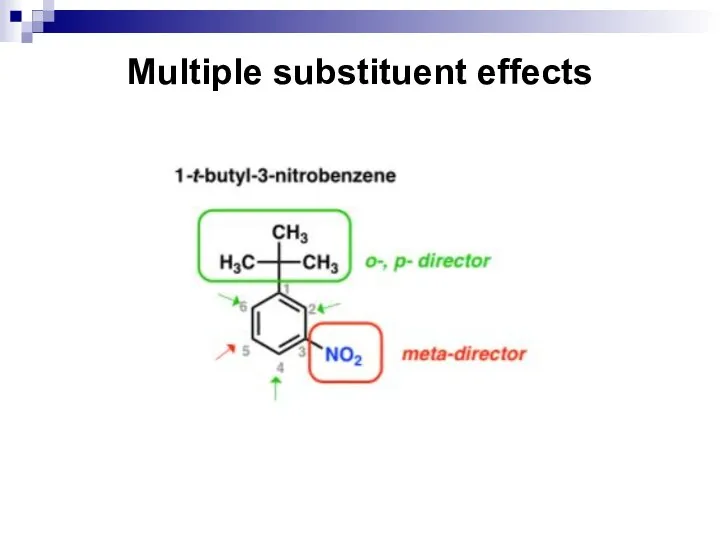
- 72. Multiple substituent effects
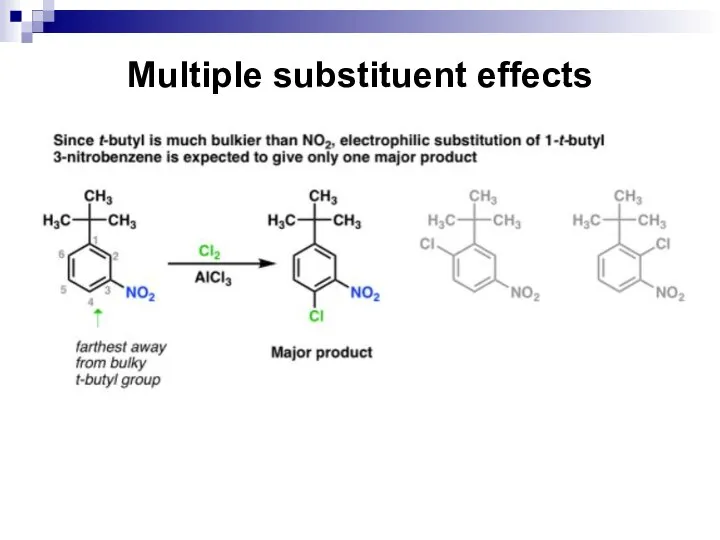
- 73. Multiple substituent effects
- 74. Multiple substituent effects
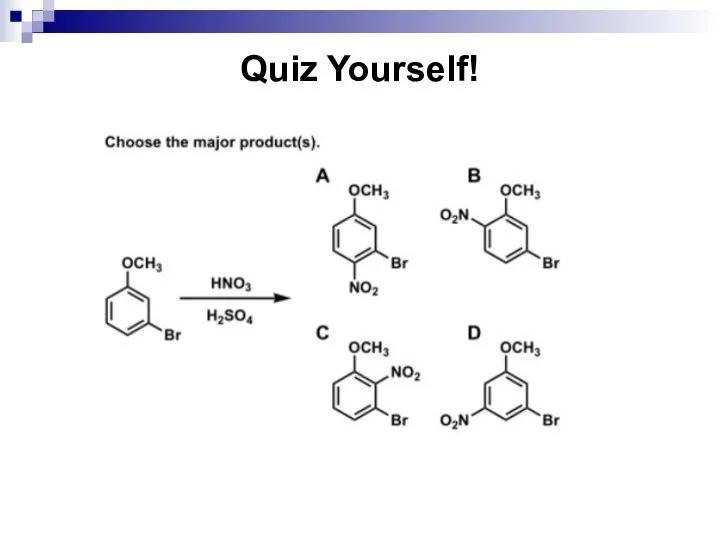
- 75. Quiz Yourself!
- 76. Summary Benzene is very important for the synthesis of dyes, pharmaceuticals and perfumes etc. Electrophilic substitution
- 77. Questions and Assignments What are the aromatic hydrocarbons? Explain the contemporary understanding of the structure of
- 78. Halogenated Aromatic Hydrocarbons Topic 3
- 79. Outline of the lecture Aryl Halides Nucleophilic Substitution on an Aromatic Ring
- 80. Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May 2012]. Available from World
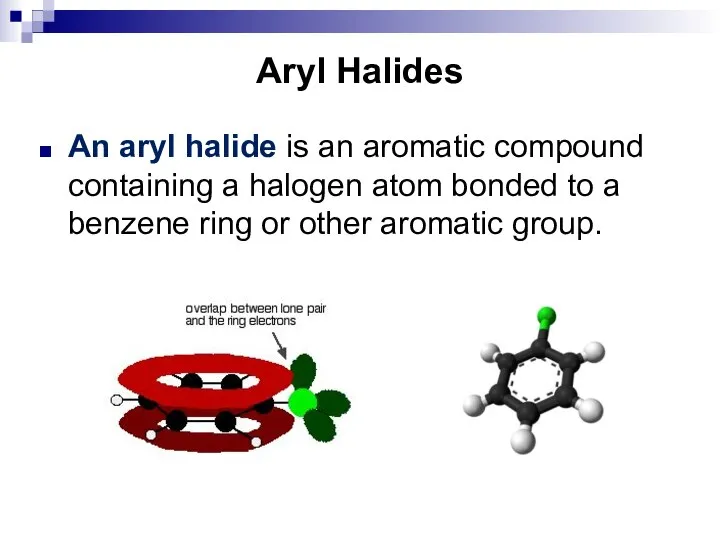
- 81. Aryl Halides An aryl halide is an aromatic compound containing a halogen atom bonded to a
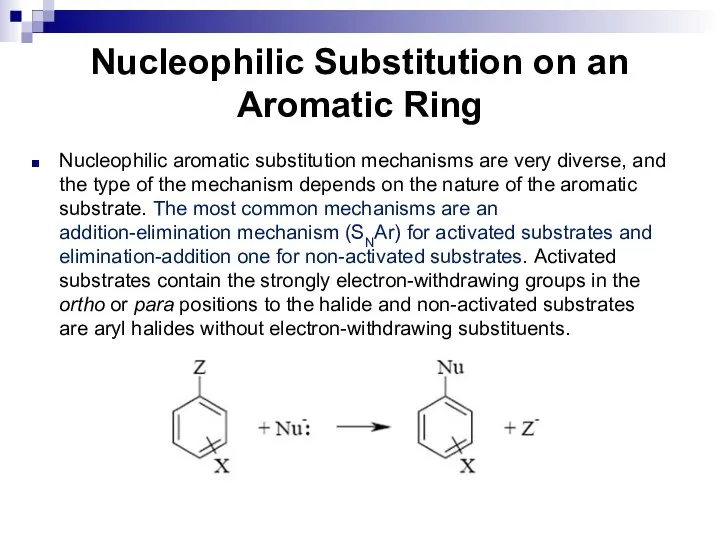
- 82. Nucleophilic Substitution on an Aromatic Ring Nucleophilic aromatic substitution mechanisms are very diverse, and the type
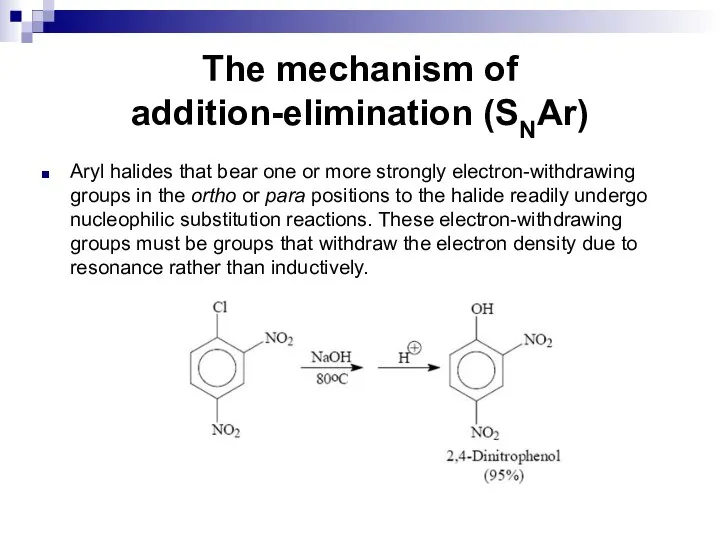
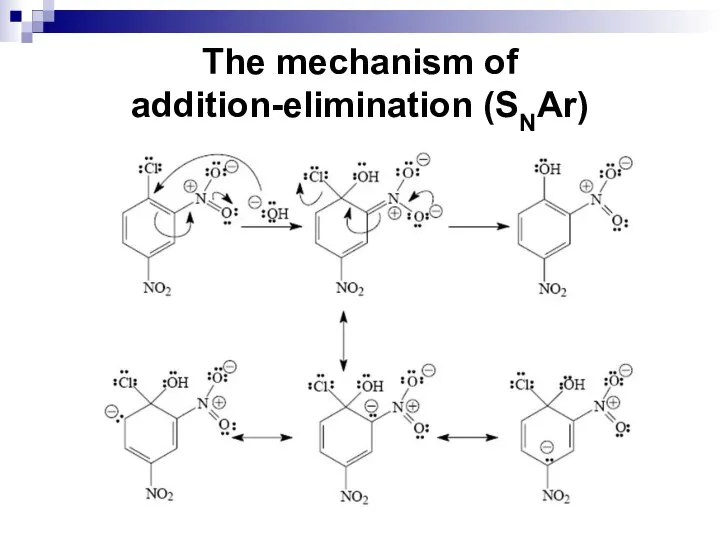
- 83. The mechanism of addition-elimination (SNAr) Aryl halides that bear one or more strongly electron-withdrawing groups in
- 84. The mechanism of addition-elimination (SNAr)
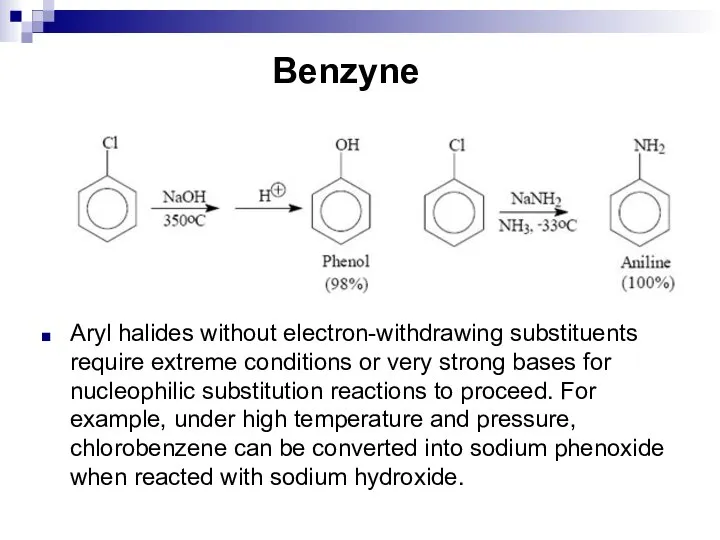
- 85. Benzyne Aryl halides without electron-withdrawing substituents require extreme conditions or very strong bases for nucleophilic substitution
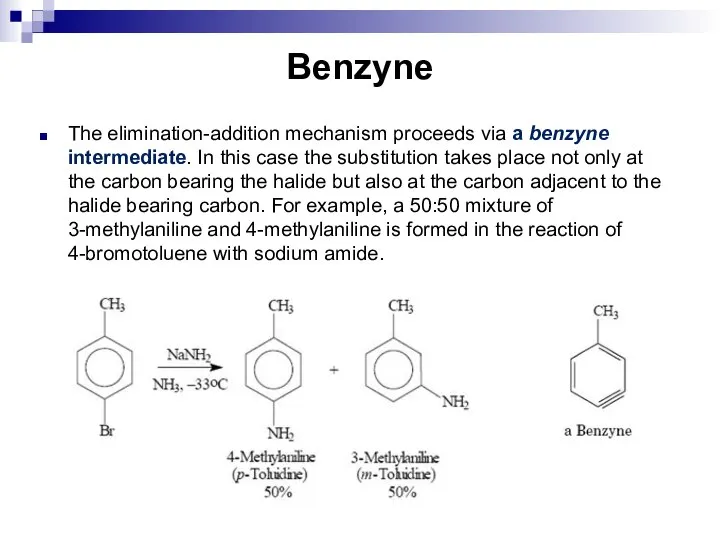
- 86. Benzyne The elimination-addition mechanism proceeds via a benzyne intermediate. In this case the substitution takes place
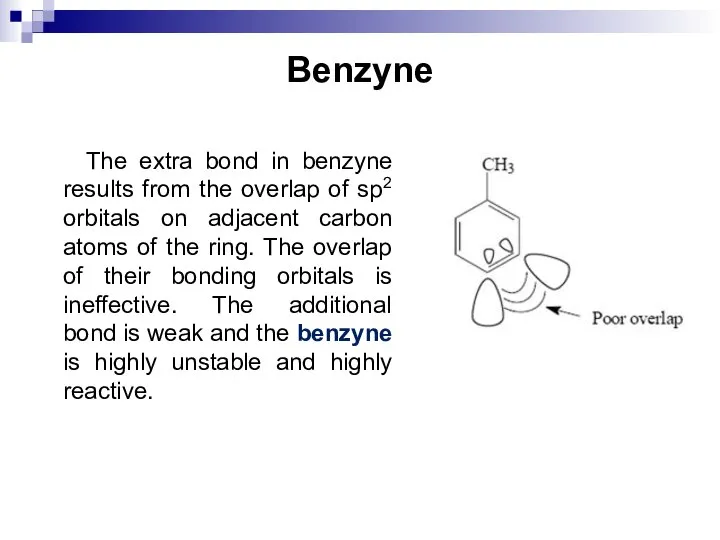
- 87. Benzyne The extra bond in benzyne results from the overlap of sp2 orbitals on adjacent carbon
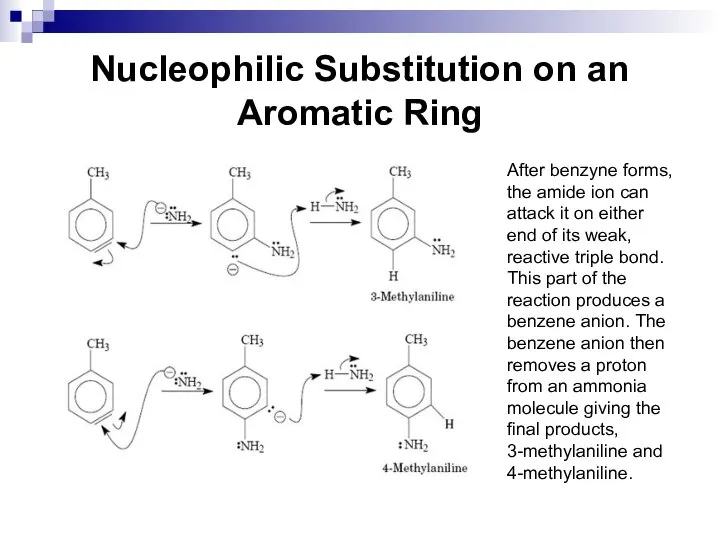
- 88. Nucleophilic Substitution on an Aromatic Ring After benzyne forms, the amide ion can attack it on
- 89. Summary In this lecture structure and chemical properties of aryl halides are considered. Mechanisms of nucleophilic
- 90. Questions and Assignments Discuss structure and reactivity of aryl halides. What are chemical properties of aryl
- 91. Topic 4 Aromatic Sulfonic Acids
- 92. Outline of the lecture Aromatic sulfonic acids Mechanism of the sulfonation reaction Isolation and identification of
- 93. Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May 2012]. Available from World
- 94. Aromatic sulfonic acids Aromatic sulfonic acids are derivatives of arenes containing the SO3H sulfo group. Aromatic
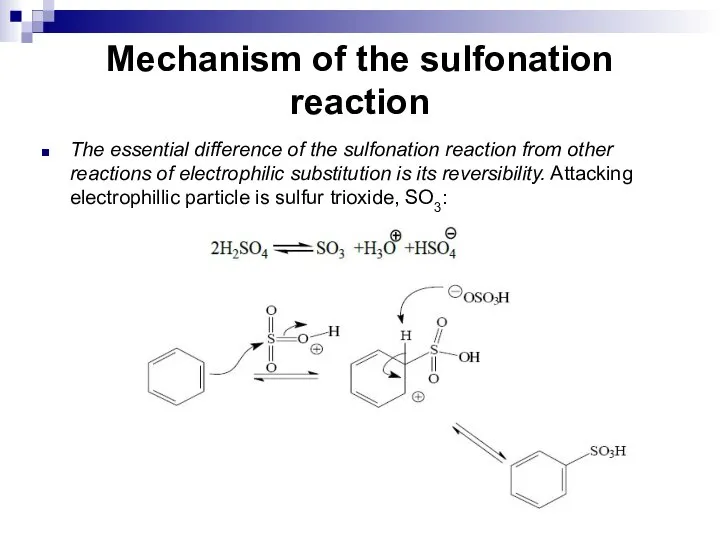
- 95. Mechanism of the sulfonation reaction The essential difference of the sulfonation reaction from other reactions of
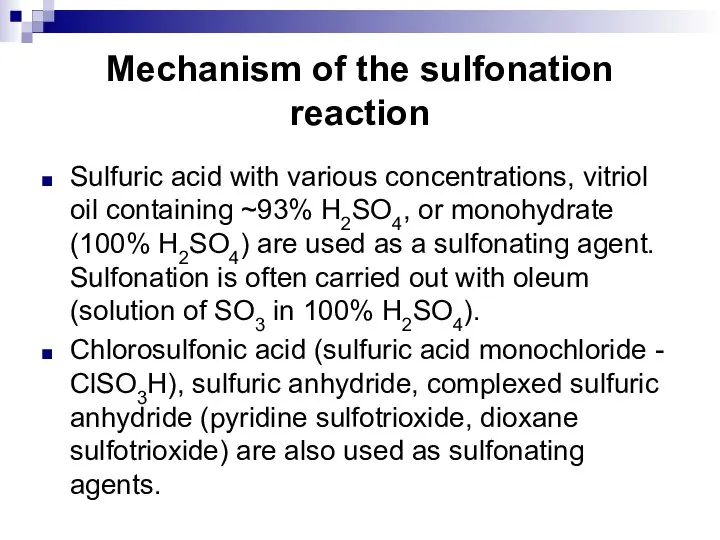
- 96. Mechanism of the sulfonation reaction Sulfuric acid with various concentrations, vitriol oil containing ~93% H2SO4, or
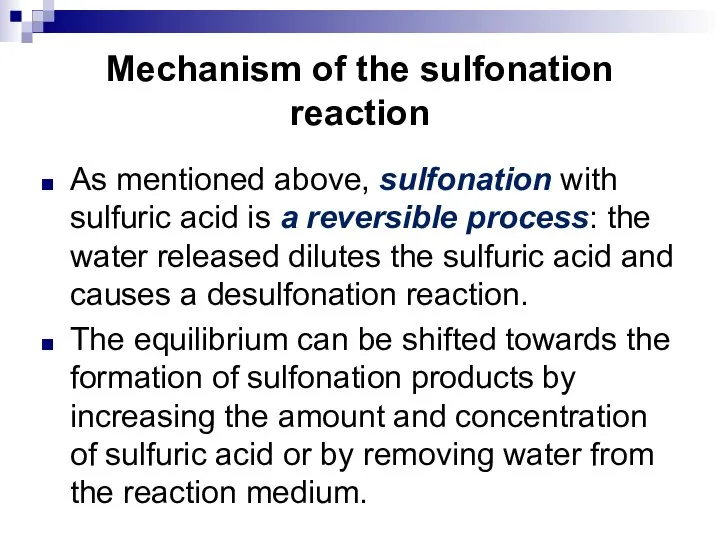
- 97. Mechanism of the sulfonation reaction As mentioned above, sulfonation with sulfuric acid is a reversible process:
