Слайд 2Arenes:
compounds containing both aliphatic and aromatic parts.
Alkylbenzenes
Alkenylbenzenes
Alkynylbenzenes
Etc.
Emphasis on the effect that

one part has on the chemistry of the other half.
Reactivity & orientation
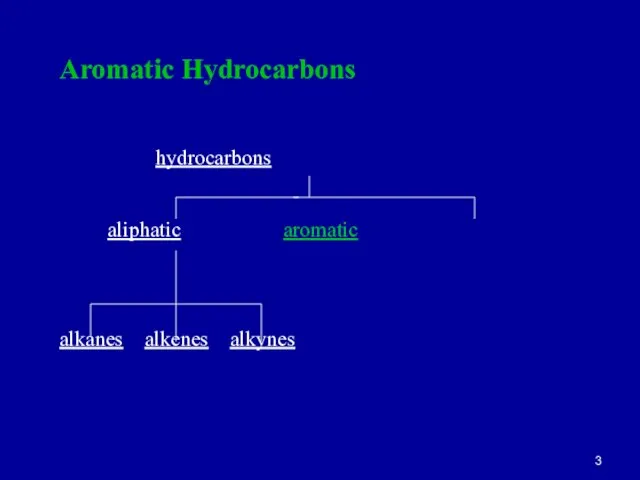
Слайд 3Aromatic Hydrocarbons
hydrocarbons
aliphatic aromatic
alkanes alkenes alkynes
Слайд 4Aliphatic compounds: open-chain compounds and ring compounds that are chemically similar to

open-chain compounds. Alkanes, alkenes, alkynes, dienes, alicyclics, etc.
Aromatic compounds: unsaturated ring compounds that are far more stable than they should be and resist the addition reactions typical of unsaturated aliphatic compounds. Benzene and related compounds.
Слайд 7Systematic Nomenclature
Monosubstituted benzenes
Hydrocarbon with benzene as parent
C6H5Br = bromobenzene
C6H5NO2 = nitrobenzene
C6H5CH2CH2CH3
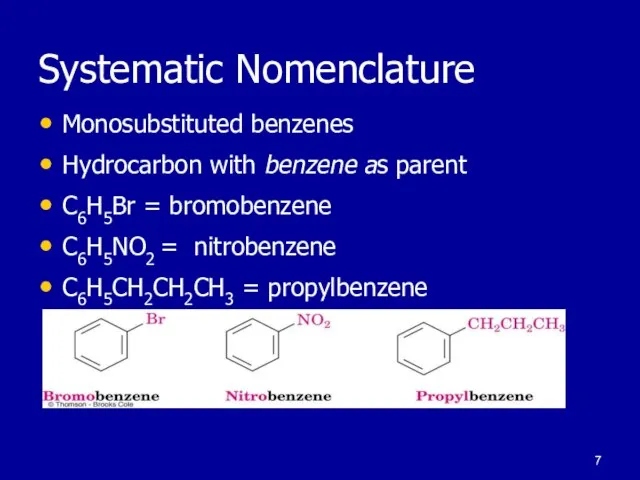
= propylbenzene
Слайд 8others named as “alkylbenzenes”:

Слайд 9The Phenyl Group
When a benzene ring is a substituent, the term phenyl
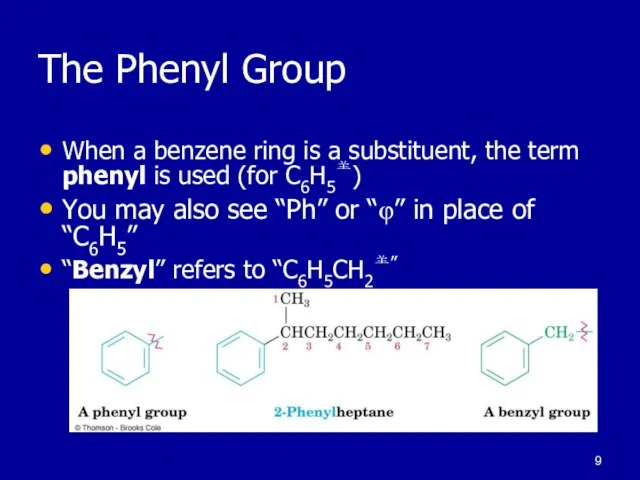