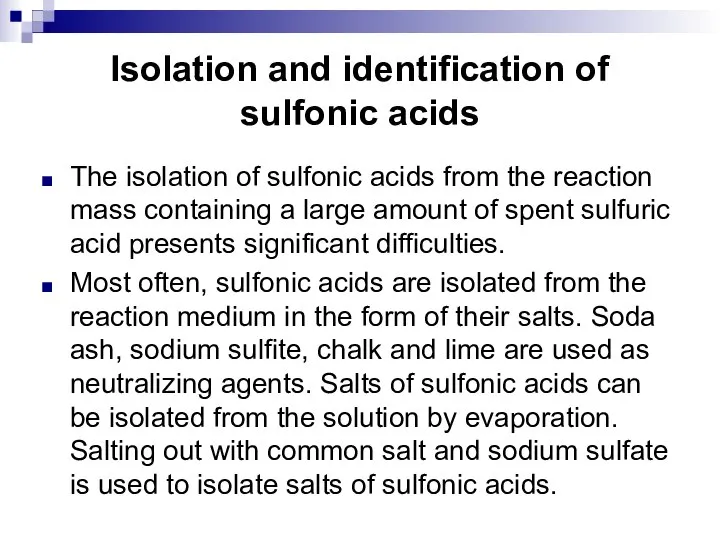
- 98. Isolation and identification of sulfonic acids The isolation of sulfonic acids from the reaction mass containing
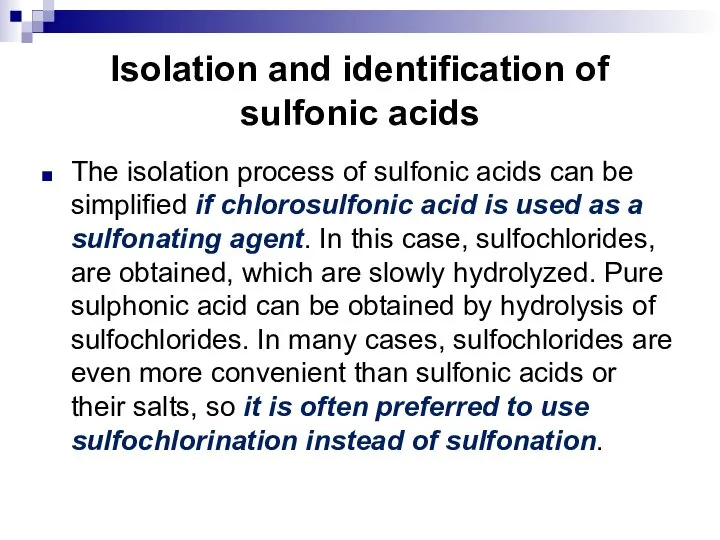
- 99. Isolation and identification of sulfonic acids The isolation process of sulfonic acids can be simplified if
- 100. Derivatives of sulfonic acids Sulfonic acids are compounds difficult to characterize, often do not have certain
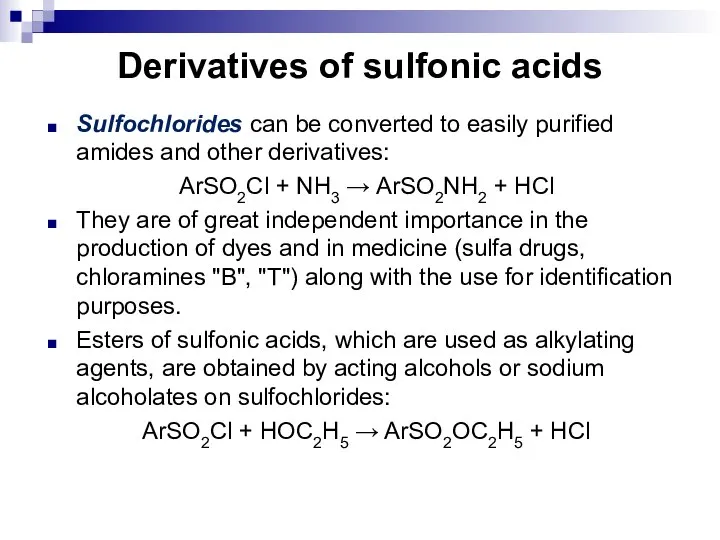
- 101. Derivatives of sulfonic acids Sulfochlorides can be converted to easily purified amides and other derivatives: ArSO2Cl
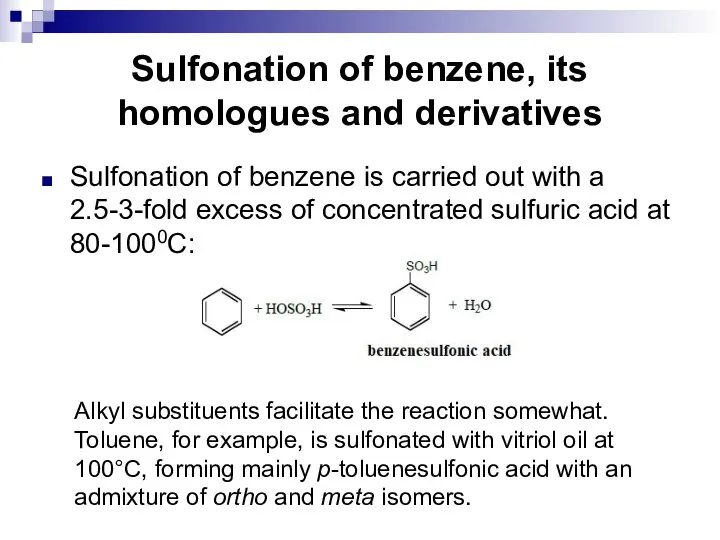
- 102. Sulfonation of benzene, its homologues and derivatives Sulfonation of benzene is carried out with a 2.5-3-fold
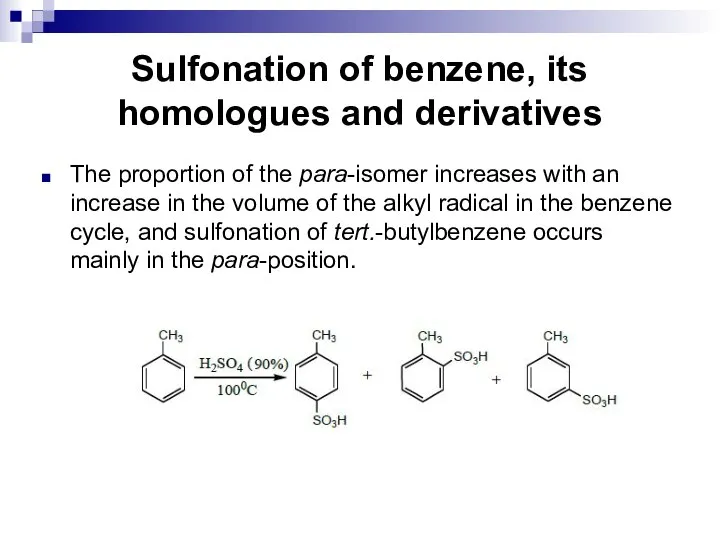
- 103. Sulfonation of benzene, its homologues and derivatives The proportion of the para-isomer increases with an increase
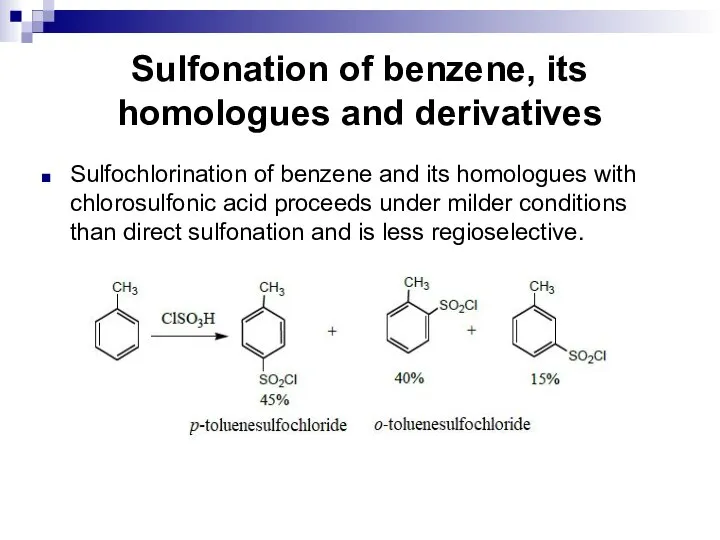
- 104. Sulfonation of benzene, its homologues and derivatives Sulfochlorination of benzene and its homologues with chlorosulfonic acid
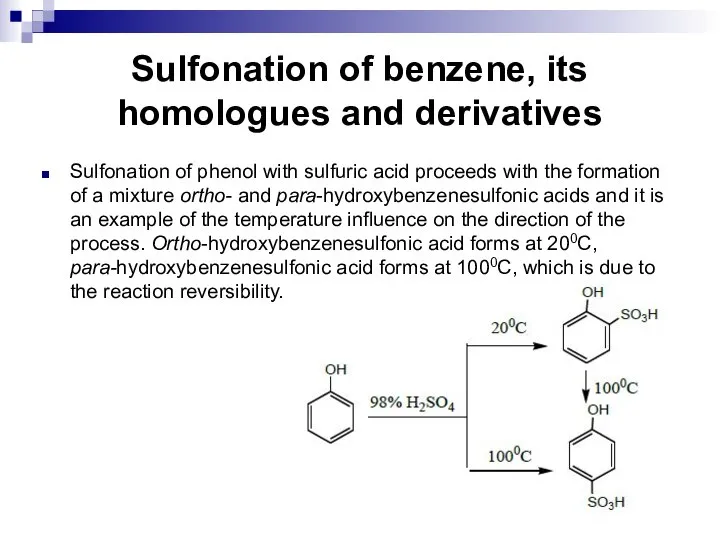
- 105. Sulfonation of benzene, its homologues and derivatives Sulfonation of phenol with sulfuric acid proceeds with the
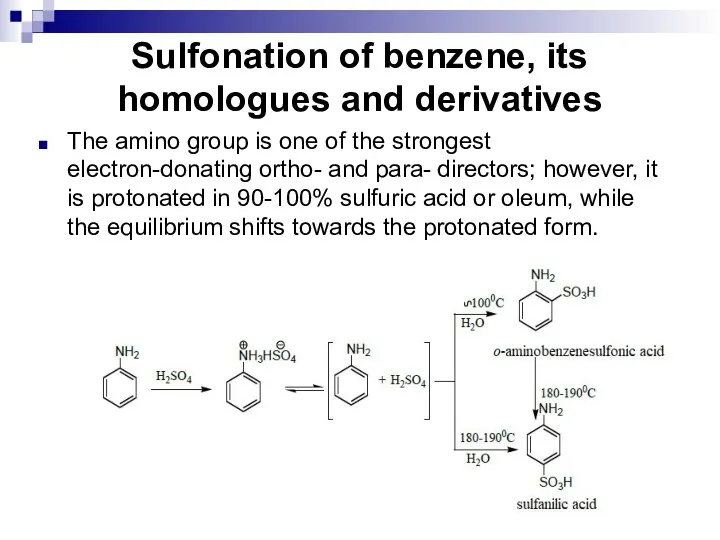
- 106. Sulfonation of benzene, its homologues and derivatives The amino group is one of the strongest electron-donating
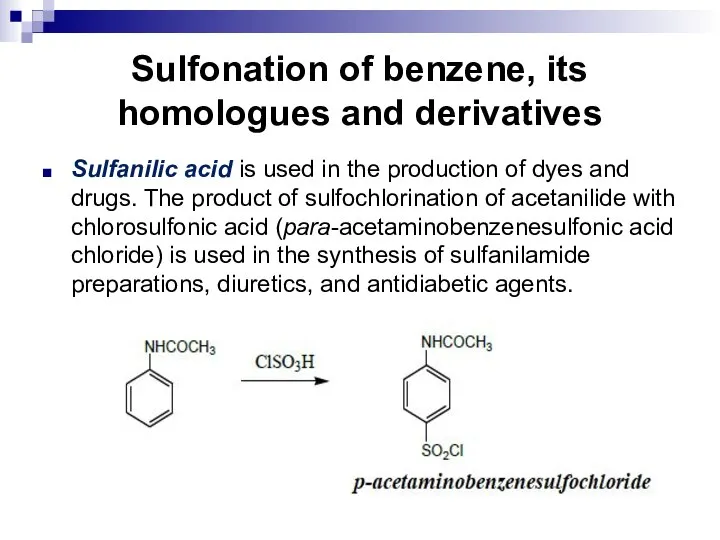
- 107. Sulfonation of benzene, its homologues and derivatives Sulfanilic acid is used in the production of dyes
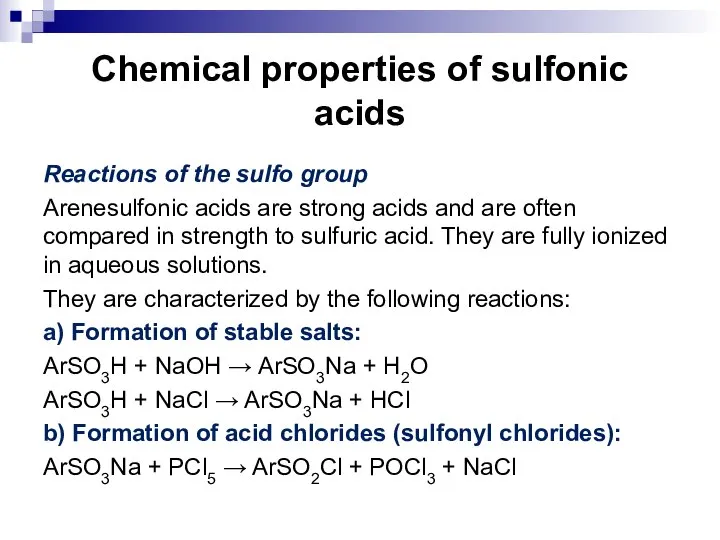
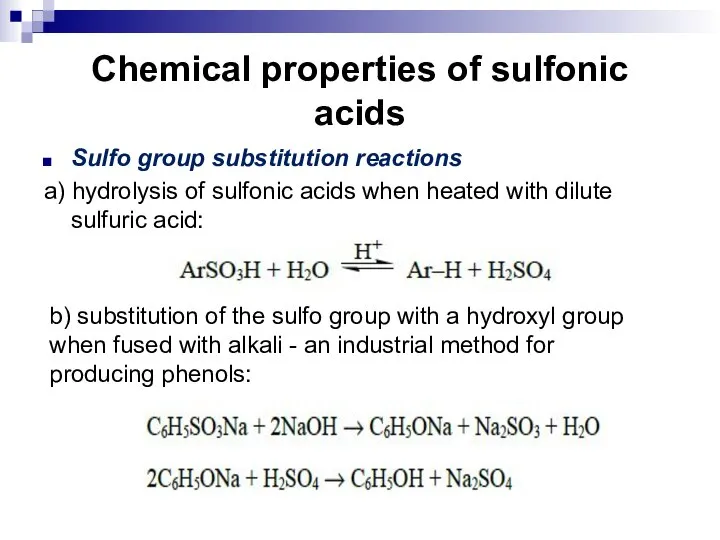
- 108. Chemical properties of sulfonic acids Reactions of the sulfo group. Substitution reactions of the sulfo group.
- 109. Chemical properties of sulfonic acids Reactions of the sulfo group Arenesulfonic acids are strong acids and
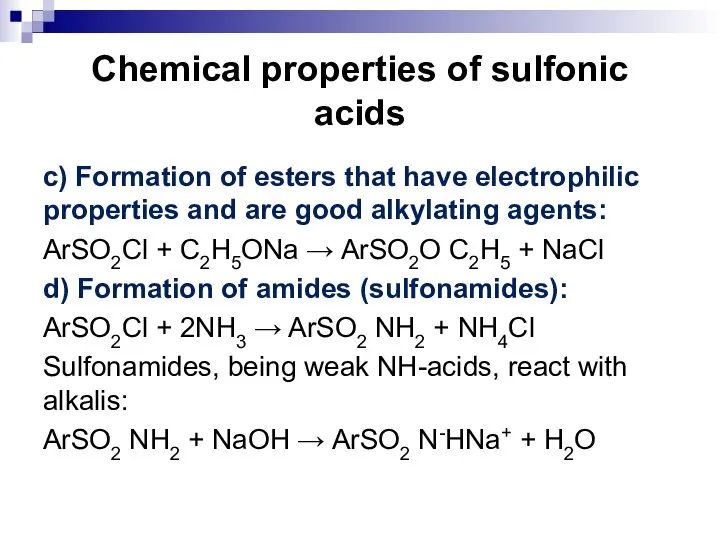
- 110. Chemical properties of sulfonic acids c) Formation of esters that have electrophilic properties and are good
- 111. Chemical properties of sulfonic acids Sulfo group substitution reactions a) hydrolysis of sulfonic acids when heated
- 112. Chemical properties of sulfonic acids c) when salts of sulfonic acids are fused with cyanides, nitriles
- 113. Summary In this lecture chemical properties of aromatic sulfonic acids are considered. Derivatives of aromatic sulfonic
- 114. Questions and Assignments What are aromatic sulfonic acids? Discuss mechanism of the sulfonation reaction. Explain how
- 115. Topic 5 Aromatic Nitro Compounds
- 116. Outline of the lecture General Information Nitro Compounds Reactions of Nitro Compounds Reduction in Neutral Medium
- 117. Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May 2012]. Available from World
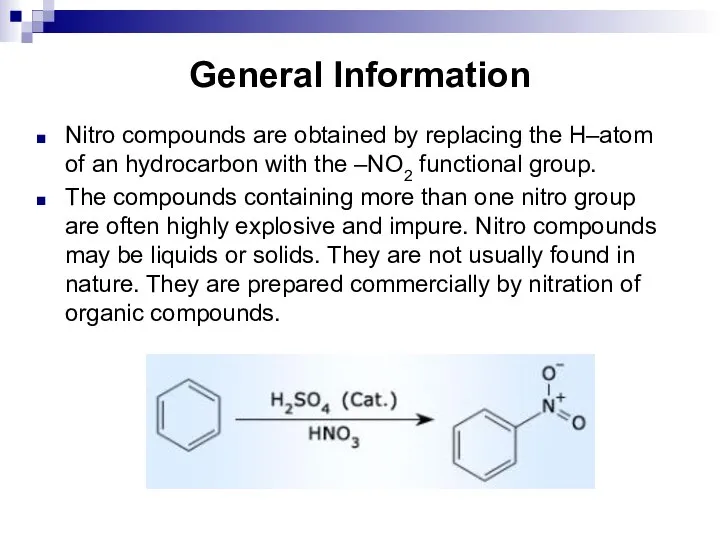
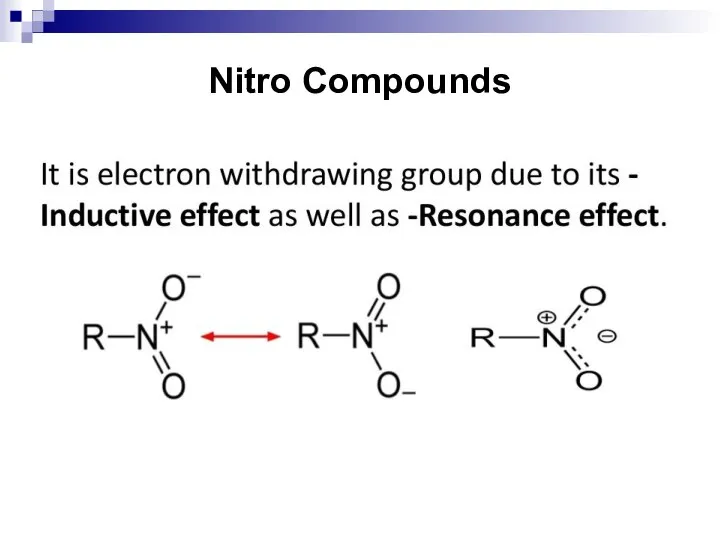
- 118. General Information Nitro compounds are obtained by replacing the H–atom of an hydrocarbon with the –NO2
- 119. Nitro Compounds Nitro compounds are present in the following forms in the nature: 3–Nitropropionic acid found
- 120. Nitro Compounds
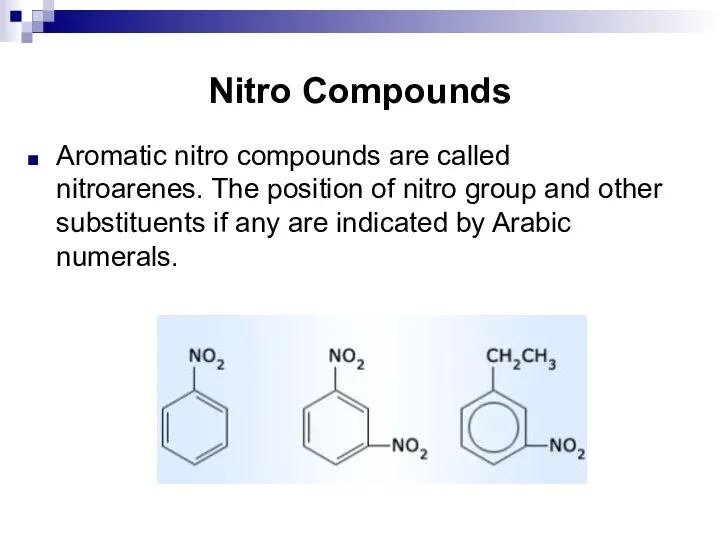
- 121. Nitro Compounds Aromatic nitro compounds are called nitroarenes. The position of nitro group and other substituents
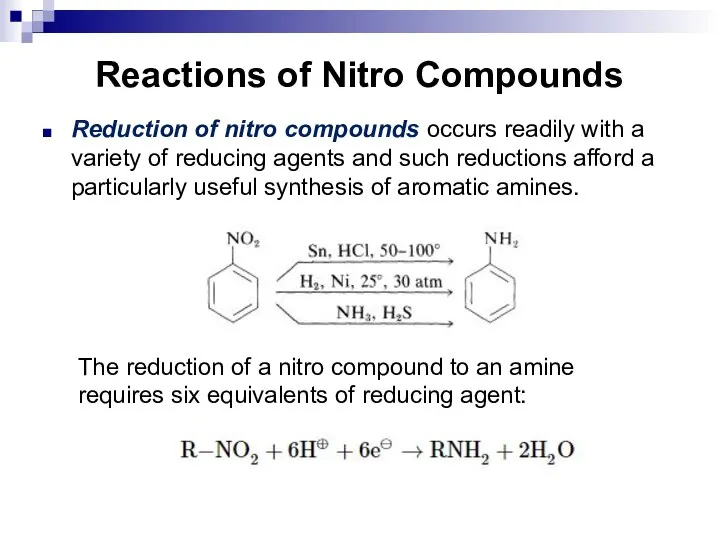
- 122. Reactions of Nitro Compounds Reduction of nitro compounds occurs readily with a variety of reducing agents
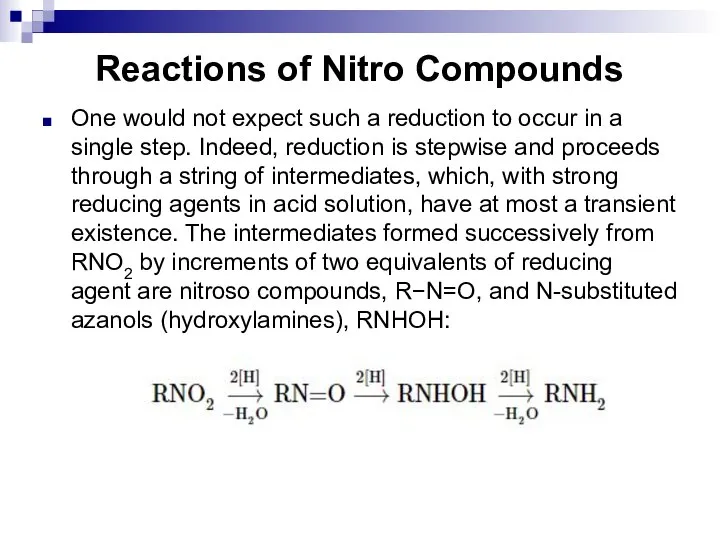
- 123. Reactions of Nitro Compounds One would not expect such a reduction to occur in a single
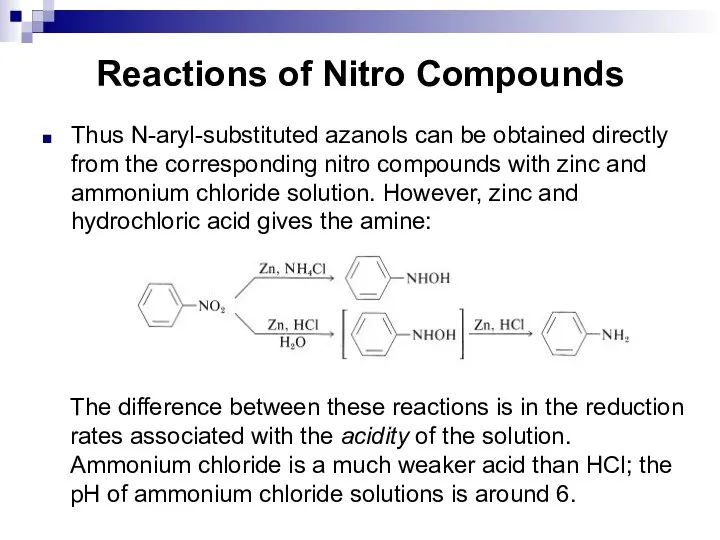
- 124. Reactions of Nitro Compounds Thus N-aryl-substituted azanols can be obtained directly from the corresponding nitro compounds
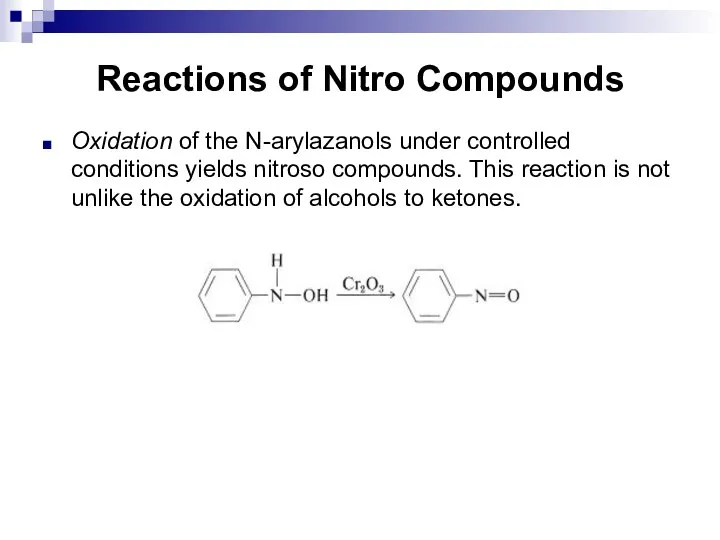
- 125. Reactions of Nitro Compounds Oxidation of the N-arylazanols under controlled conditions yields nitroso compounds. This reaction
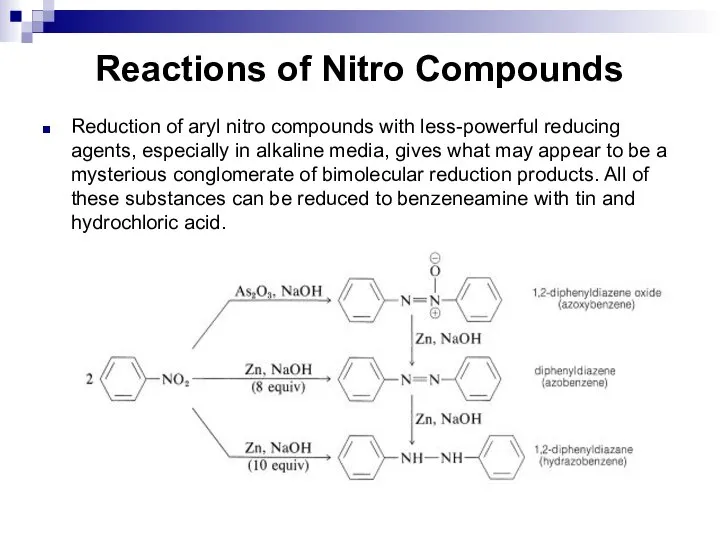
- 126. Reactions of Nitro Compounds Reduction of aryl nitro compounds with less-powerful reducing agents, especially in alkaline
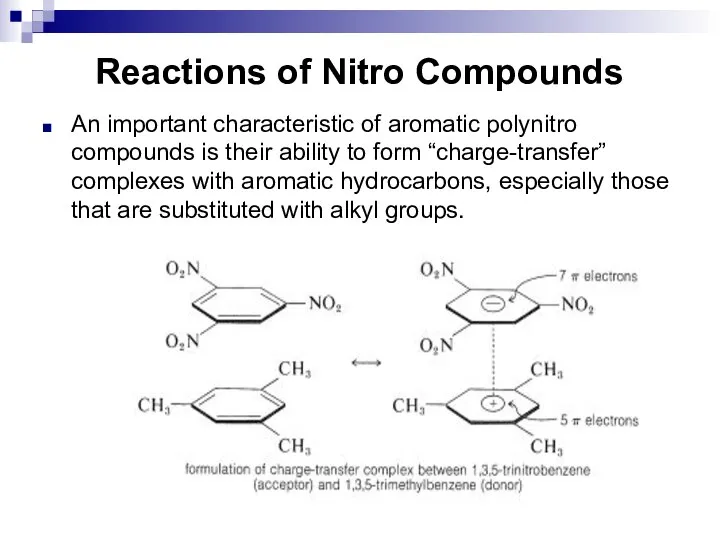
- 127. Reactions of Nitro Compounds An important characteristic of aromatic polynitro compounds is their ability to form
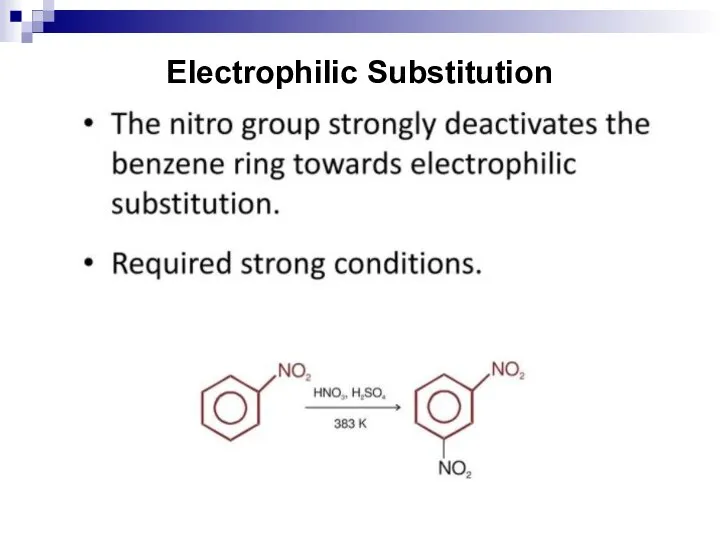
- 128. Electrophilic Substitution
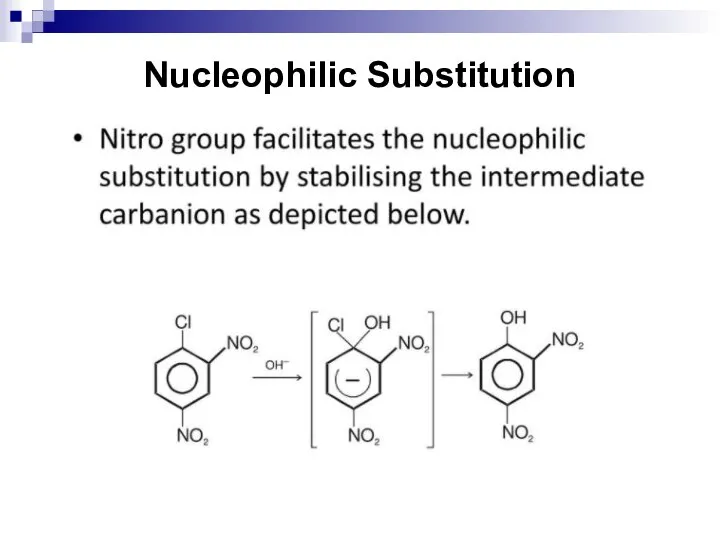
- 129. Nucleophilic Substitution
- 130. Summary In this lecture chemical properties of aromatic nitro compounds are considered. Reduction reactions are discussed
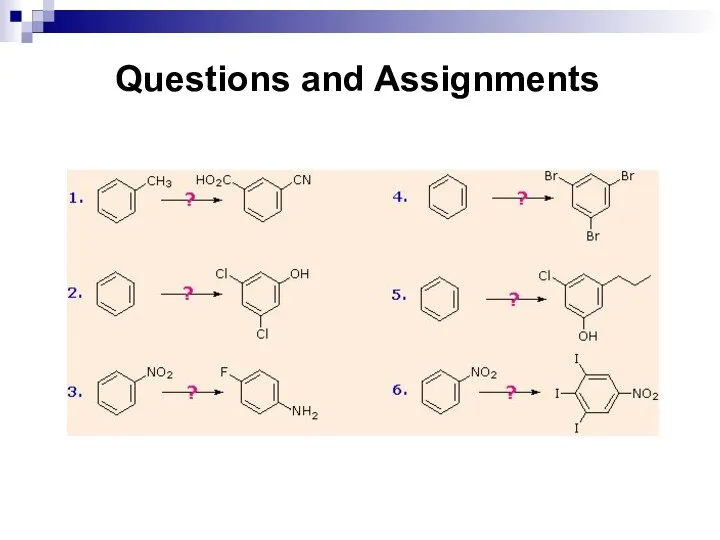
- 131. Questions and Assignments
- 132. Topic 6 Aromatic Amines
- 133. Outline of the lecture Aromatic Amines Naming Aromatic Amines Basicity of Aromatic Amines Reactions of Aromatic
- 134. Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May 2012]. Available from World
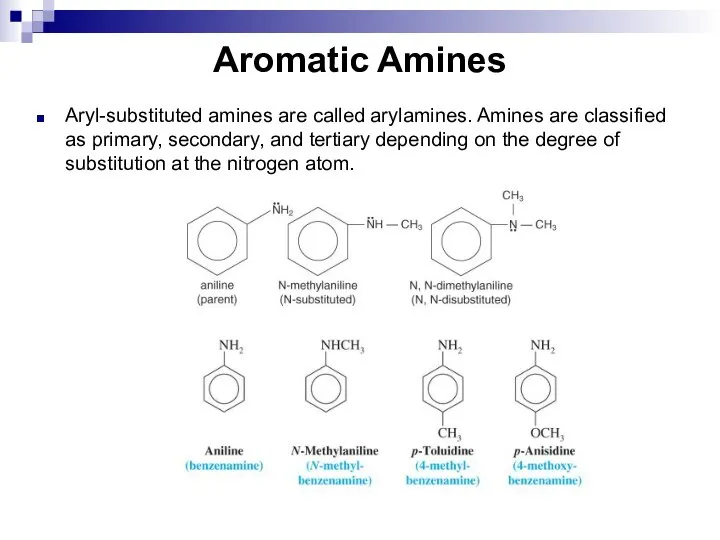
- 135. Aromatic Amines Aryl-substituted amines are called arylamines. Amines are classified as primary, secondary, and tertiary depending
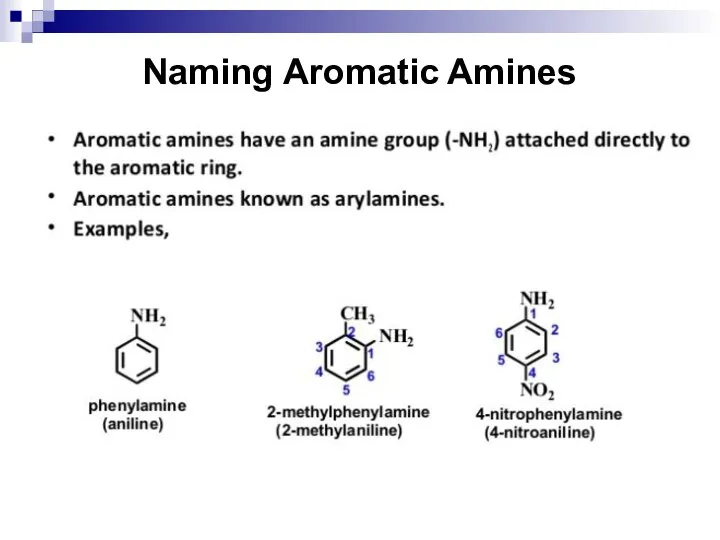
- 136. Naming Aromatic Amines
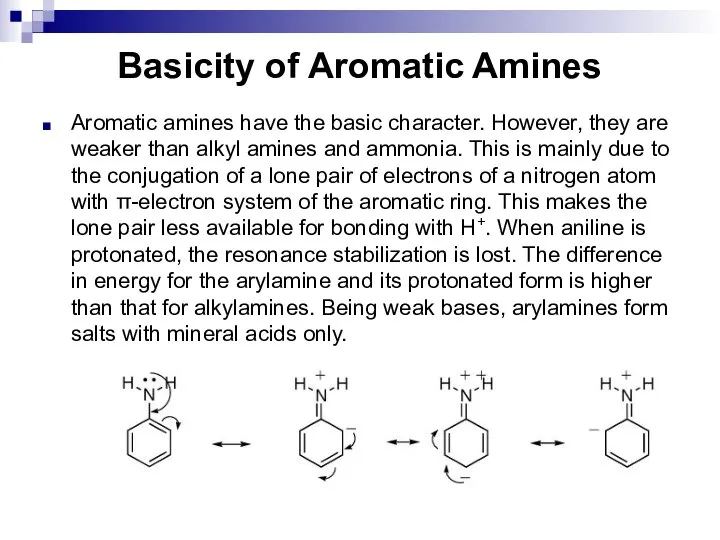
- 137. Basicity of Aromatic Amines Aromatic amines have the basic character. However, they are weaker than alkyl
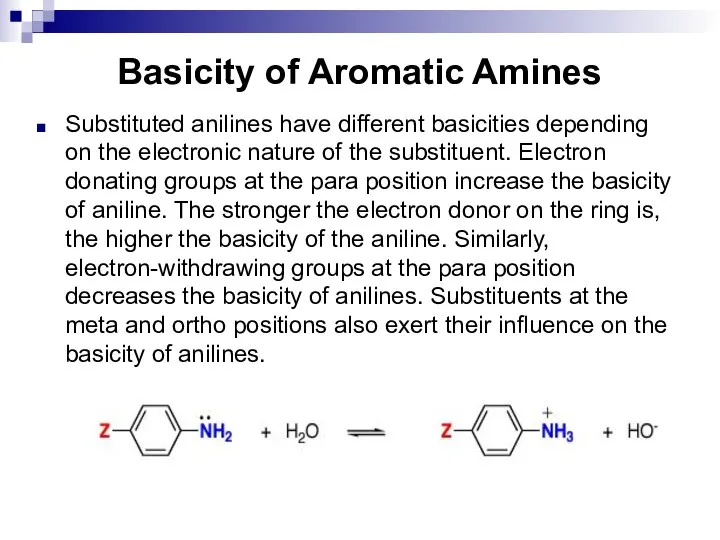
- 138. Basicity of Aromatic Amines Substituted anilines have different basicities depending on the electronic nature of the
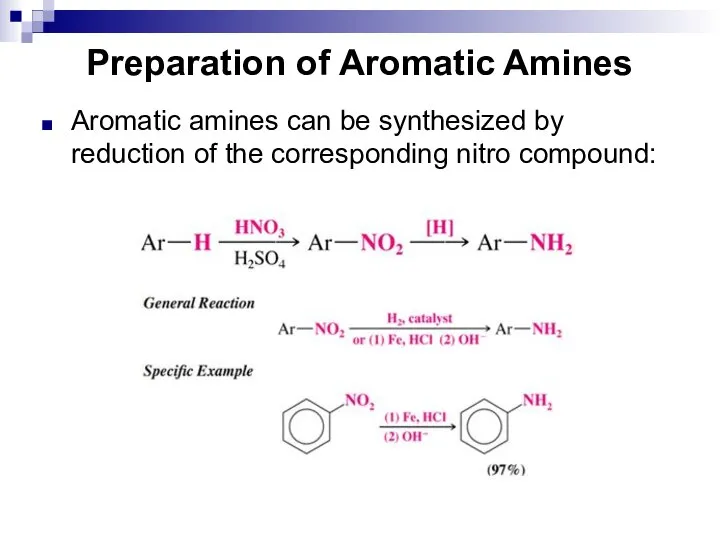
- 139. Preparation of Aromatic Amines Aromatic amines can be synthesized by reduction of the corresponding nitro compound:
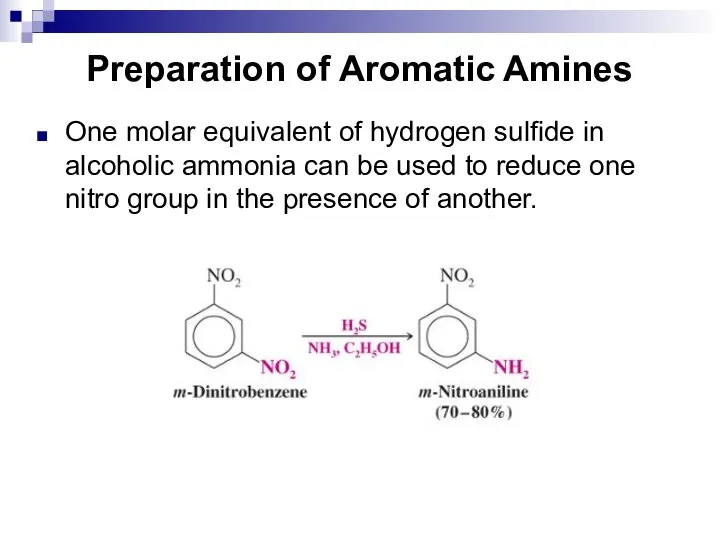
- 140. Preparation of Aromatic Amines One molar equivalent of hydrogen sulfide in alcoholic ammonia can be used
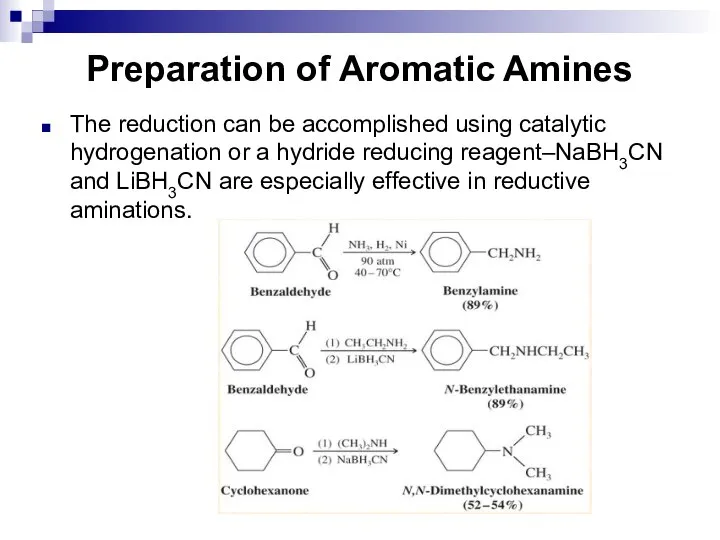
- 141. Preparation of Aromatic Amines The reduction can be accomplished using catalytic hydrogenation or a hydride reducing
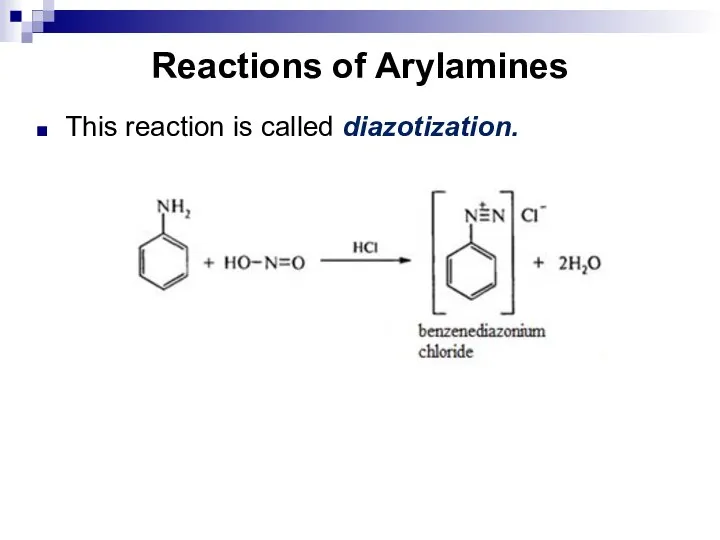
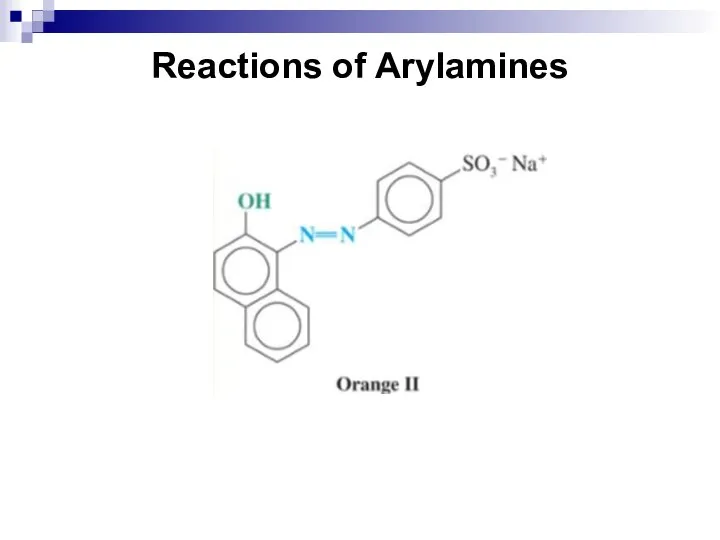
- 142. Reactions of Arylamines This reaction is called diazotization.