is used (for C6H5)
You may also see “Ph” or “φ” in place of “C6H5”
“Benzyl” refers to “C6H5CH2”
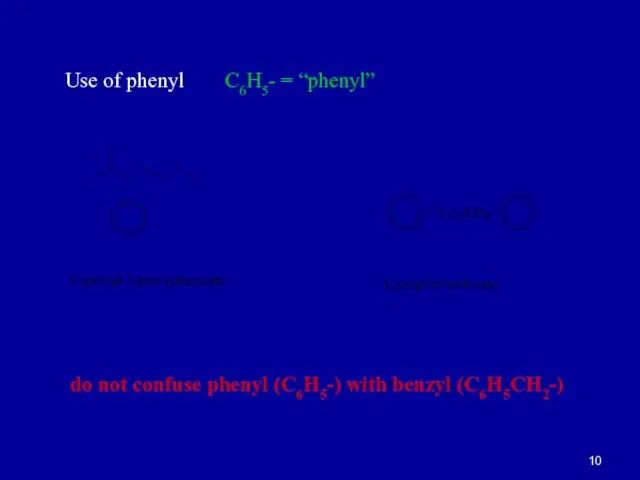
Слайд 10Use of phenyl C6H5- = “phenyl”
do not confuse phenyl (C6H5-) with benzyl (C6H5CH2-)
Слайд 11Nomenclature: Side Chains
If side chain has < 6 carbons
Alkyl benzene
If side chain
has > 6 carbons
Phenyl alkane
Слайд 15Nomenclature Disubstituted Benzene
Relative positions on a benzene ring
ortho- (o) on adjacent carbons
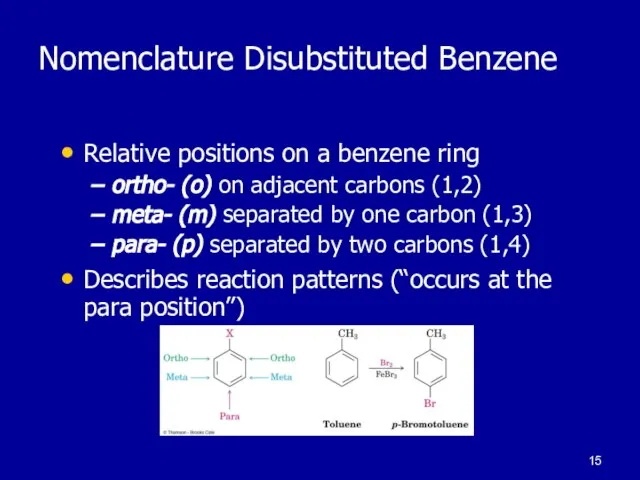
(1,2)
meta- (m) separated by one carbon (1,3)
para- (p) separated by two carbons (1,4)
Describes reaction patterns (“occurs at the para position”)
Слайд 16Nomenclature More Than Two Substituents
Choose numbers to get lowest possible values
List
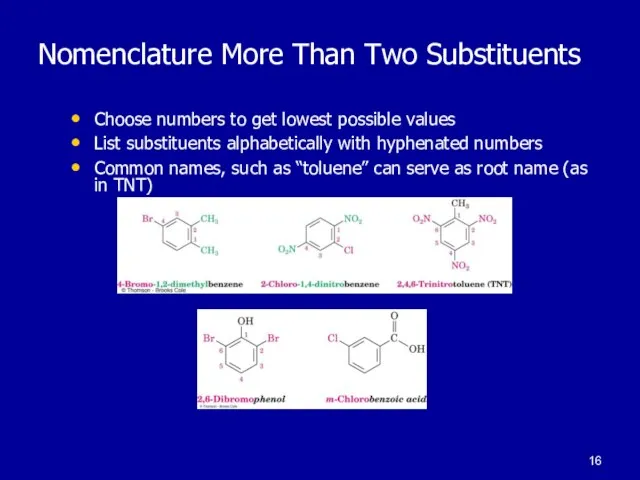
substituents alphabetically with hyphenated numbers
Common names, such as “toluene” can serve as root name (as in TNT)
Слайд 17Benzene
Three double bonds
Unreactive towards normal reagents (compare to alkenes)
Very stable
Why?
How can

we get benzene to react?
Can we control these reactions?