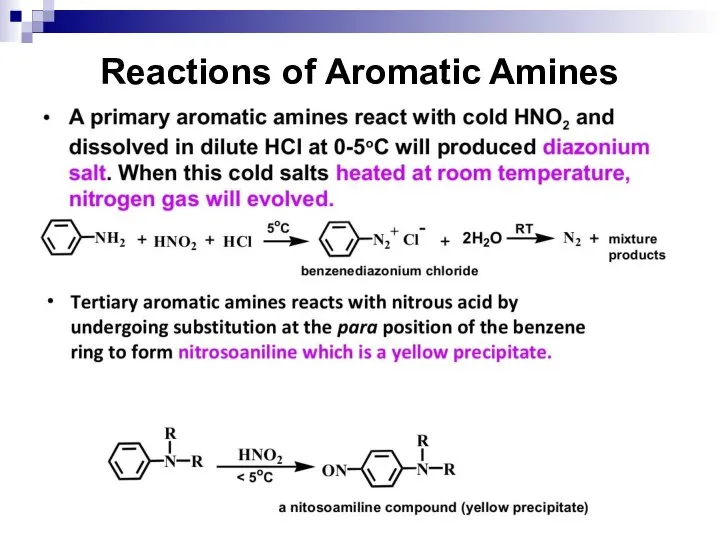
- 143. Reactions of Aromatic Amines
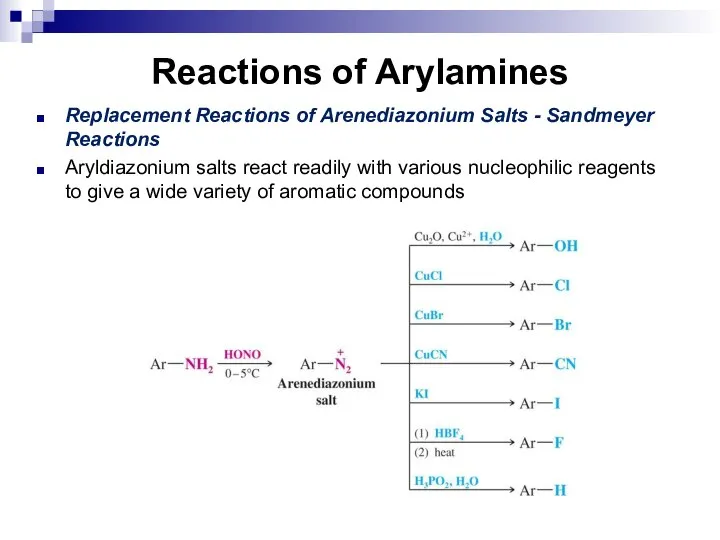
- 144. Reactions of Arylamines Replacement Reactions of Arenediazonium Salts - Sandmeyer Reactions Aryldiazonium salts react readily with
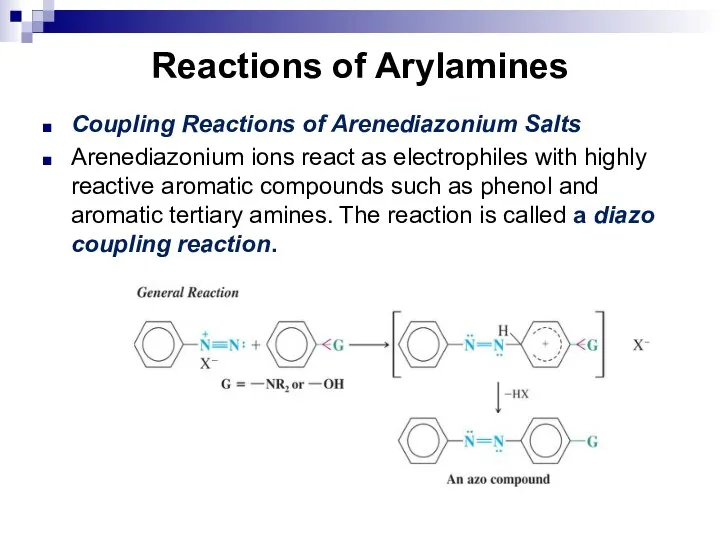
- 145. Reactions of Arylamines Coupling Reactions of Arenediazonium Salts Arenediazonium ions react as electrophiles with highly reactive
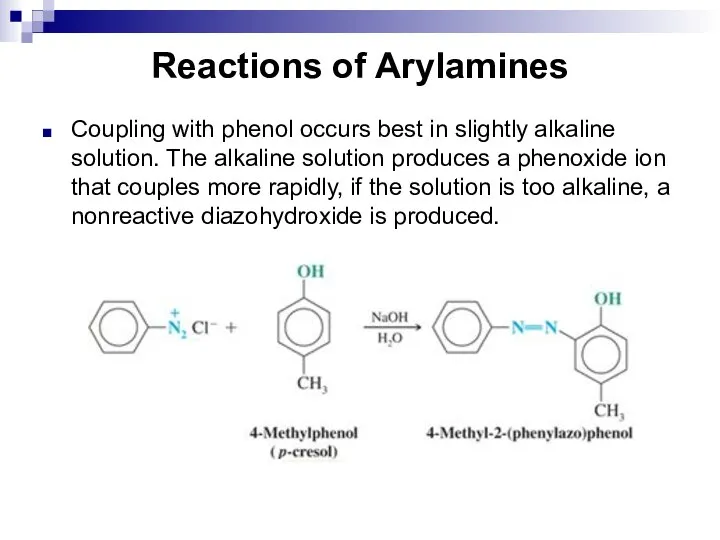
- 146. Reactions of Arylamines Coupling with phenol occurs best in slightly alkaline solution. The alkaline solution produces
- 147. Reactions of Arylamines Phenol and aniline derivatives undergo coupling almost exclusively at the para position unless
- 148. Reactions of Arylamines
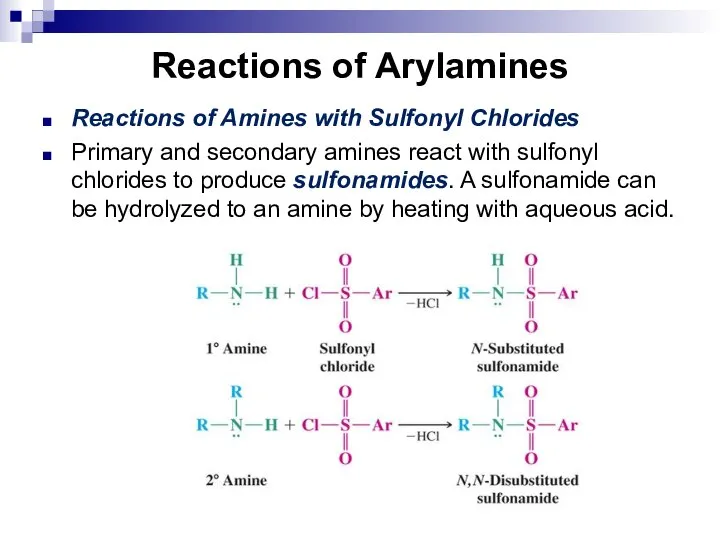
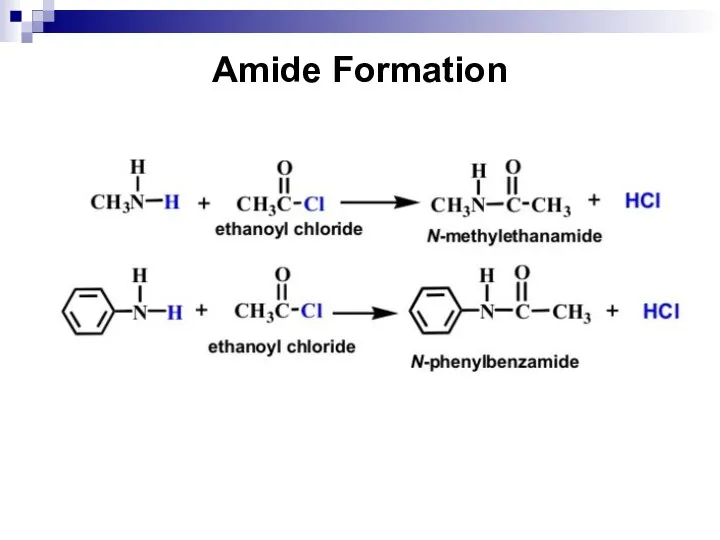
- 149. Reactions of Arylamines Reactions of Amines with Sulfonyl Chlorides Primary and secondary amines react with sulfonyl
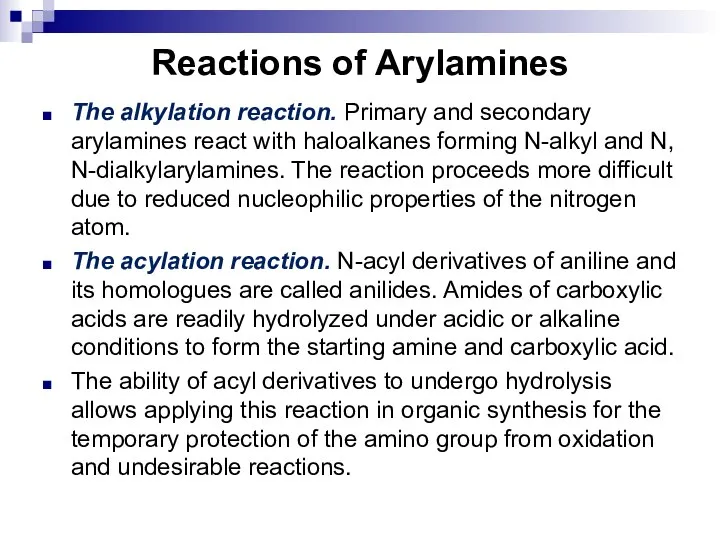
- 150. Reactions of Arylamines The alkylation reaction. Primary and secondary arylamines react with haloalkanes forming N-alkyl and
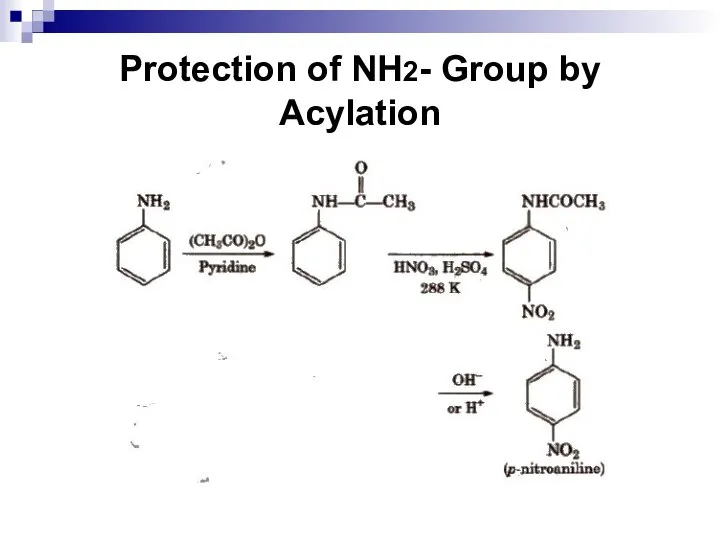
- 151. Protection of NH2- Group by Acylation
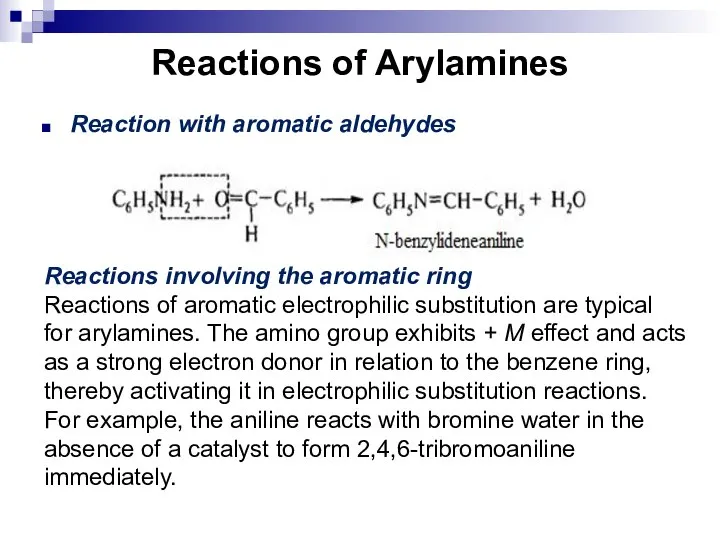
- 152. Reactions of Arylamines Reaction with aromatic aldehydes Reactions involving the aromatic ring Reactions of aromatic electrophilic
- 153. Amide Formation
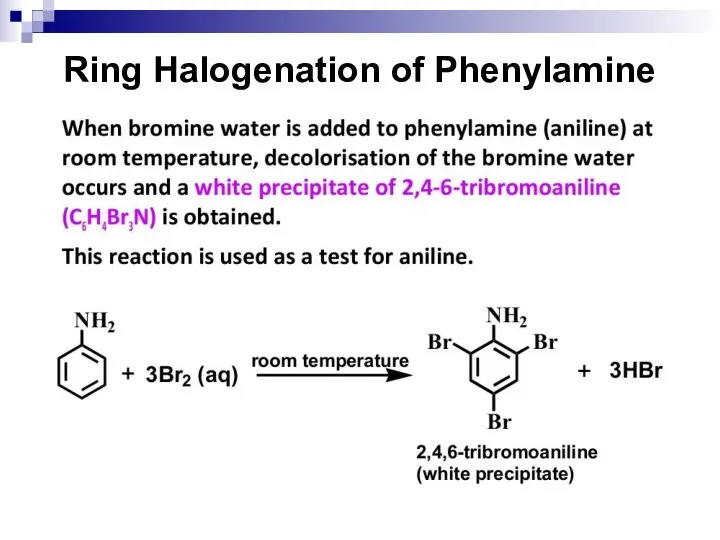
- 154. Ring Halogenation of Phenylamine
- 155. Summary In this lecture nomenclature, methods of synthesis as well as chemical properties of aromatic nitro
- 156. Questions and Assignments
- 157. Diazo and azo compounds Topic 7
- 158. Outline of the lecture Diazonium salts Preparation of Diazonium Salts Chemical Properties of Diazonium Salts
- 159. Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May 2012]. Available from World
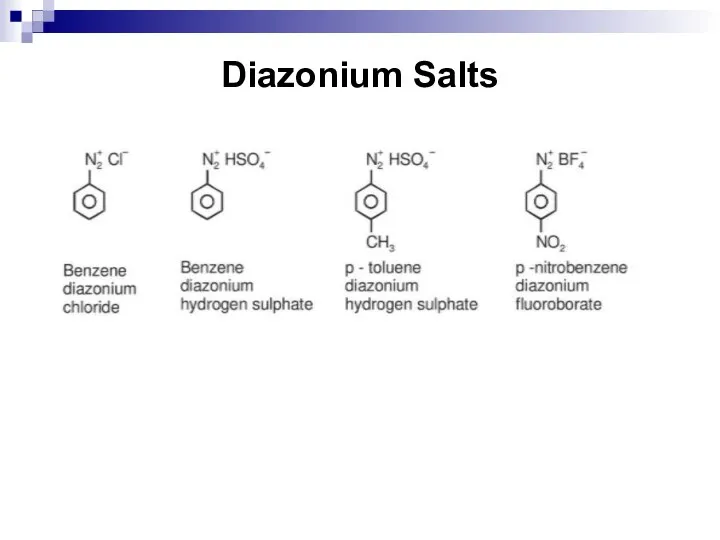
- 160. Diazonium Salts
- 161. Diazonium Salts
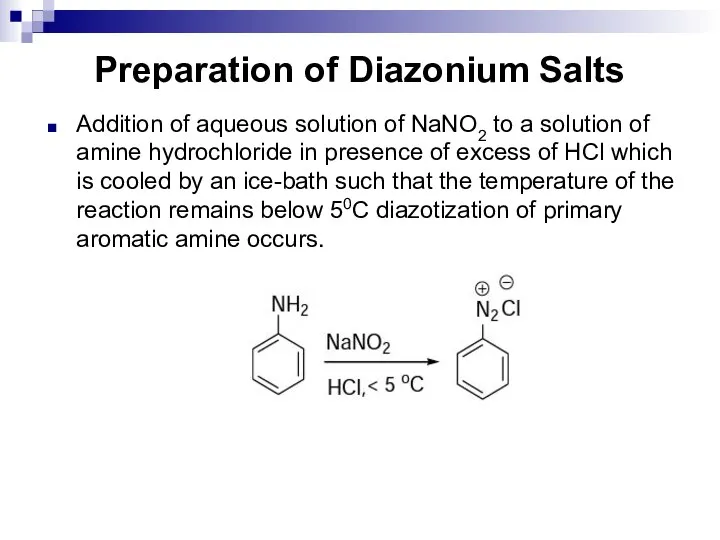
- 162. Preparation of Diazonium Salts Addition of aqueous solution of NaNO2 to a solution of amine hydrochloride
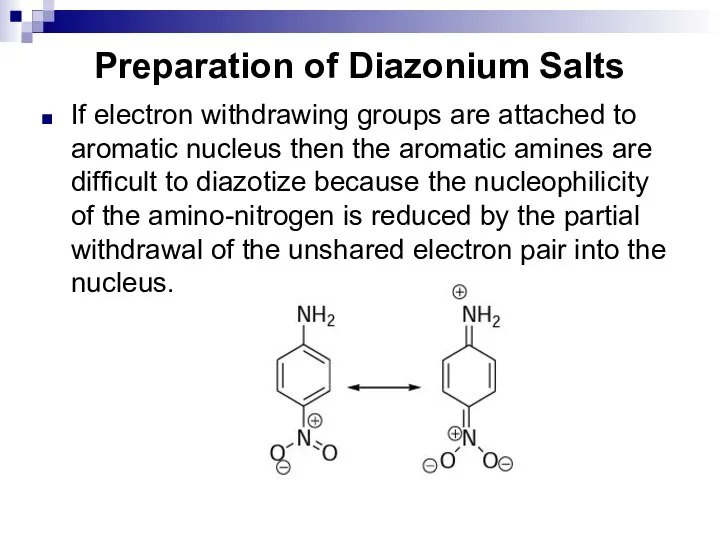
- 163. Preparation of Diazonium Salts If electron withdrawing groups are attached to aromatic nucleus then the aromatic
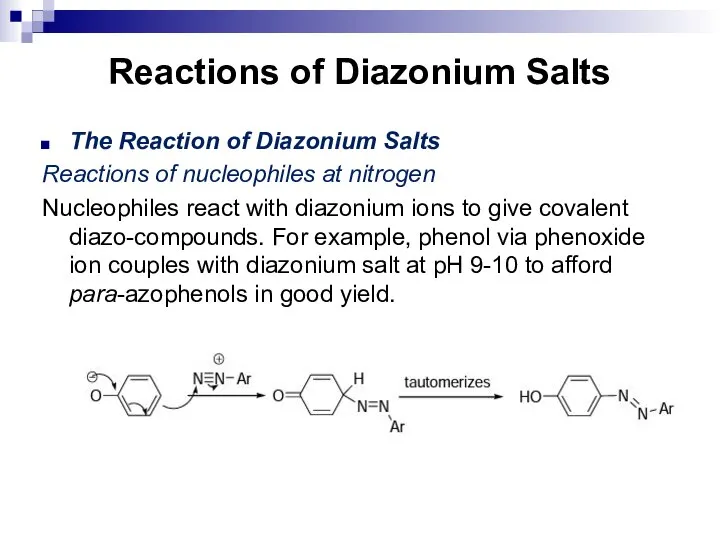
- 164. Reactions of Diazonium Salts The Reaction of Diazonium Salts Reactions of nucleophiles at nitrogen Nucleophiles react
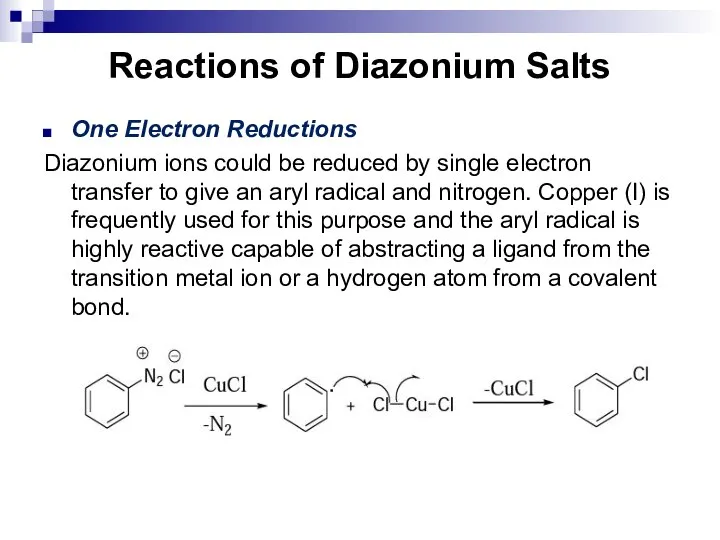
- 165. Reactions of Diazonium Salts One Electron Reductions Diazonium ions could be reduced by single electron transfer
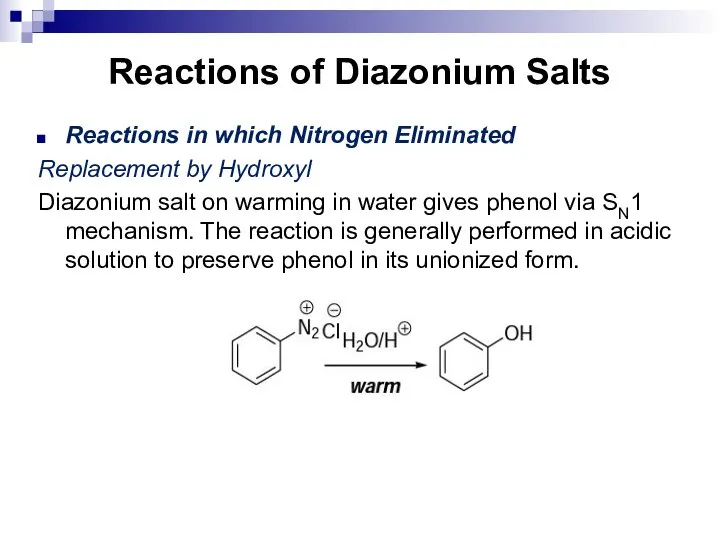
- 166. Reactions of Diazonium Salts Reactions in which Nitrogen Eliminated Replacement by Hydroxyl Diazonium salt on warming
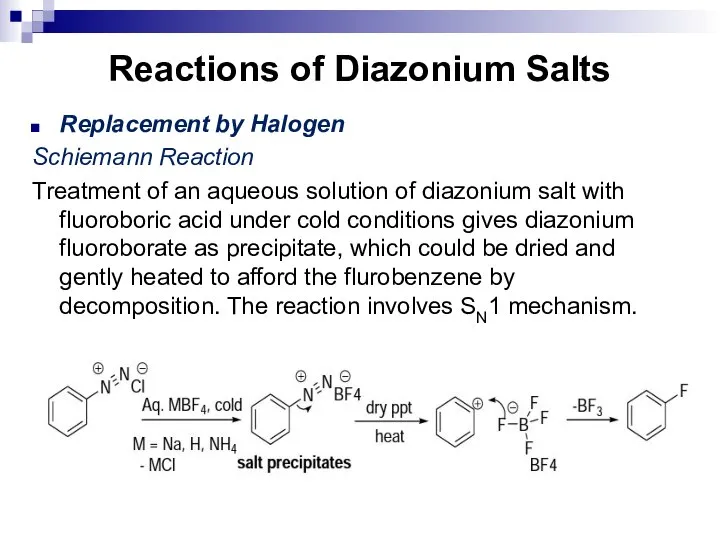
- 167. Reactions of Diazonium Salts Replacement by Halogen Schiemann Reaction Treatment of an aqueous solution of diazonium
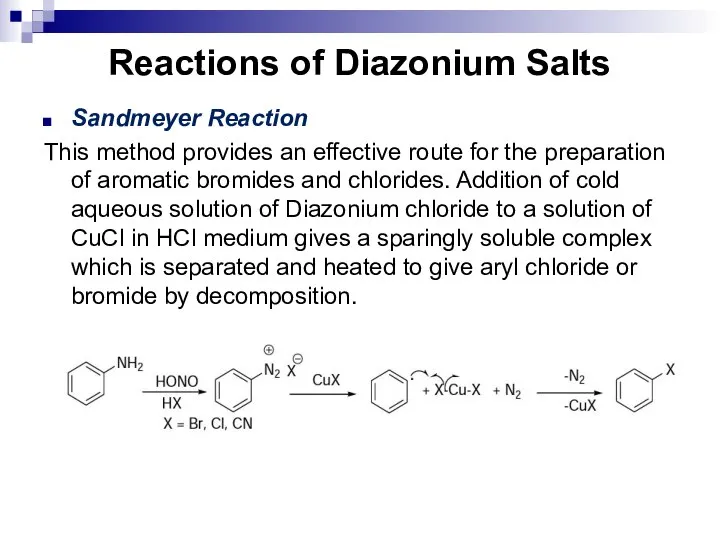
- 168. Reactions of Diazonium Salts Sandmeyer Reaction This method provides an effective route for the preparation of
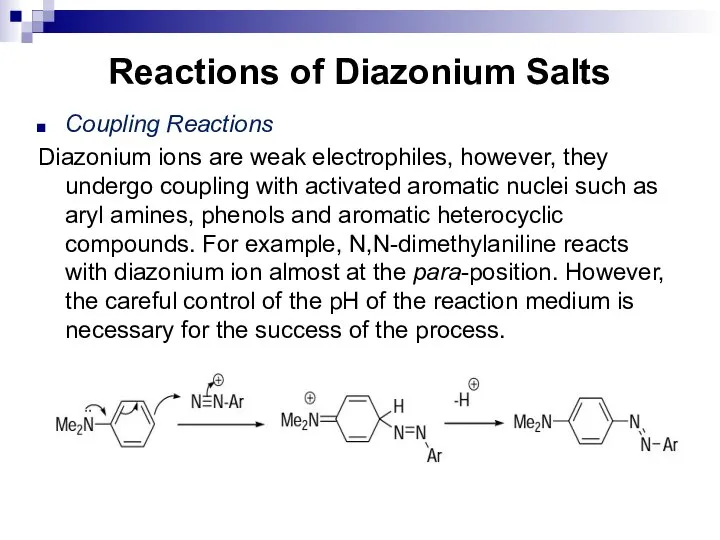
- 169. Reactions of Diazonium Salts Coupling Reactions Diazonium ions are weak electrophiles, however, they undergo coupling with
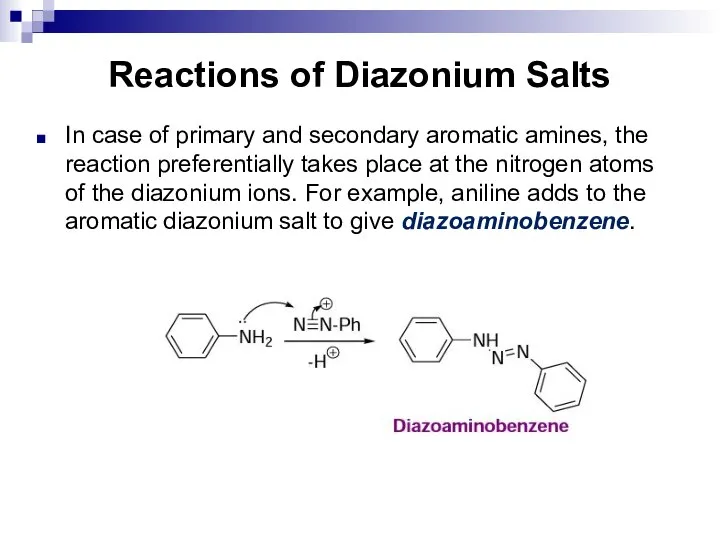
- 170. Reactions of Diazonium Salts In case of primary and secondary aromatic amines, the reaction preferentially takes
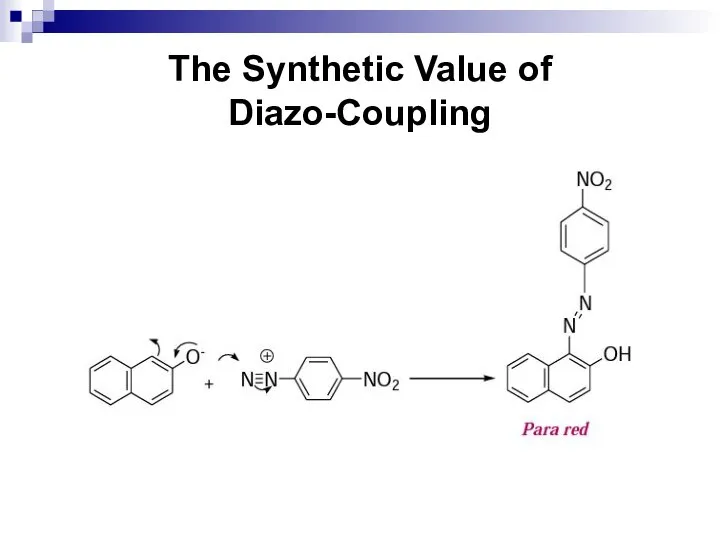
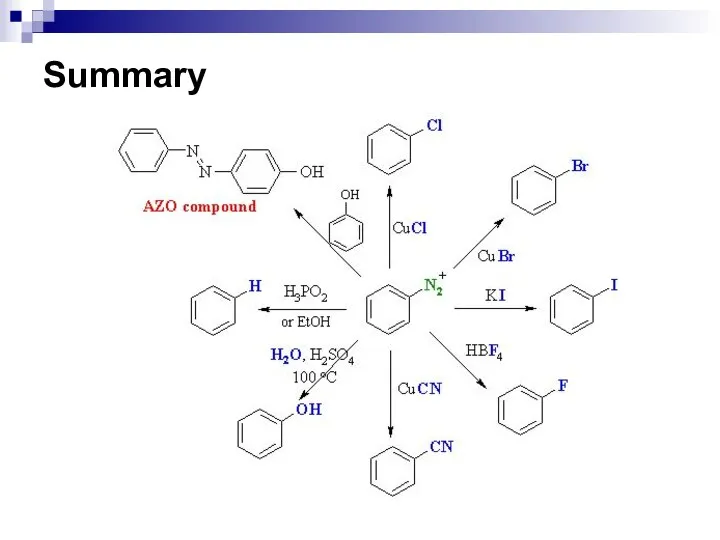
- 171. The Synthetic Value of Diazo-Coupling Dye-stuffs Aromatic azo-compounds are coloured. Several of those compounds synthesized by
- 172. The Synthetic Value of Diazo-Coupling
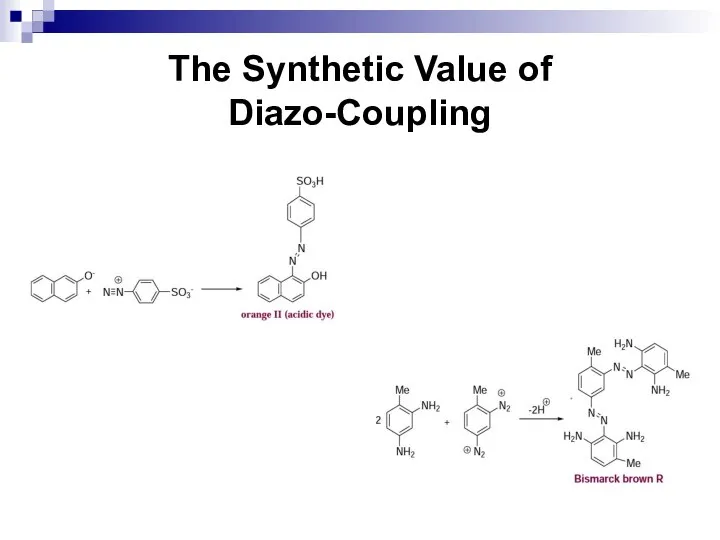
- 173. The Synthetic Value of Diazo-Coupling The second group of azo-compounds posses either a sulfonic acid group
- 174. The Synthetic Value of Diazo-Coupling
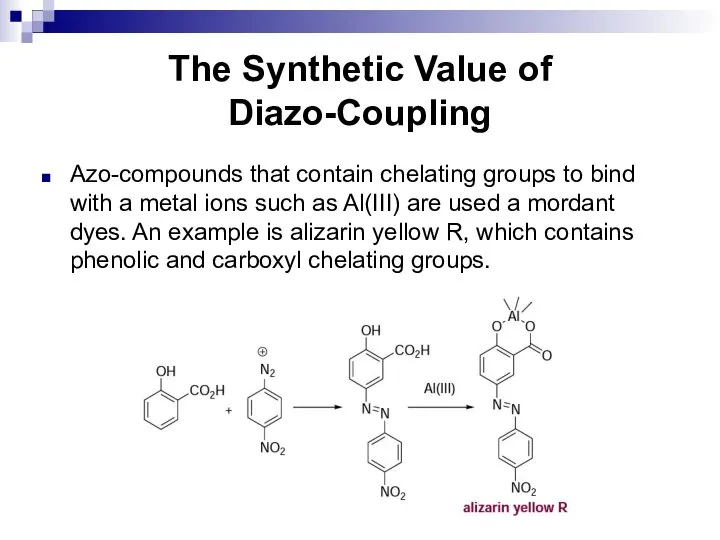
- 175. The Synthetic Value of Diazo-Coupling Azo-compounds that contain chelating groups to bind with a metal ions
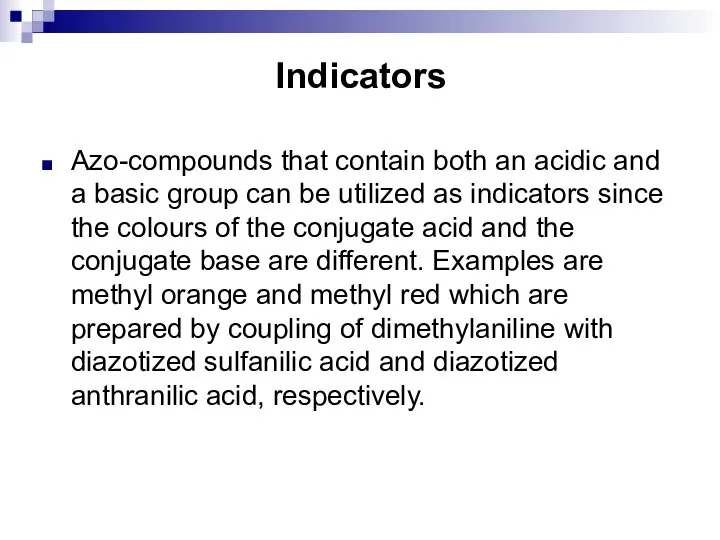
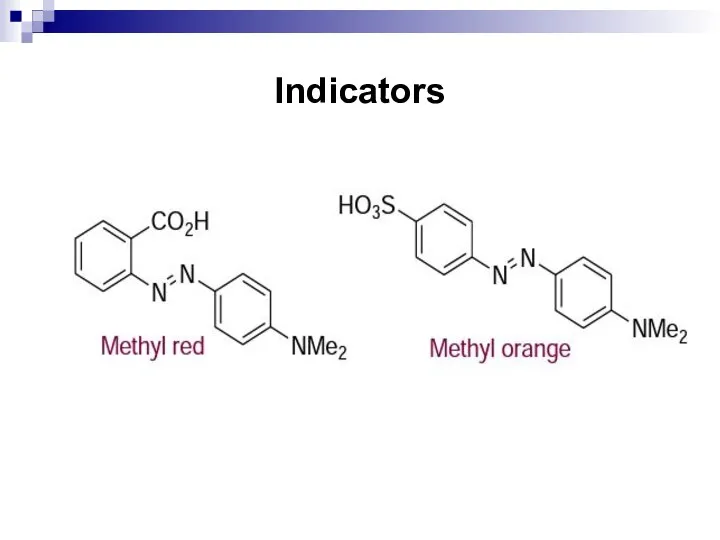
- 176. Indicators Azo-compounds that contain both an acidic and a basic group can be utilized as indicators
- 177. Indicators
- 178. Application of Azo Dyes
- 179. Summary
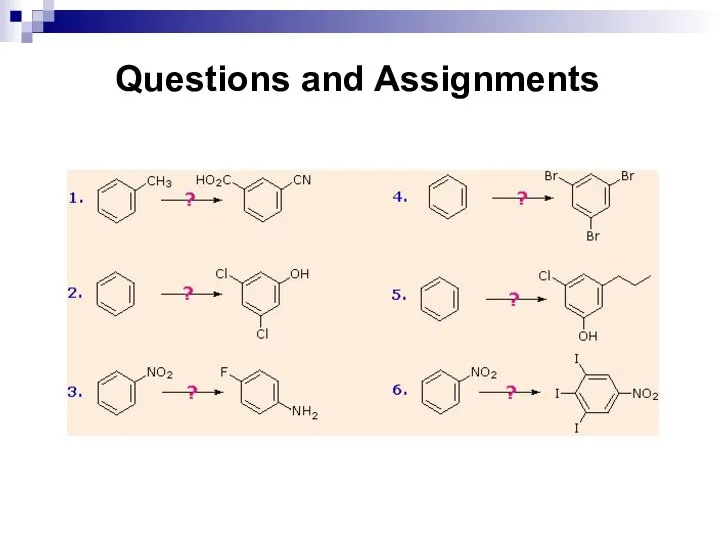
- 180. Questions and Assignments
- 181. Topic 8 Phenols
- 182. Outline of the lecture Phenols Naming Phenols Chemical Properties of Phenols. Acidity Ester Formation Ether Formation
- 183. Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May 2012]. Available from World
- 184. Phenols Phenol is a compound that has a hydroxyl group bonded to one carbon atom in
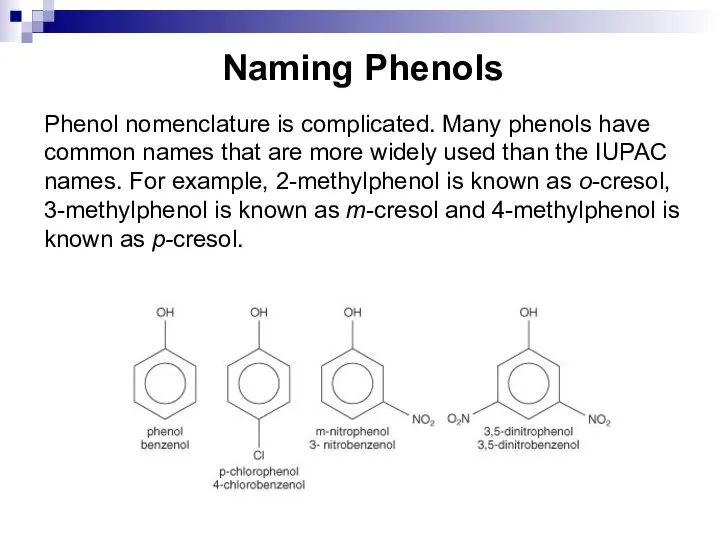
- 185. Naming Phenols Phenol nomenclature is complicated. Many phenols have common names that are more widely used
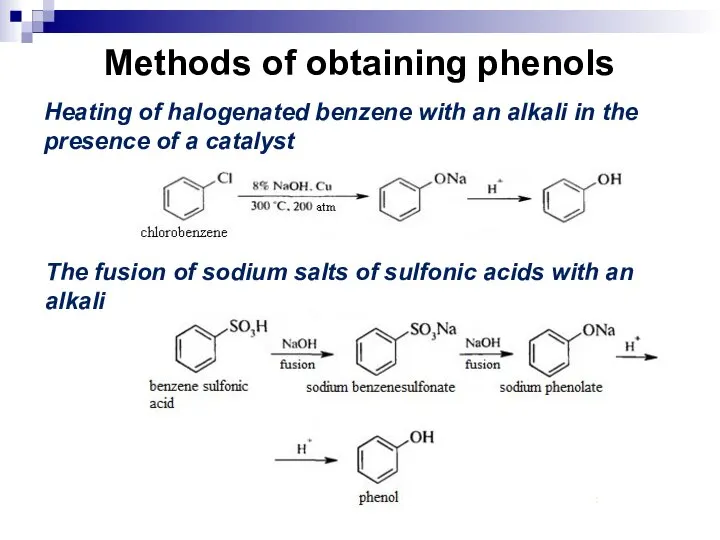
- 186. Methods of obtaining phenols Heating of halogenated benzene with an alkali in the presence of a
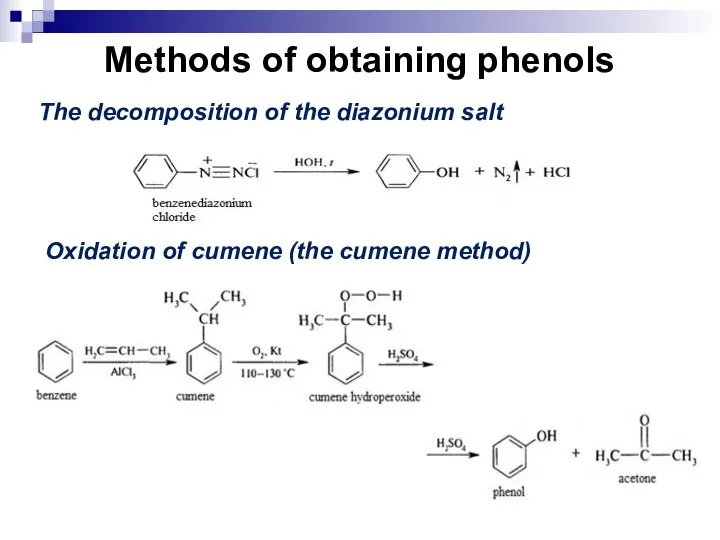
- 187. Methods of obtaining phenols The decomposition of the diazonium salt Oxidation of cumene (the cumene method)
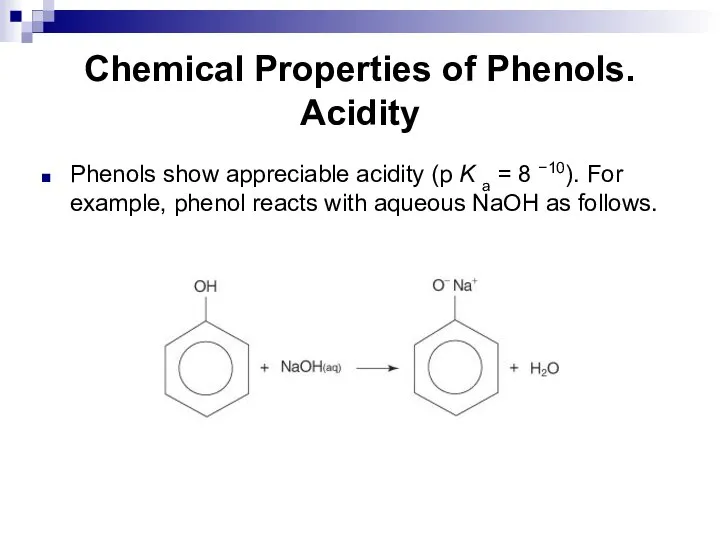