Слайд 18Observations: Reactions of Benzene
Benzene reacts slowly with Br2
Product is bromobenzene
Substitution
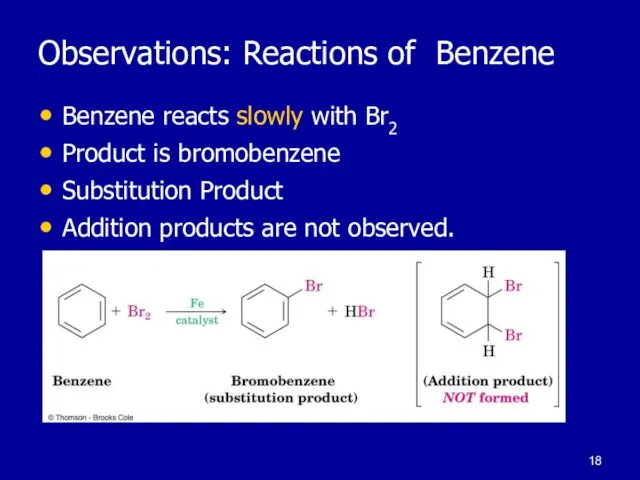
Product
Addition products are not observed.
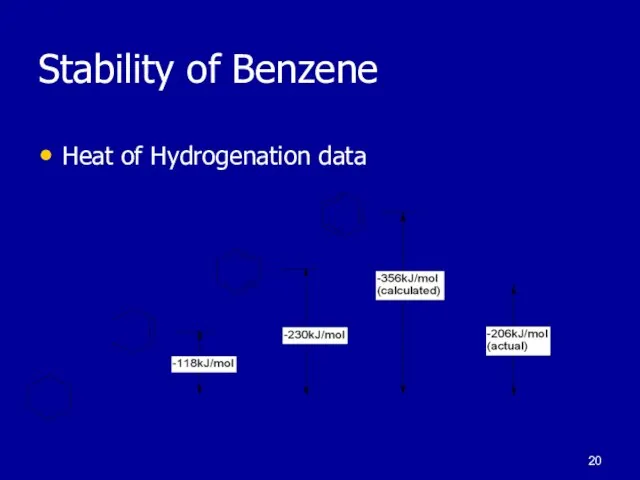
Слайд 19Stability of Benzene
KMnO4
Reacts with alkenes
No reaction with benzene
HCl
Reacts with alkenes
No reaction with

benzene
HBr
Reacts with alkenes
No reaction with benzene
Слайд 20Stability of Benzene
Heat of Hydrogenation data
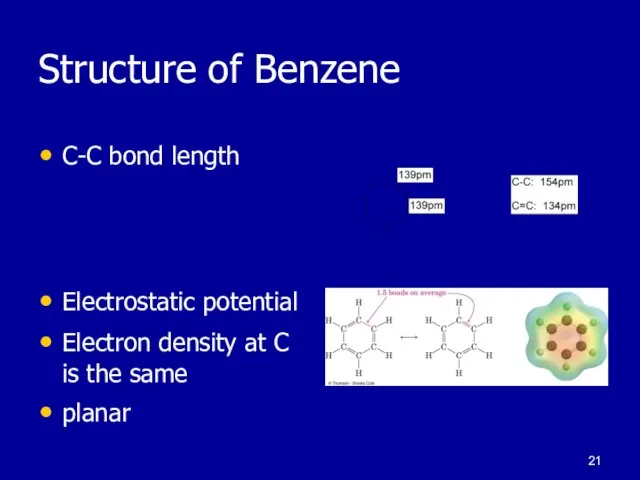
Слайд 21Structure of Benzene
C-C bond length
Electrostatic potential
Electron density at C is the same
planar
Слайд 22Structure of Benzene
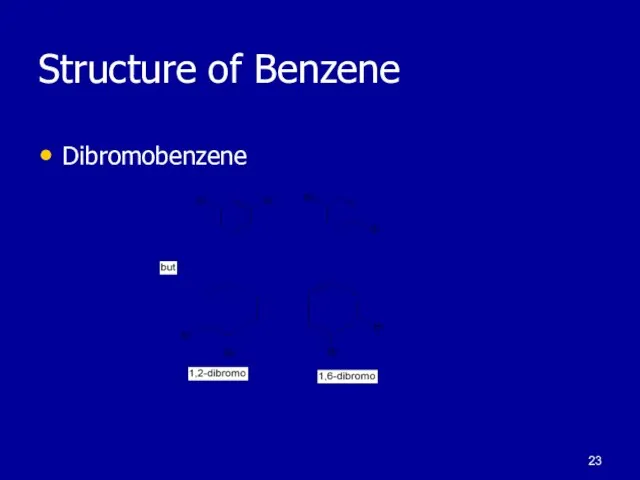
August Kekule proposed:
1,3,5-cyclohexatriene structure
Explained single monobromo product

Слайд 23Structure of Benzene
Dibromobenzene
Слайд 24Structure of Benzene
Issue was resolved by Kekule

Слайд 25Structure of Benzene
Explains the observed products
Does not explain
Unreactive nature of benzene
Observation of