- 188. Chemical Properties of Phenols. Acidity Phenols show appreciable acidity (p K a = 8 −10). For
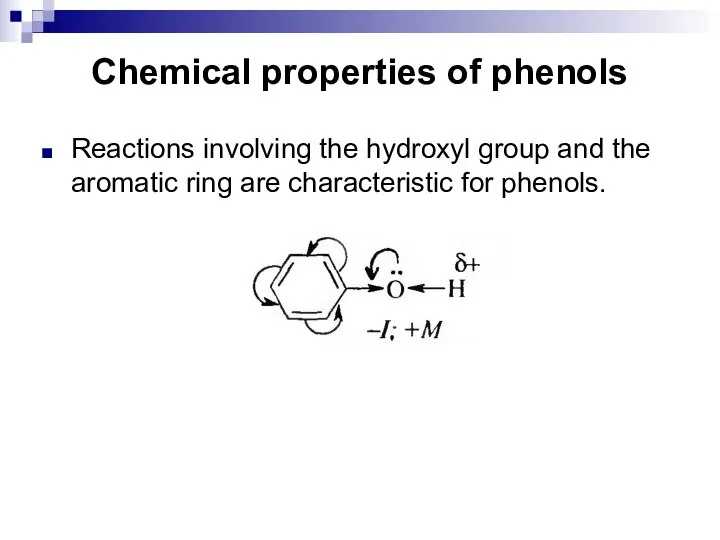
- 189. Chemical properties of phenols Reactions involving the hydroxyl group and the aromatic ring are characteristic for
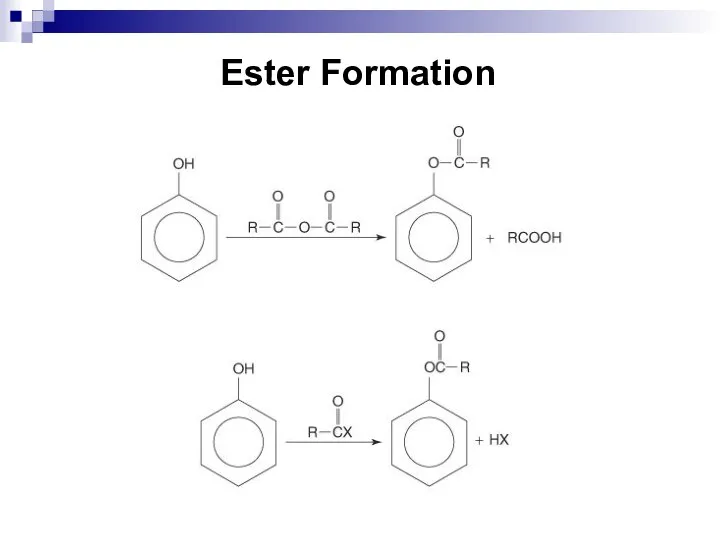
- 190. Ester Formation
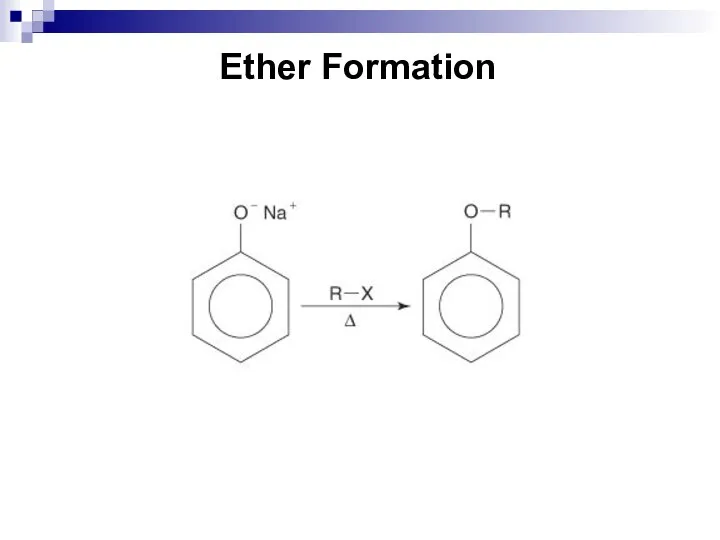
- 191. Ether Formation
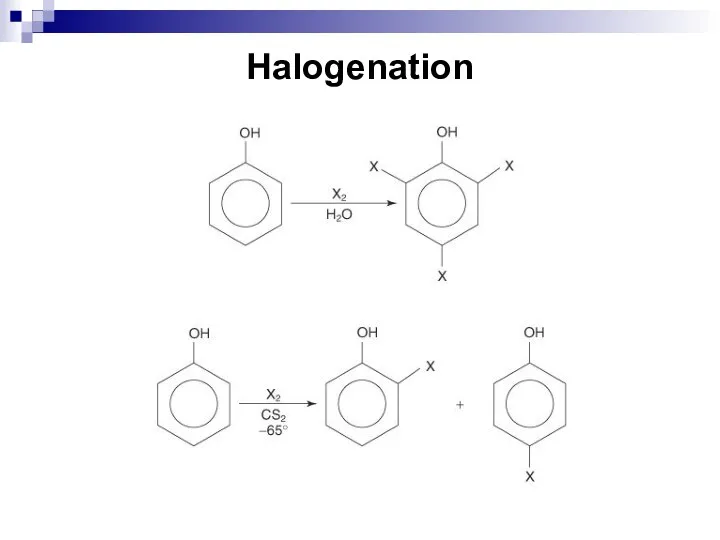
- 192. Halogenation
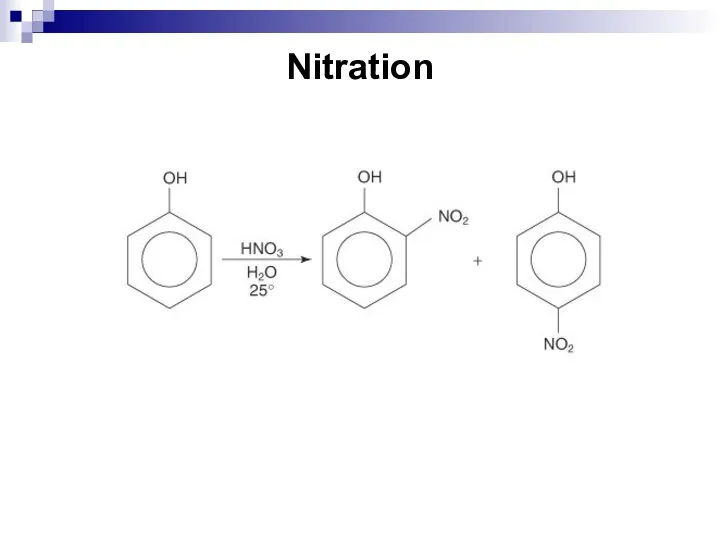
- 193. Nitration
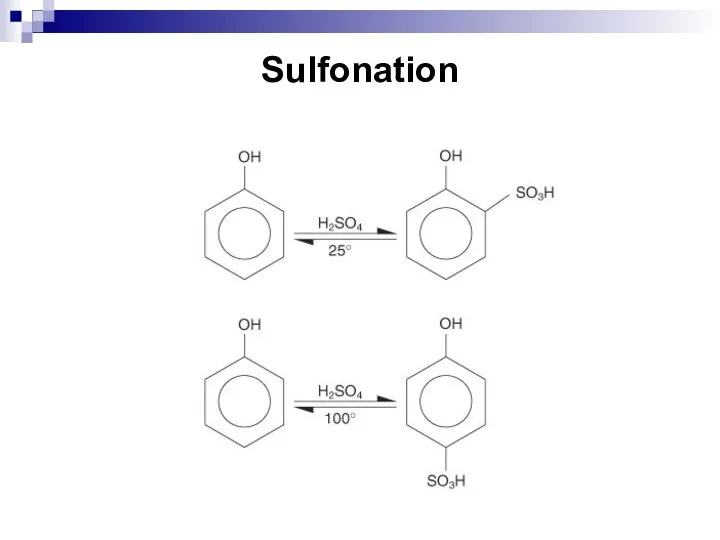
- 194. Sulfonation
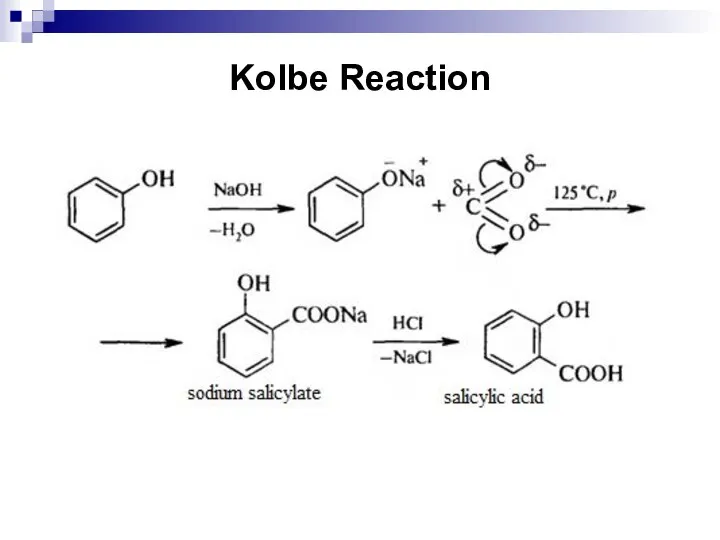
- 195. Kolbe Reaction
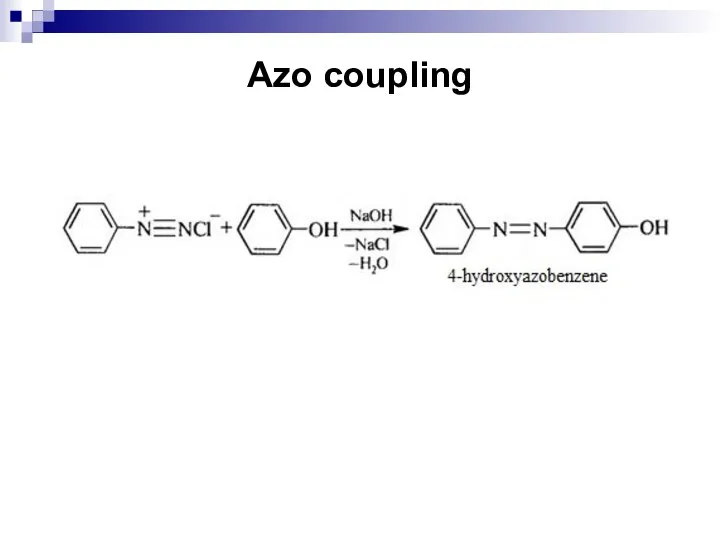
- 196. Azo coupling
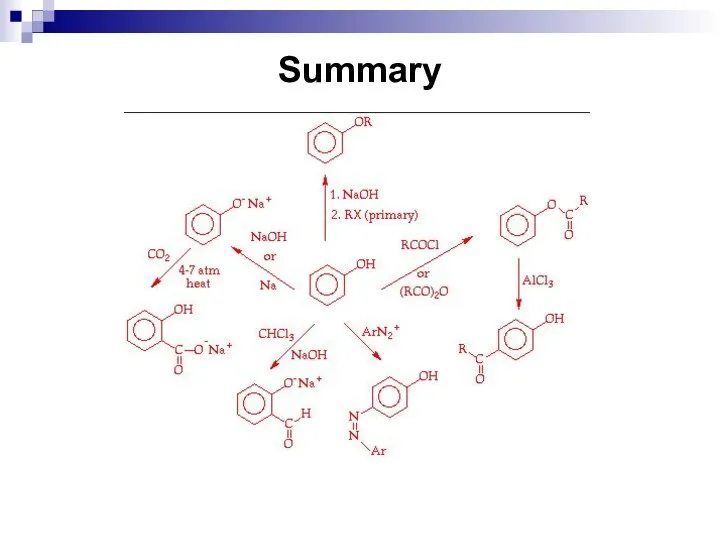
- 197. Summary
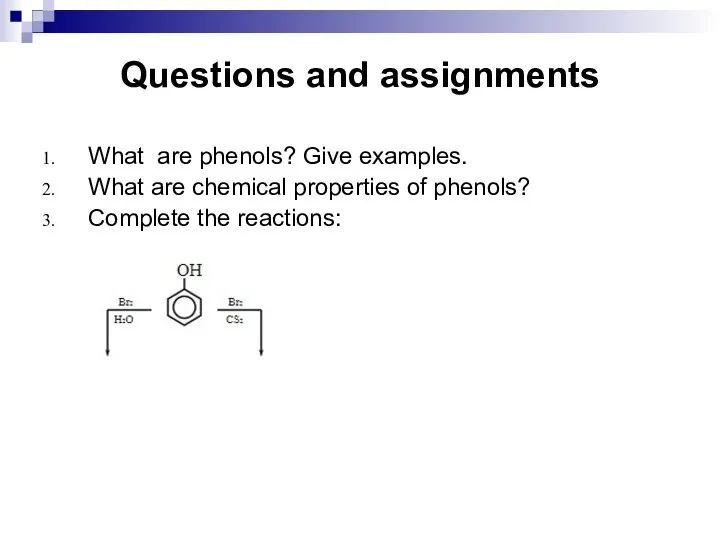
- 198. Questions and assignments What are phenols? Give examples. What are chemical properties of phenols? Complete the
- 199. Aromatic Aldehydes and Ketones Topic 9
- 200. Outline of the lecture Aromatic Aldehydes and Ketones Friedel-Crafts Acylation Chemical Properties of Aromatic Aldehydes and
- 201. Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May 2012]. Available from World
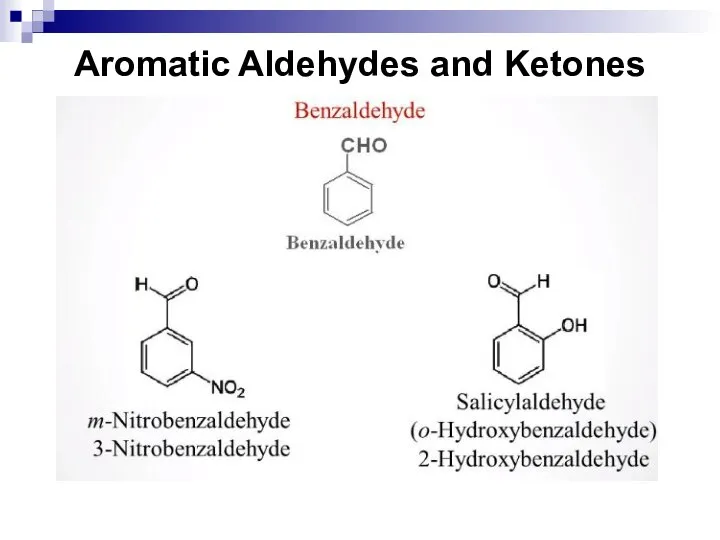
- 202. Aromatic Aldehydes and Ketones
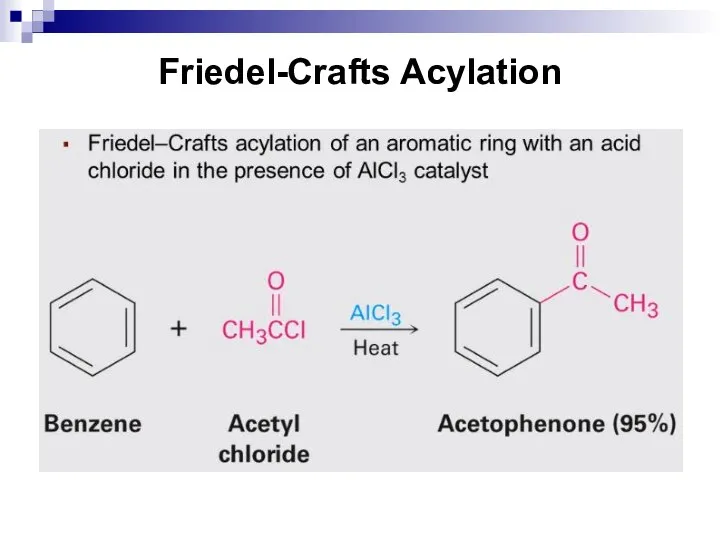
- 203. Friedel-Crafts Acylation
- 204. Chemical Properties of Aromatic Aldehydes and Ketones Aromatic aldehydes mostly have the same properties as aliphatic
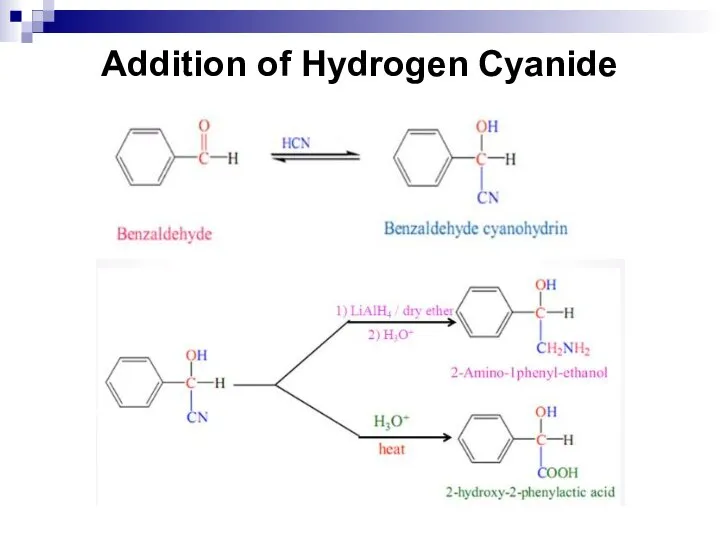
- 205. Addition of Hydrogen Cyanide
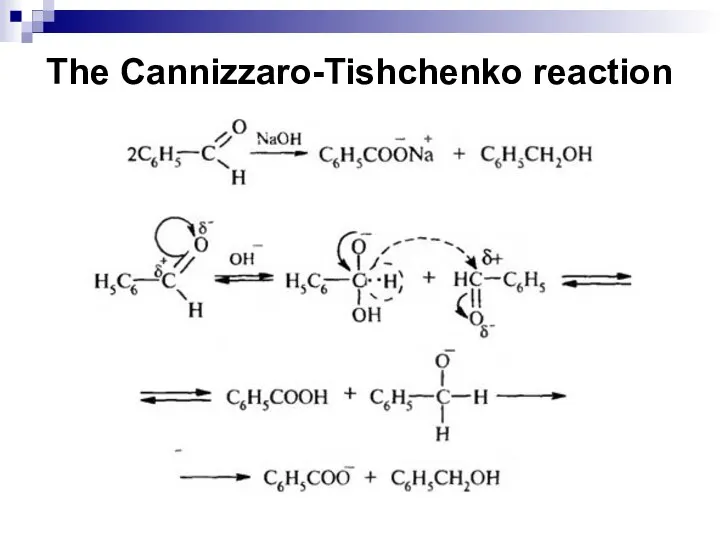
- 206. The Cannizzaro-Tishchenko reaction
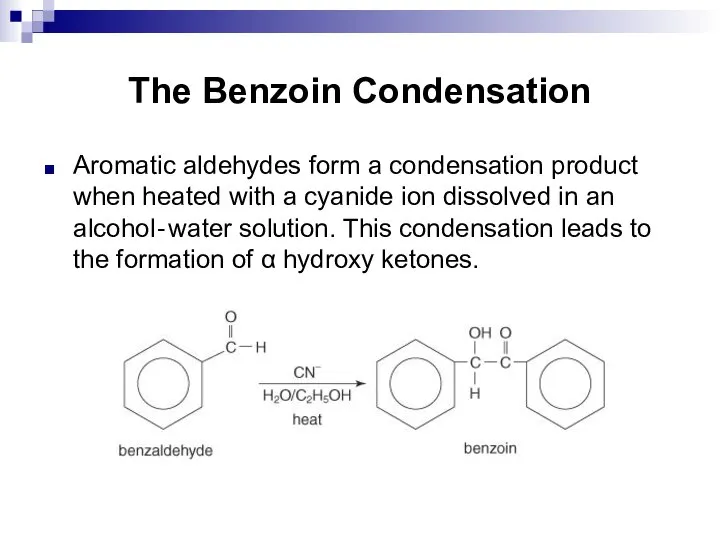
- 207. The Benzoin Condensation Aromatic aldehydes form a condensation product when heated with a cyanide ion dissolved
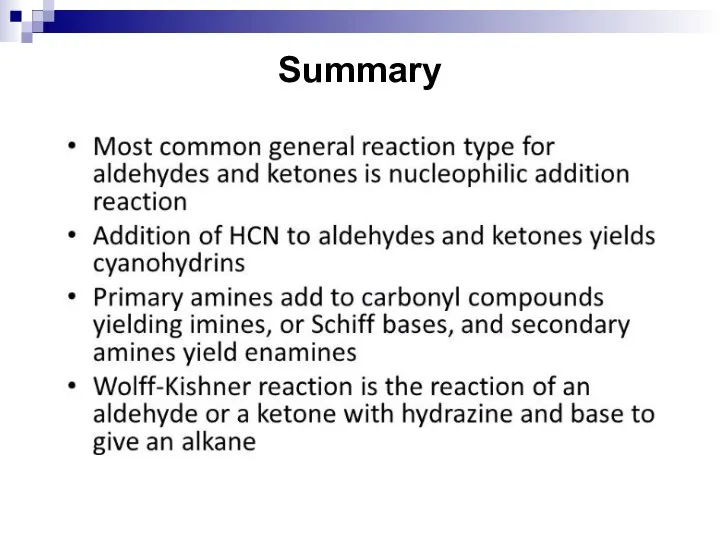
- 208. Summary
- 209. Questions and Assignments In which following reactions aromatic aldehyde is treated with acid anhydride in presence
- 210. Aromatic Carboxylic Acids and Their Derivatives Topic 10
- 211. Outline of the lecture Aromatic Carboxylic acids Side Chain Oxidation of Alkylbenzenes Synthesis of Amides Reduction
- 212. Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May 2012]. Available from World
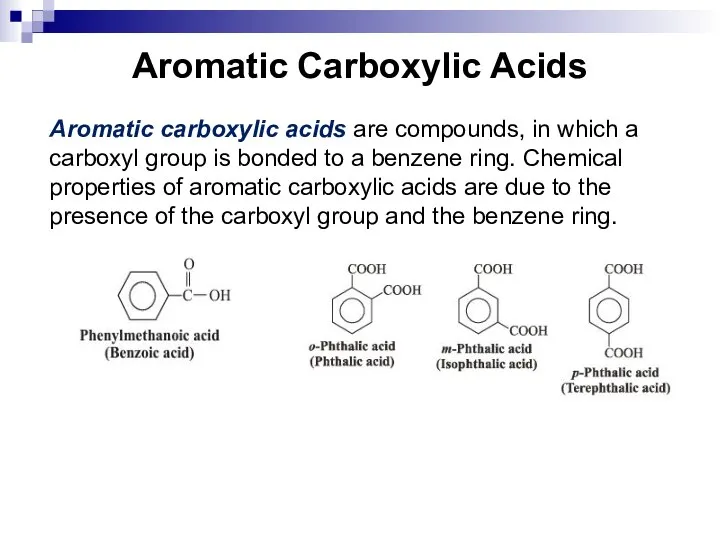
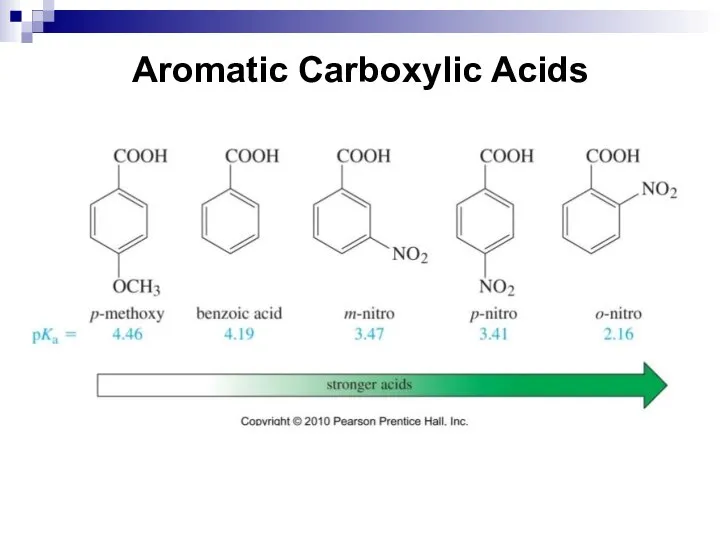
- 213. Aromatic Carboxylic Acids Aromatic carboxylic acids are compounds, in which a carboxyl group is bonded to
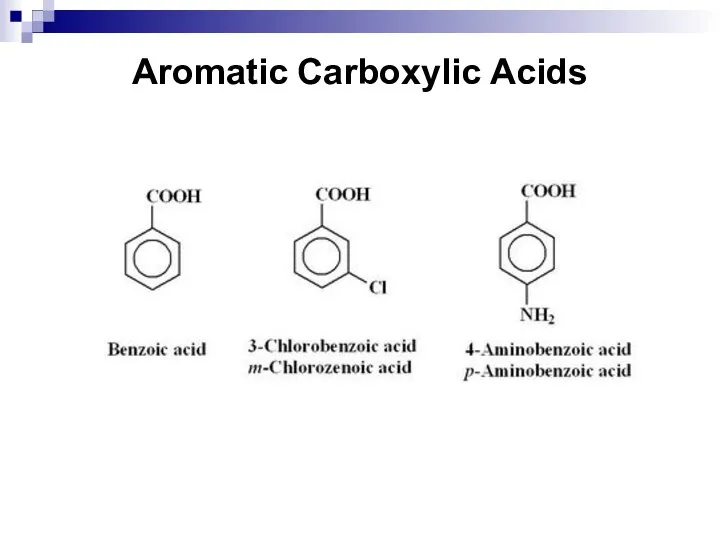
- 214. Aromatic Carboxylic Acids
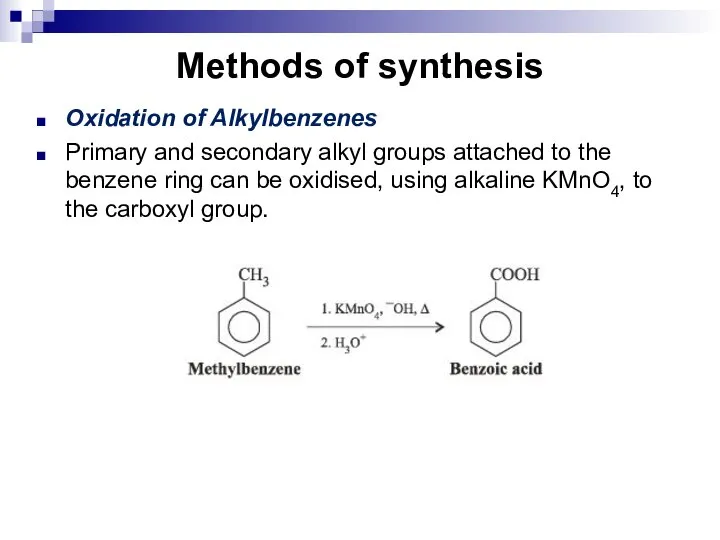
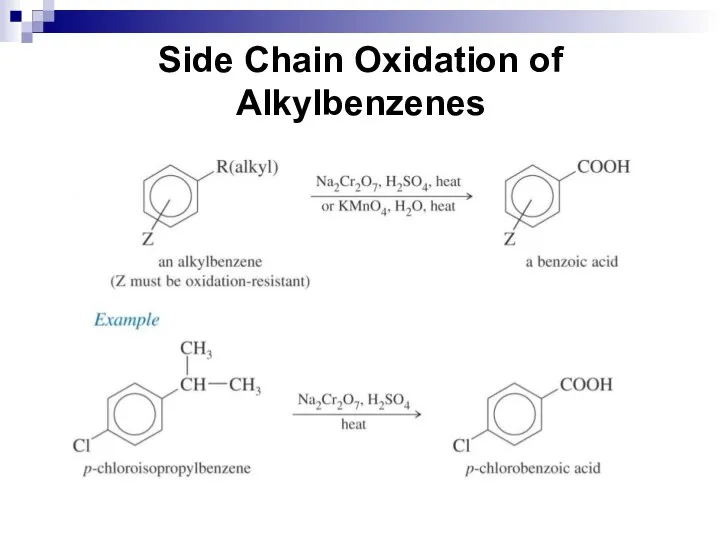
- 215. Methods of synthesis Oxidation of Alkylbenzenes Primary and secondary alkyl groups attached to the benzene ring
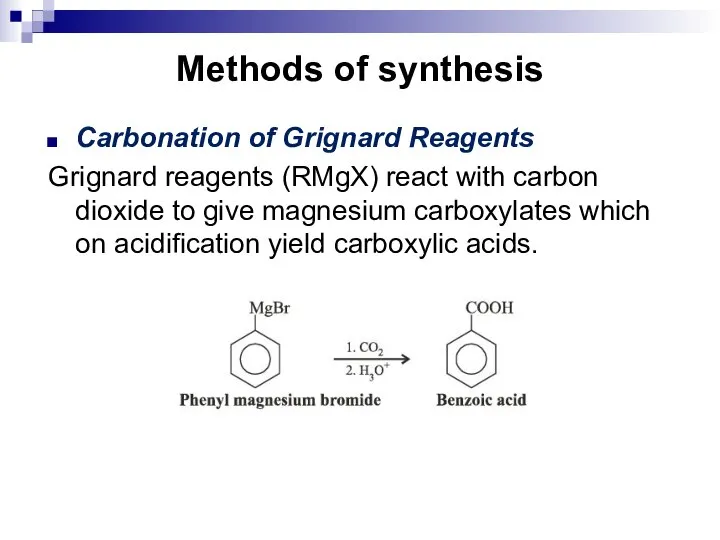
- 216. Methods of synthesis Carbonation of Grignard Reagents Grignard reagents (RMgX) react with carbon dioxide to give
- 217. Aromatic Carboxylic Acids
- 218. Side Chain Oxidation of Alkylbenzenes
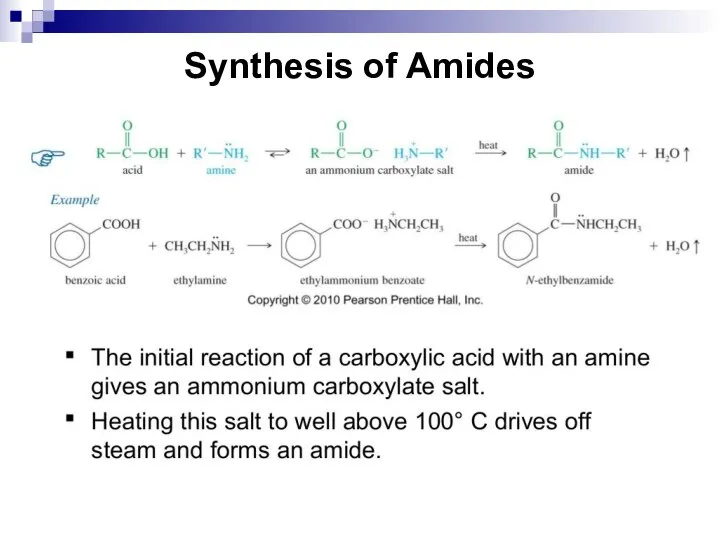
- 219. Synthesis of Amides
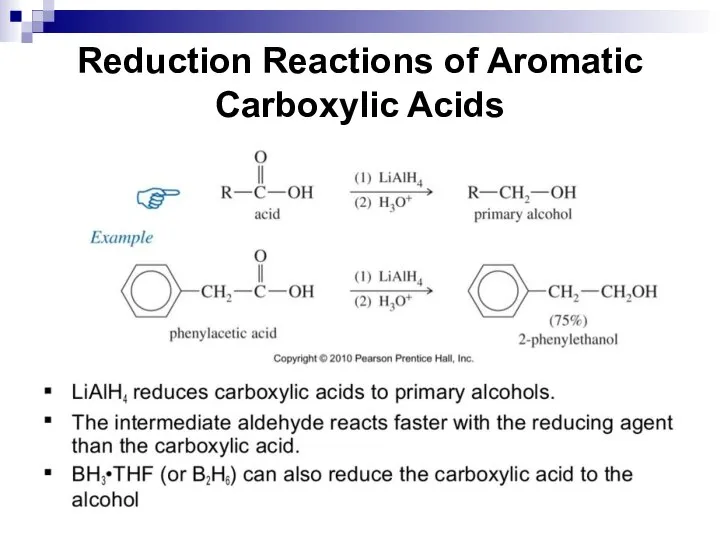
- 220. Reduction Reactions of Aromatic Carboxylic Acids
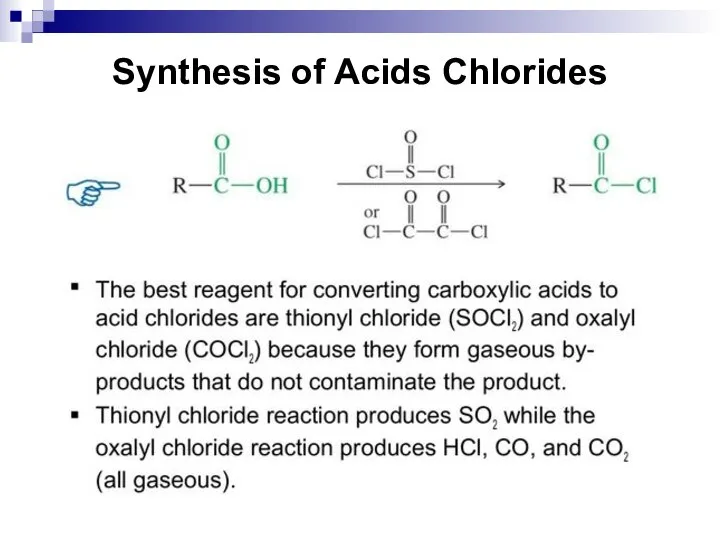
- 221. Synthesis of Acids Chlorides
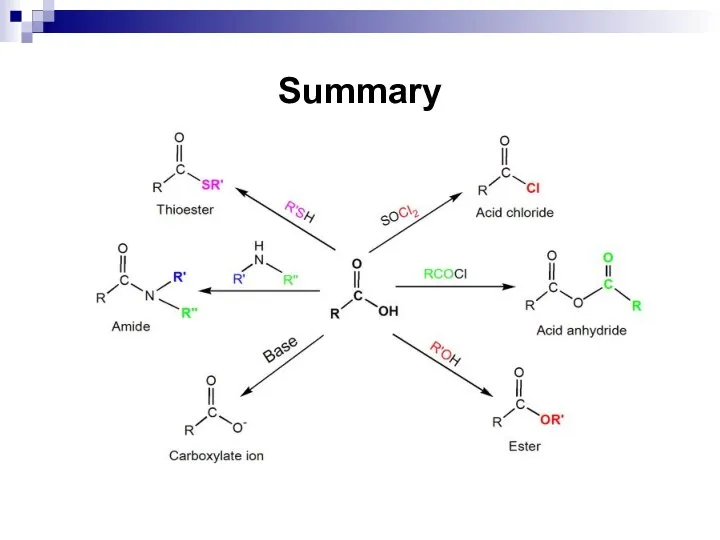
- 222. Summary
- 223. Questions and Assignments What are carboxylic acids? Give examples. Draw a structure of p-aminobenzoic acid. What
- 224. Polynuclear Aromatic Compounds Topic 11
- 225. Outline of the lecture Polynuclear Aromatic Compounds SStructure and Structure and CStructure and Chemical Structure and
- 226. Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May 2012]. Available from World
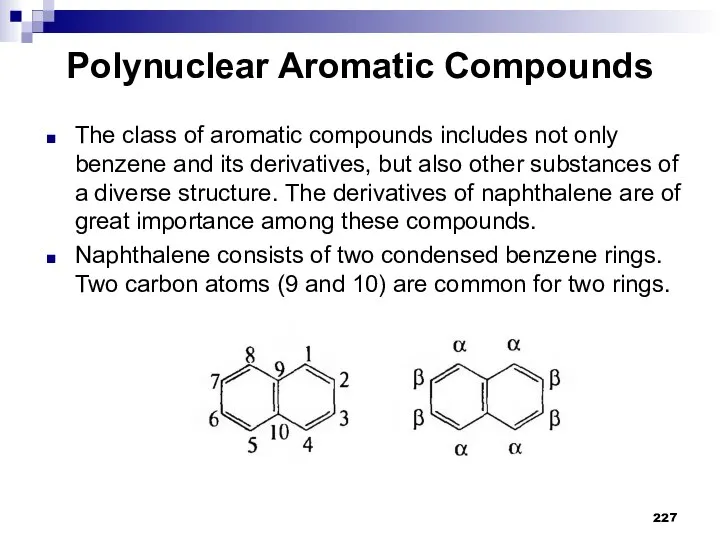
- 227. Polynuclear Aromatic Compounds The class of aromatic compounds includes not only benzene and its derivatives, but
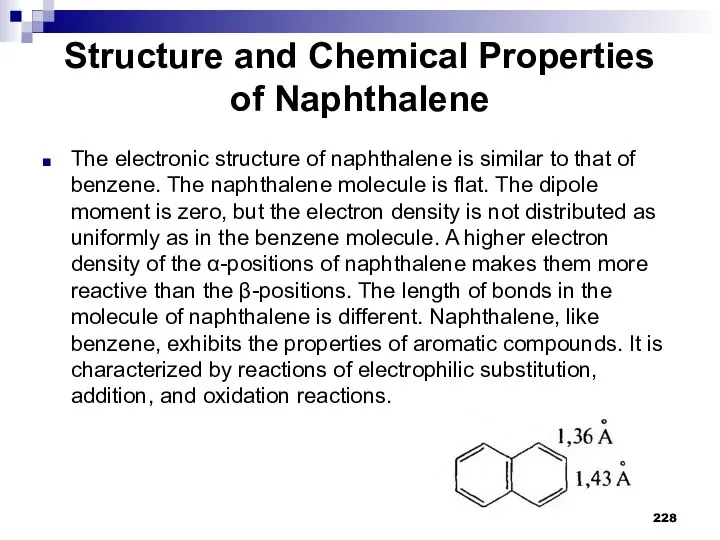
- 228. Structure and Chemical Properties of Naphthalene The electronic structure of naphthalene is similar to that of
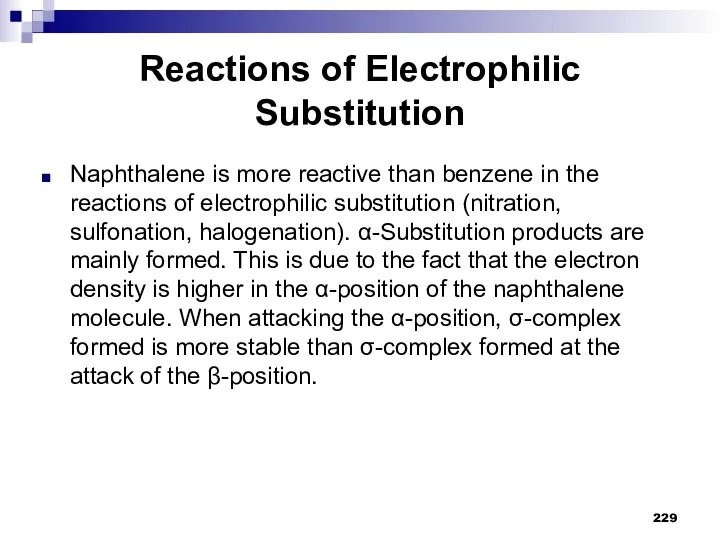
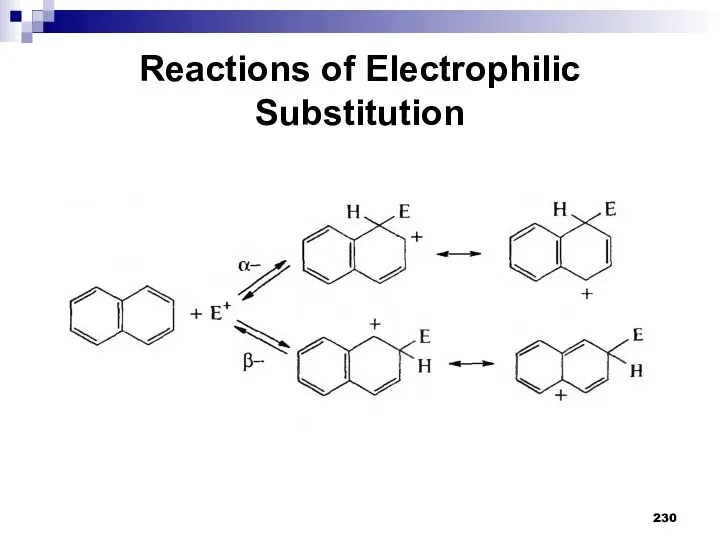
- 229. Reactions of Electrophilic Substitution Naphthalene is more reactive than benzene in the reactions of electrophilic substitution
- 230. Reactions of Electrophilic Substitution
- 231. Reactions of Electrophilic Substitution When attacking α-positions, the delocalization of the positive charge in the σ-complex
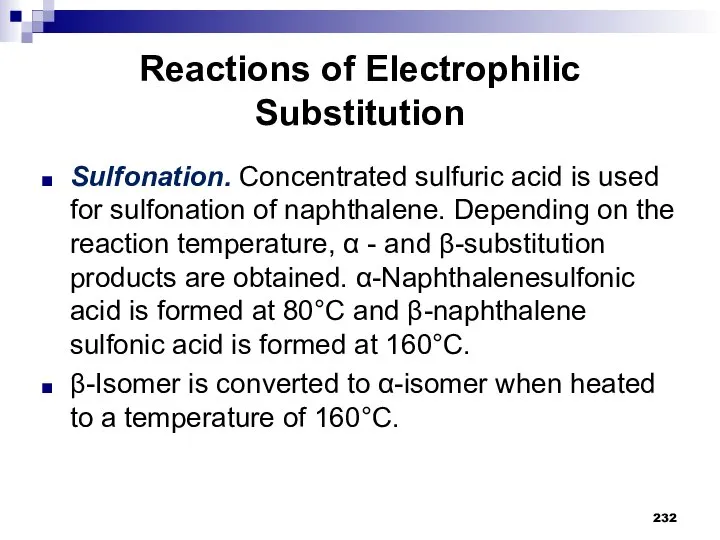
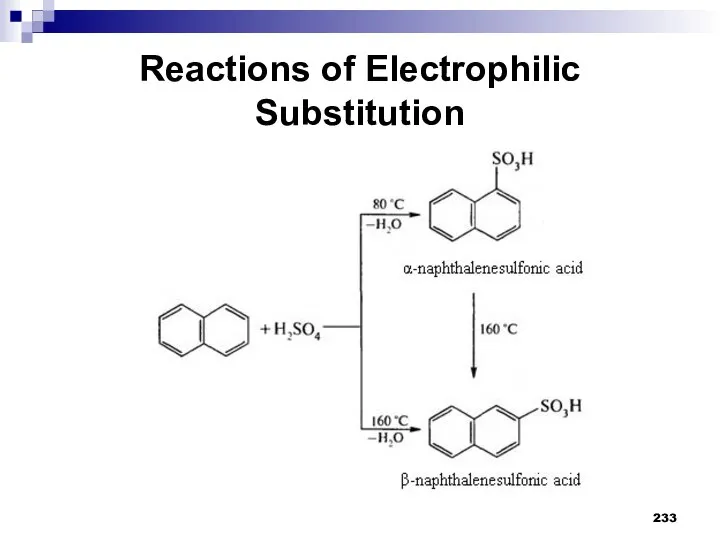
- 232. Reactions of Electrophilic Substitution Sulfonation. Concentrated sulfuric acid is used for sulfonation of naphthalene. Depending on
- 233. Reactions of Electrophilic Substitution
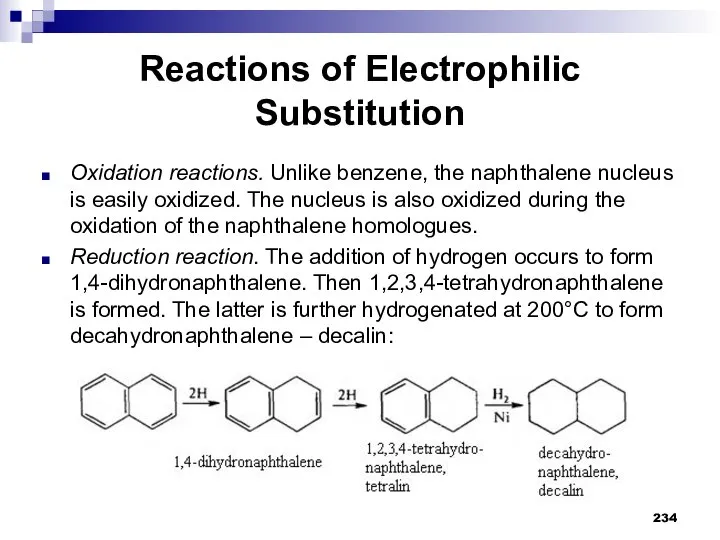
- 234. Reactions of Electrophilic Substitution Oxidation reactions. Unlike benzene, the naphthalene nucleus is easily oxidized. The nucleus
- 235. Polynuclear Aromatic Compounds More complex condensed systems are also known. They are anthracene and phenanthrene. The
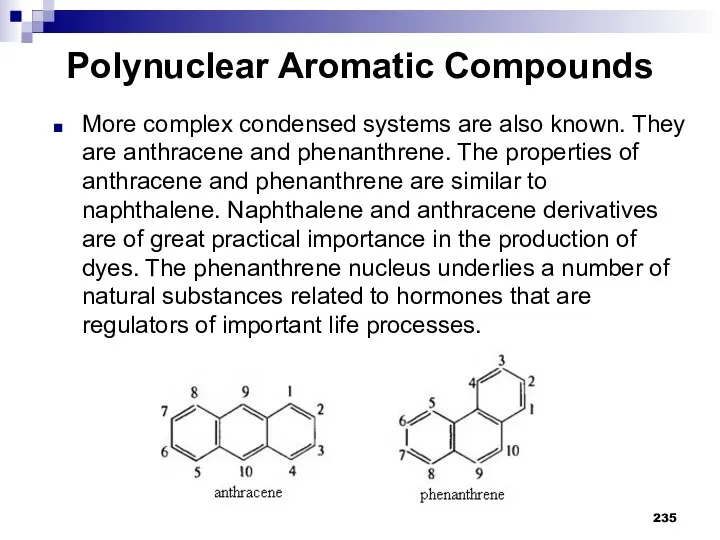
- 236. Polynuclear Aromatic Compounds Benzene rings can be combined with each other in different ways forming other
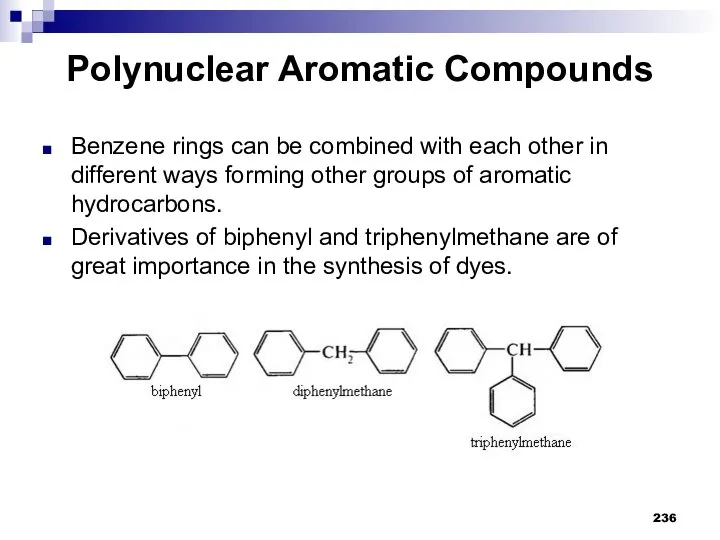
- 237. Questions and Assignments What are polynuclear aromatic compounds called? What are examples of polynuclear aromatic compounds?