only substitution products
A triene
As reactive as any alkene
Would give addition products
Not expected to be more stable
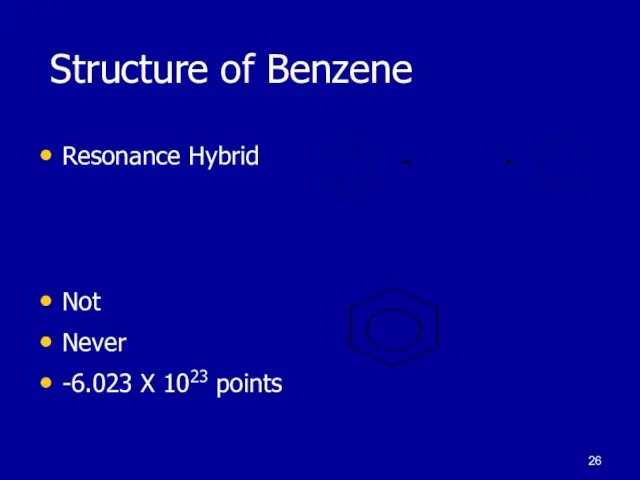
Слайд 26 Structure of Benzene
Resonance Hybrid
Not
Never
-6.023 X 1023 points
Слайд 27Stability of Benzene
MO Description
6 p atomic orbitals combine in cyclic manner
Generate 6
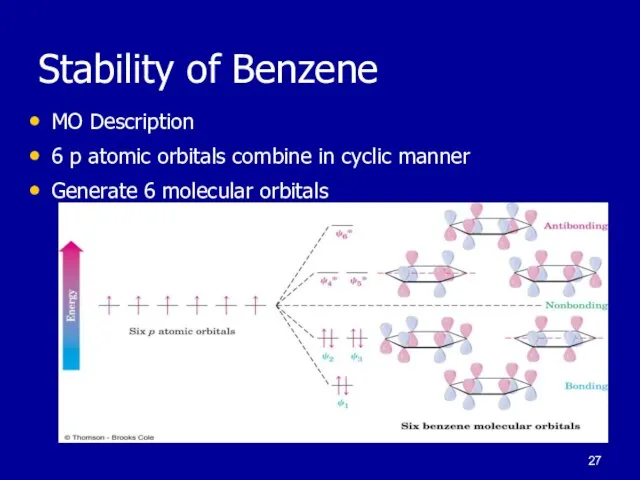
molecular orbitals
Слайд 28Key Ideas on Benzene
Unusually stable
heat of hydrogenation 150 kJ/mol lower than a

cyclic triene
Planar hexagon:
bond angles are 120°
carbon–carbon bond lengths 139 pm
Undergoes substitution not addition
Resonance hybrid
One more important factor is the number of electrons in the cyclic orbital
Слайд 29Aromaticity
E Huckel (1931)
Aromaticity is a property of certain molecules
Chemistry would be similar
to benzene
Meet the following criteria
Planar
Mono cyclic system
Conjugated pi system
Contains 4n + 2 π electrons
Can apply rules to variety of compounds and determine aromatic nature.
Led to wild chase to make compounds
Met the rules
Violated the rules
Слайд 30Aromaticity and the 4n + 2 Rule
Huckel’s rule, based on calculations –
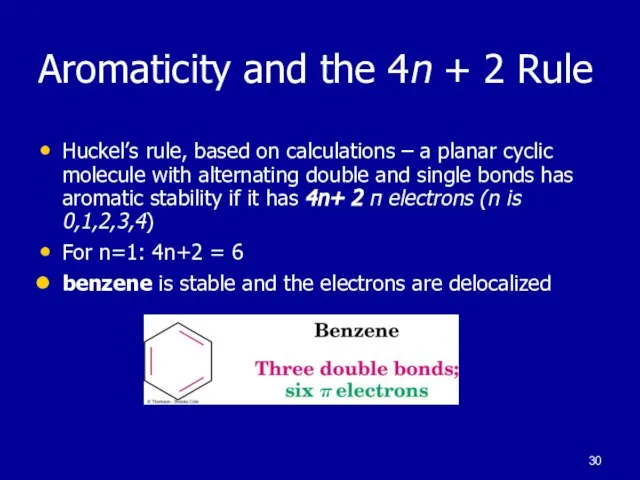
a planar cyclic molecule with alternating double and single bonds has aromatic stability if it has 4n+ 2 π electrons (n is 0,1,2,3,4)
For n=1: 4n+2 = 6
benzene is stable and the electrons are delocalized
Слайд 31Compounds With 4n π Electrons Are Not Aromatic (May be Anti-aromatic)
Planar, cyclic

molecules with 4 n π electrons are much less stable than expected (anti-aromatic)
They will distort out of plane and behave like ordinary alkenes
4- and 8-electron compounds are not delocalized
Alternating single and double bonds
Слайд 32Cyclobutadiene
Cyclobutadiene is so unstable that it dimerizes by a self-Diels-Alder reaction at

low temperature
Слайд 33Cyclooctatetraene
Cyclooctatetraene has four double bonds
Behaves as if it were 4 separate alkenes
It
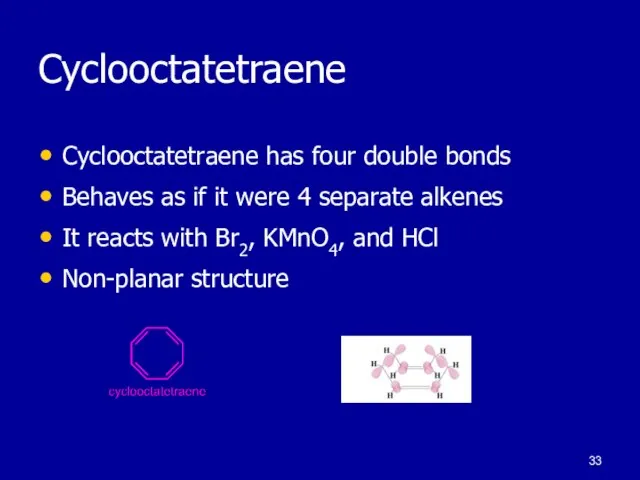
reacts with Br2, KMnO4, and HCl
Non-planar structure
Слайд 34Aromatic Heterocycles