- 238. Heterocycles Topic 12
- 239. Outline of the lecture Heterocyclic compounds Classification of Heterocycles Pyrrole Synthesis of Heterocycles Chemical Properties of
- 240. Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May 2012]. Available from World
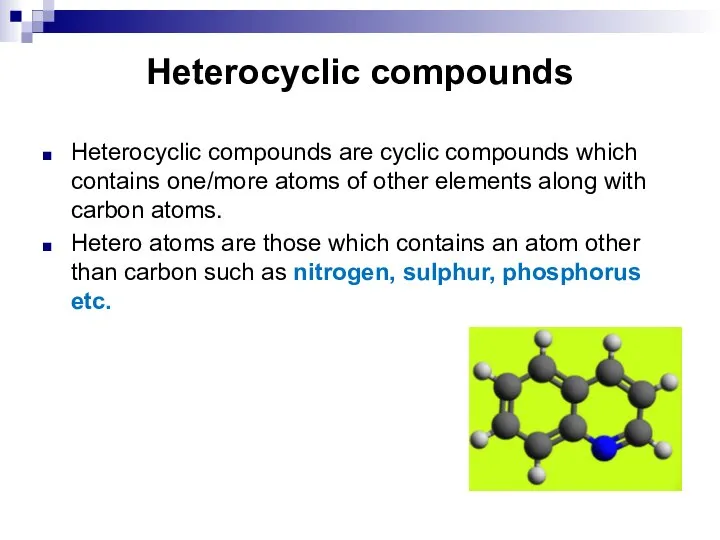
- 241. Heterocyclic compounds Heterocyclic compounds are cyclic compounds which contains one/more atoms of other elements along with
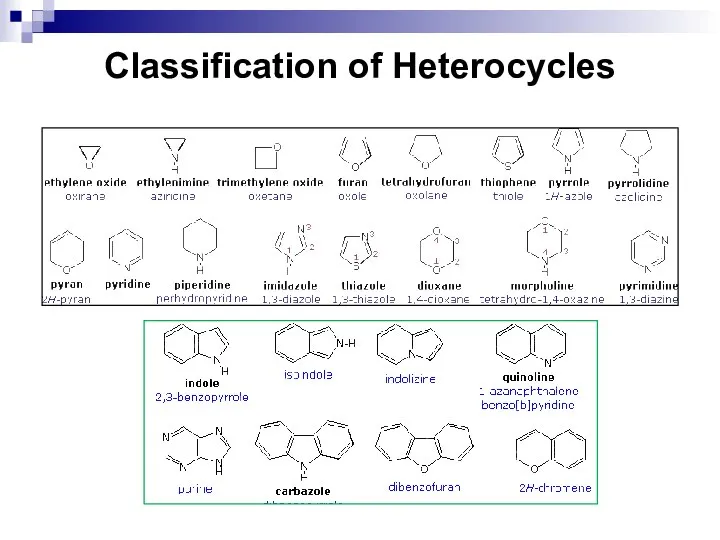
- 242. Classification of Heterocycles
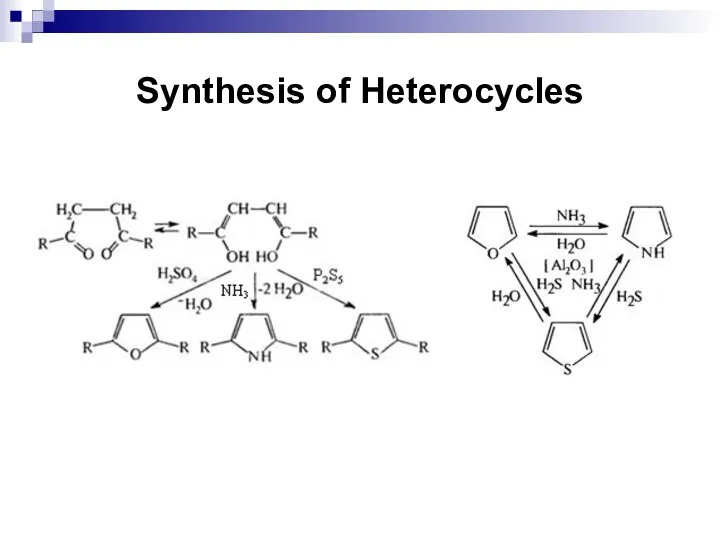
- 243. Synthesis of Heterocycles
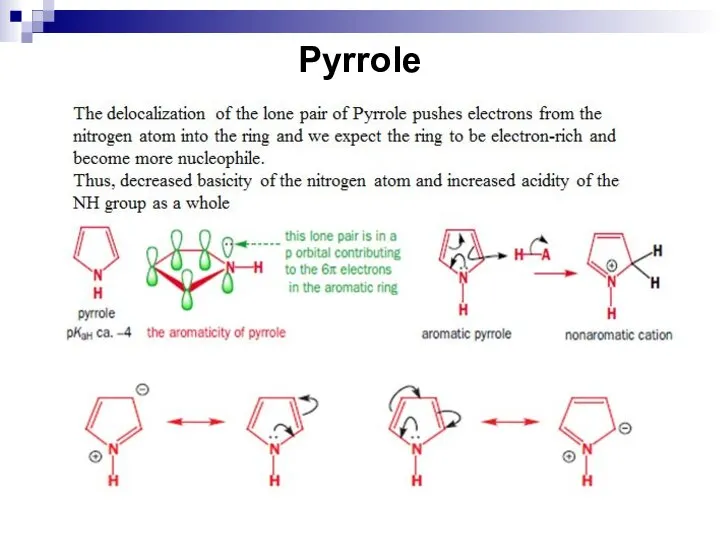
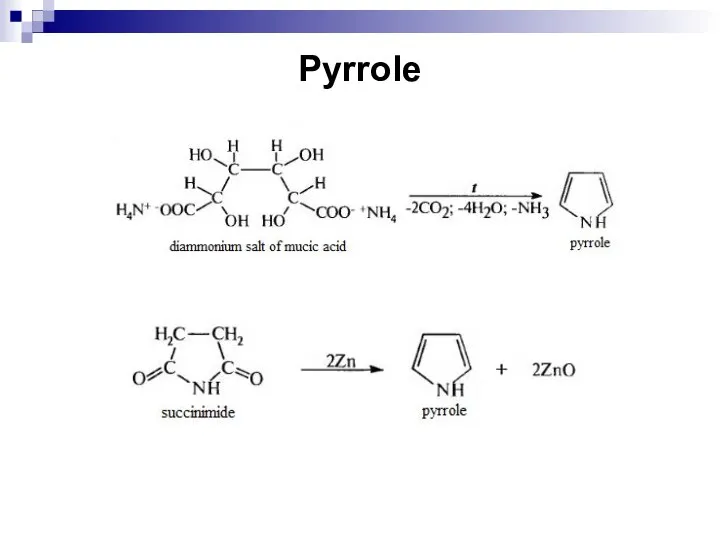
- 244. Pyrrole
- 245. Pyrrole
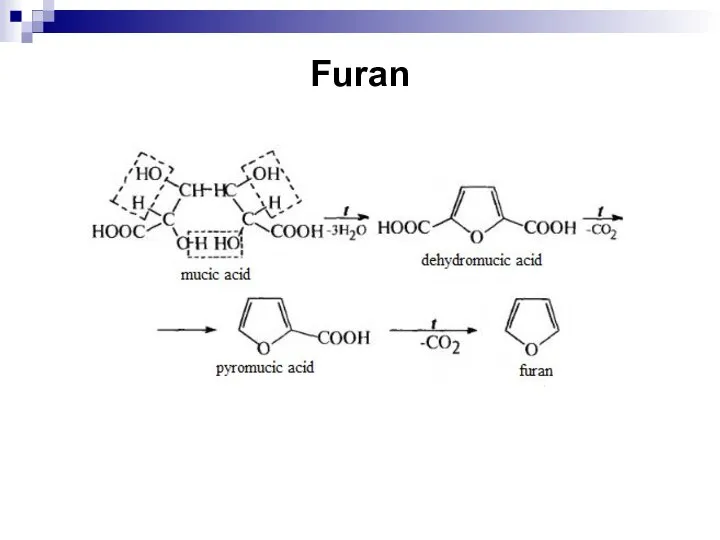
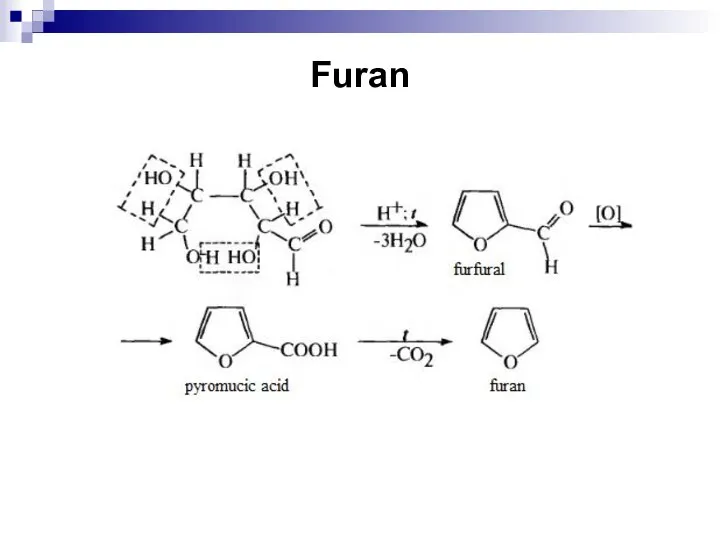
- 246. Furan
- 247. Furan
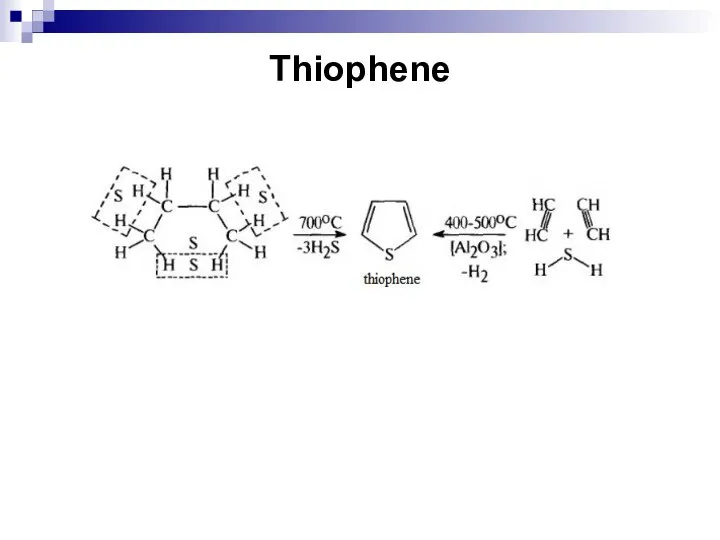
- 248. Thiophene
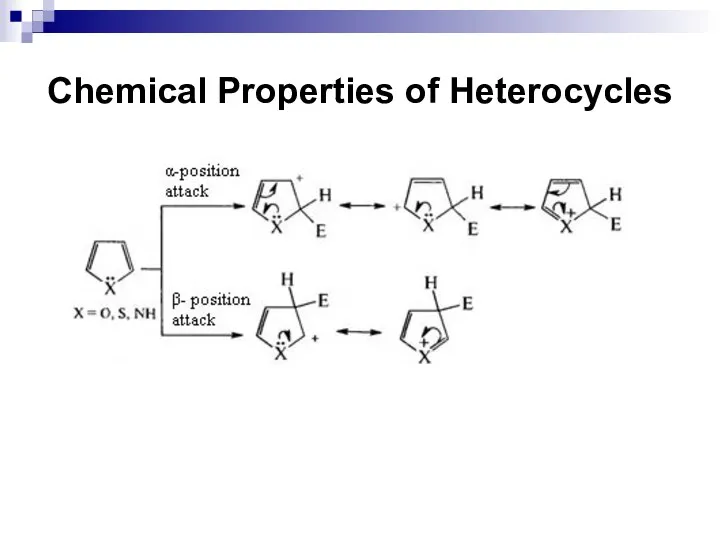
- 249. Chemical Properties of Heterocycles
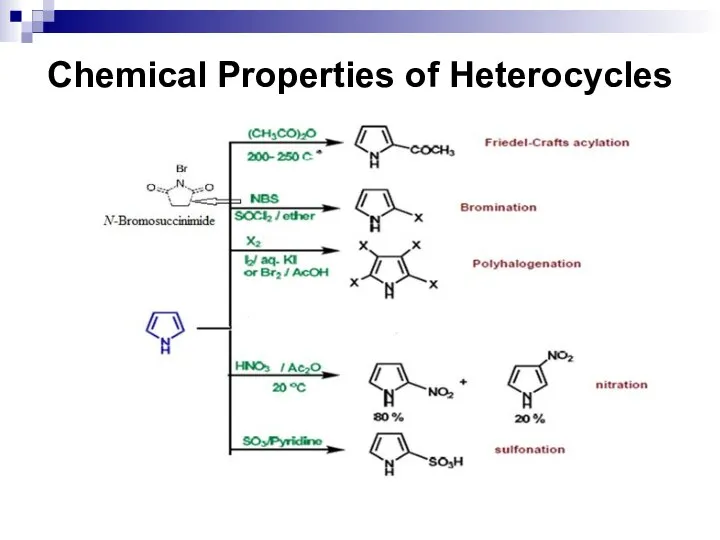
- 250. Chemical Properties of Heterocycles
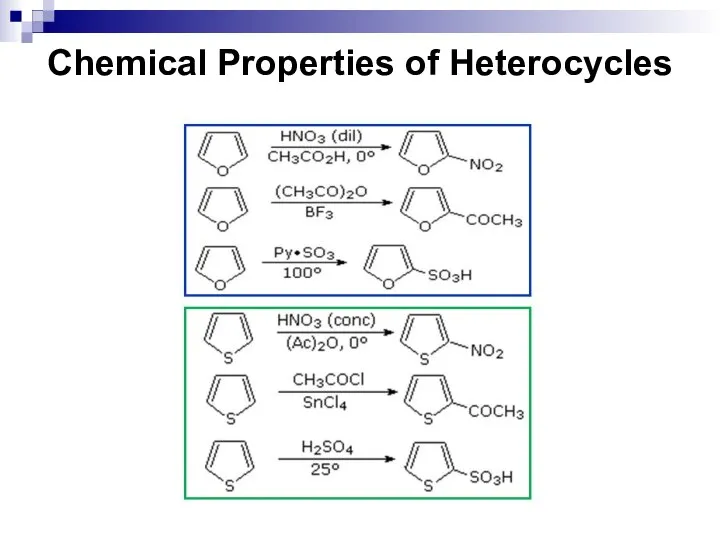
- 251. Chemical Properties of Heterocycles
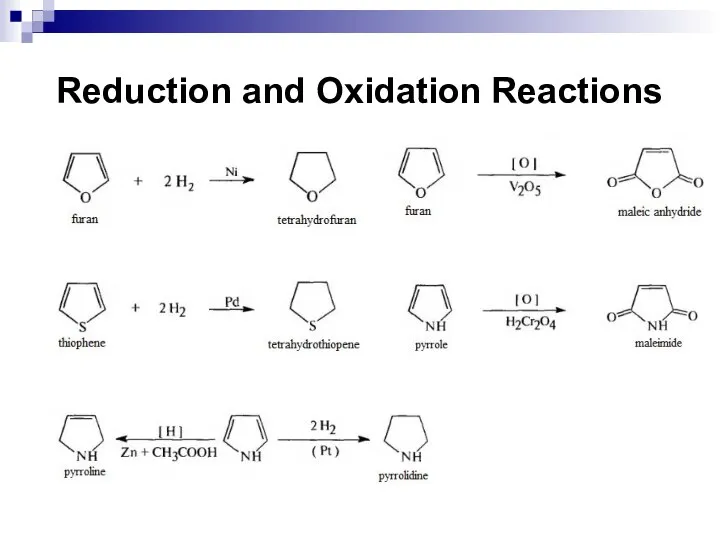
- 252. Reduction and Oxidation Reactions
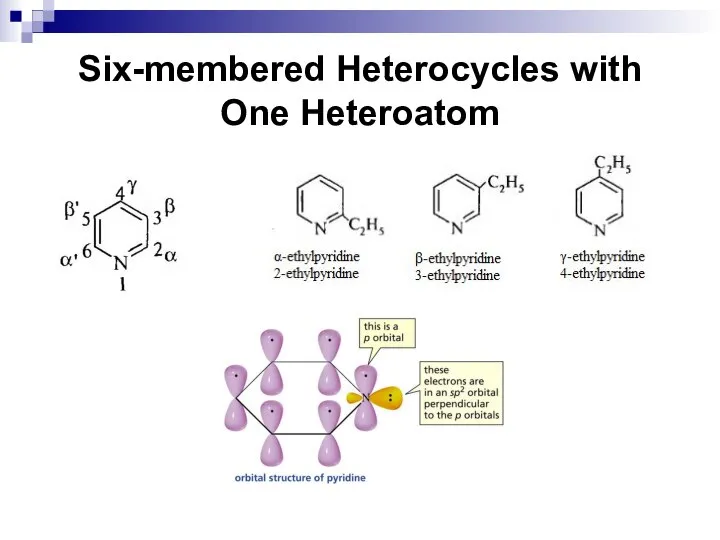
- 253. Six-membered Heterocycles with One Heteroatom
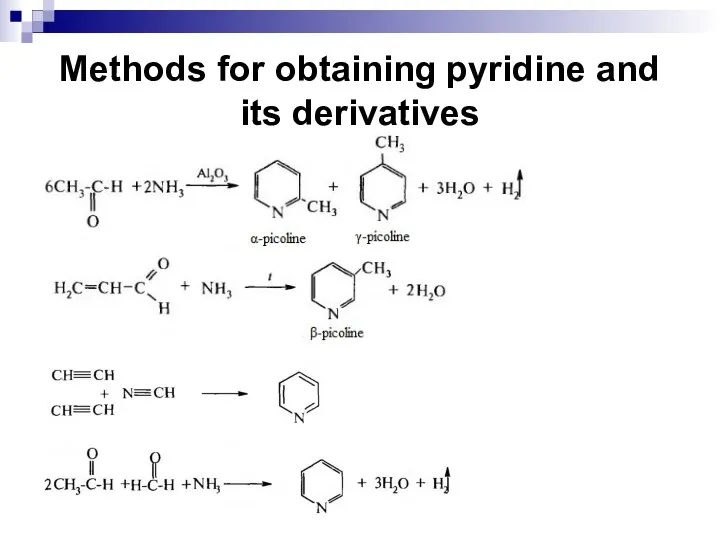
- 254. Methods for obtaining pyridine and its derivatives
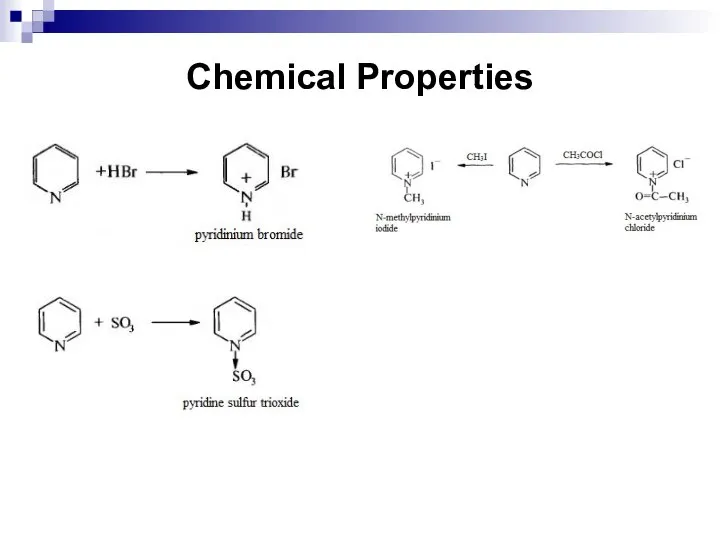
- 255. Chemical Properties
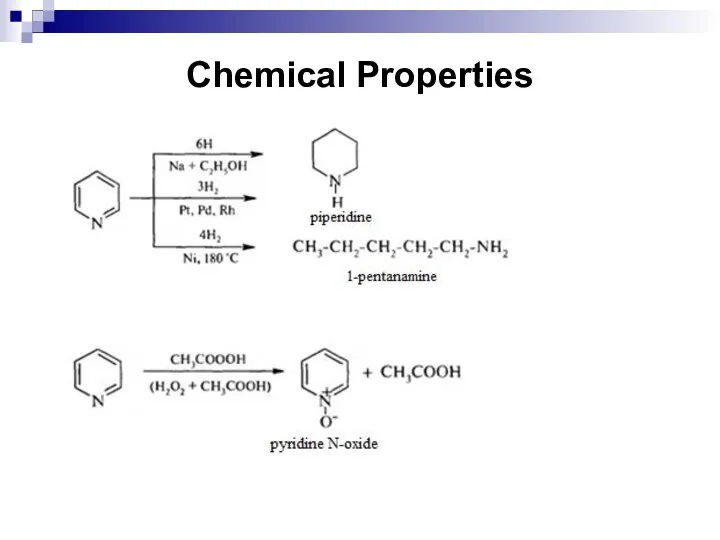
- 256. Chemical Properties
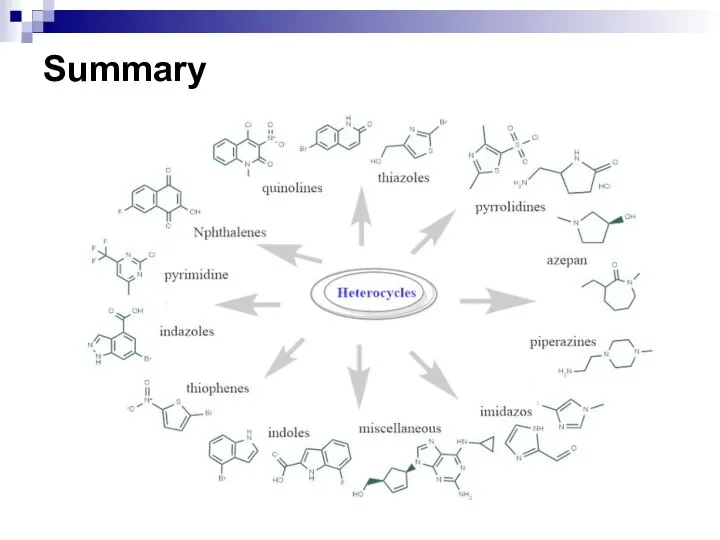
- 257. Summary
- 259. Скачать презентацию



![Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1109369/slide-4.jpg)



![Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1109369/slide-27.jpg)









![Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1109369/slide-79.jpg)



![Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1109369/slide-92.jpg)






![Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1109369/slide-116.jpg)




![Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1109369/slide-133.jpg)




![Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1109369/slide-158.jpg)






![Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1109369/slide-182.jpg)



![Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1109369/slide-200.jpg)




![Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1109369/slide-211.jpg)



![Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1109369/slide-225.jpg)



![Bibliography: Daley, R., Daley, S. 2012. Organic Chemistry. [online]. [Accessed 7 May](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1109369/slide-239.jpg)
 ПРОЕКТ «В гостях у дедушки Чуковского»
ПРОЕКТ «В гостях у дедушки Чуковского» Сеть офисов недвижимости и права G▪ R ▪ A ▪ N ▪ D
Сеть офисов недвижимости и права G▪ R ▪ A ▪ N ▪ D Ассоциация молодых строителей
Ассоциация молодых строителей Ландшафтный дизайн. Работы по инженерной подготовке территории объекта. (Лекция 1)
Ландшафтный дизайн. Работы по инженерной подготовке территории объекта. (Лекция 1) Аттестационная работа. Чайные традиции в Китае
Аттестационная работа. Чайные традиции в Китае Глагол – важная единица речи
Глагол – важная единица речи Презентация на тему Ацтеки
Презентация на тему Ацтеки Радиоволны
Радиоволны Поэзия моя, ты – из окопа...
Поэзия моя, ты – из окопа... Кафе-пекарня Амелия
Кафе-пекарня Амелия Артемида
Артемида Цифровое меню для ресторанов Subway. Специальное предложение
Цифровое меню для ресторанов Subway. Специальное предложение !!!
!!! Расчет электрической цепи
Расчет электрической цепи Комбинированное электронное представление печатных изданий
Комбинированное электронное представление печатных изданий Как древние люди представляли себе Вселенную
Как древние люди представляли себе Вселенную Файл. Файловая структура.
Файл. Файловая структура. Pricing and discounts – инструмент ценообразования и продвижения онлайн-магазинов
Pricing and discounts – инструмент ценообразования и продвижения онлайн-магазинов Презентация на тему Наши помощники - органы чувств (1 класс)
Презентация на тему Наши помощники - органы чувств (1 класс) В гостях у станционного смотрителя
В гостях у станционного смотрителя Как понять другого человека через познание самого себя?
Как понять другого человека через познание самого себя? Решение Кейса. Варианты урегулирования ПА Астрилово
Решение Кейса. Варианты урегулирования ПА Астрилово Презентация на тему Неморфологический способ образования слов
Презентация на тему Неморфологический способ образования слов Промышленное строительство
Промышленное строительство Элементы атлетической гимнастики
Элементы атлетической гимнастики How To Fake French
How To Fake French  Презентация на тему Инфекционные заболевания людей 7 класс
Презентация на тему Инфекционные заболевания людей 7 класс Модернизация розничных сетей распространения прессы
Модернизация розничных сетей распространения прессы