Heterocyclic compounds contain elements other than carbon in a ring, such
as N,S,O,P
There are many heterocyclic aromatic compounds
Cyclic compounds that contain only carbon are called carbocycles
Nomenclature is specialized
Four are important in biological chemistry
Слайд 35Pyridine
A six-membered heterocycle with a nitrogen atom in its ring
π electron structure
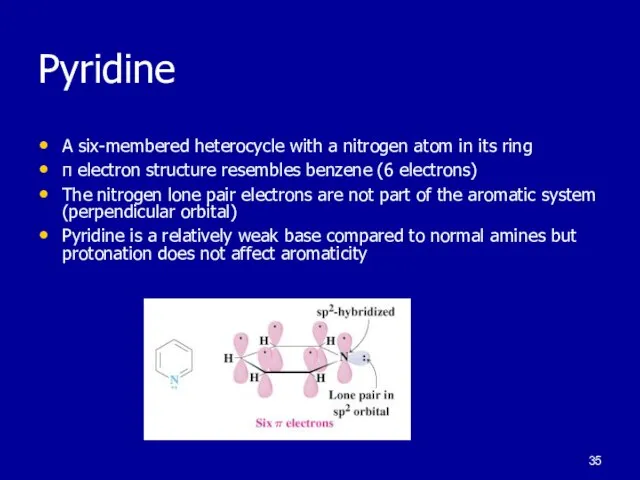
resembles benzene (6 electrons)
The nitrogen lone pair electrons are not part of the aromatic system (perpendicular orbital)
Pyridine is a relatively weak base compared to normal amines but protonation does not affect aromaticity
Слайд 36Pyrrole
A five-membered heterocycle with one nitrogen
Four sp2-hybridized carbons with 4 p orbitals
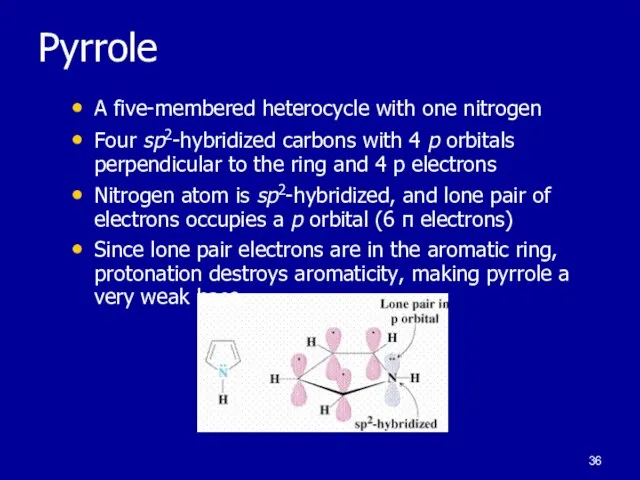
perpendicular to the ring and 4 p electrons
Nitrogen atom is sp2-hybridized, and lone pair of electrons occupies a p orbital (6 π electrons)
Since lone pair electrons are in the aromatic ring, protonation destroys aromaticity, making pyrrole a very weak base
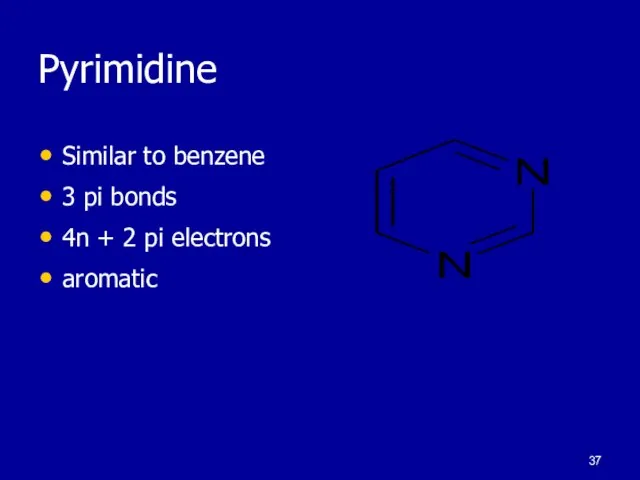
Слайд 37Pyrimidine
Similar to benzene
3 pi bonds
4n + 2 pi electrons
aromatic
Слайд 38Imidazole
Similar to pyrrole
Pair of non-bonding electrons on N used
4n + 2 pi
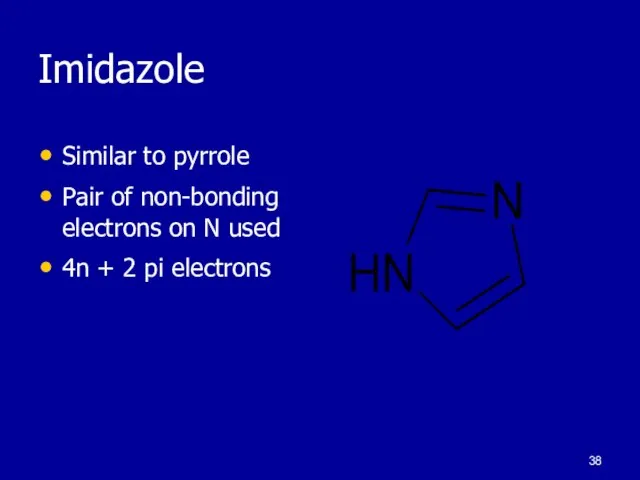
electrons
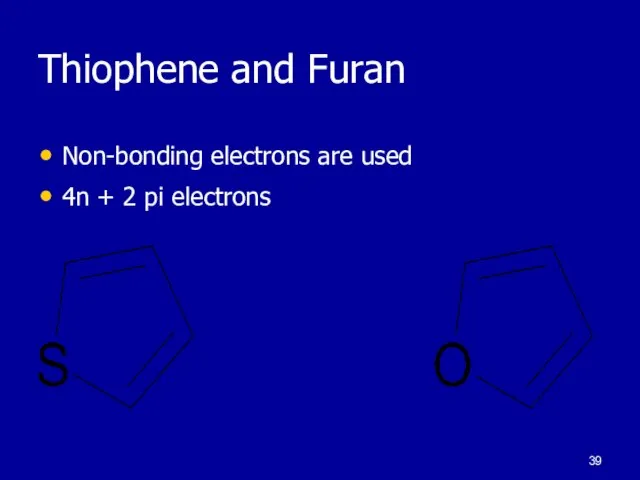
Слайд 39Thiophene and Furan
Non-bonding electrons are used
4n + 2 pi electrons
Слайд 40Substitution Reactions of Benzene
Benzene is aromatic: a cyclic conjugated compound with 6
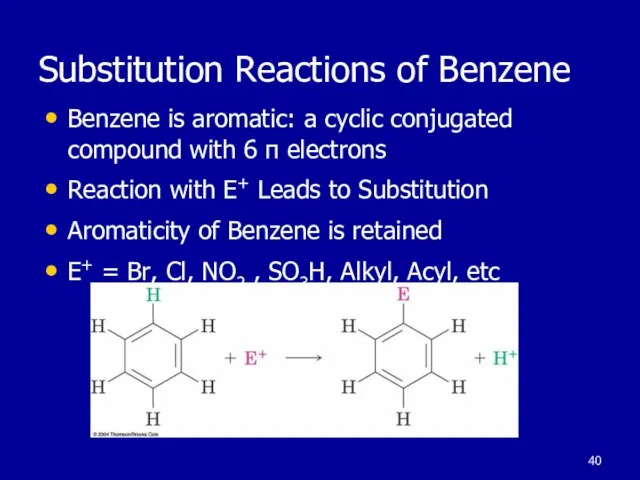
π electrons
Reaction with E+ Leads to Substitution
Aromaticity of Benzene is retained
E+ = Br, Cl, NO2 , SO3H, Alkyl, Acyl, etc
Слайд 41Aromatic Substitutions
The proposed mechanism for the reaction of benzene with electrophiles
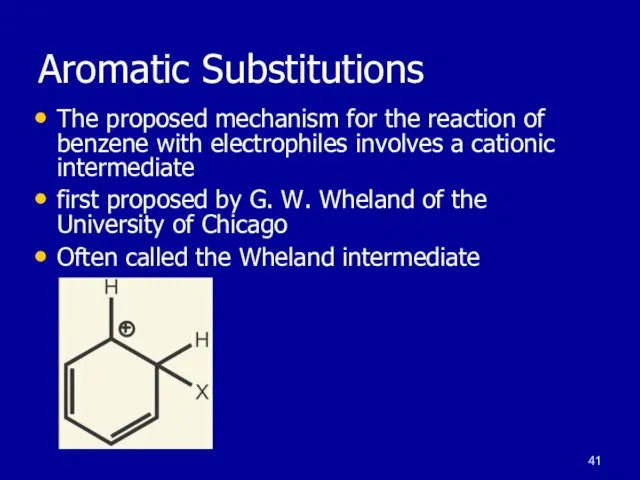
involves a cationic intermediate
first proposed by G. W. Wheland of the University of Chicago
Often called the Wheland intermediate
Слайд 42Chemistry of the Intermediate
Loss of a proton leads to rearomatization and substitution
Loss
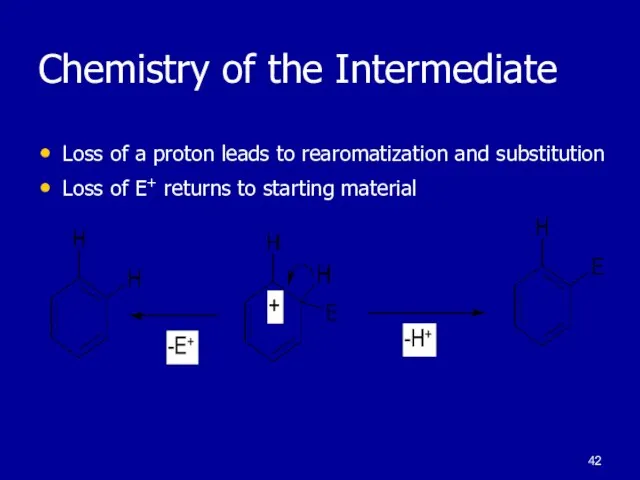
of E+ returns to starting material
Слайд 43Halogenation
Add Cl, Br, and I
Must use Lewis acid catalyst
F is too reactive
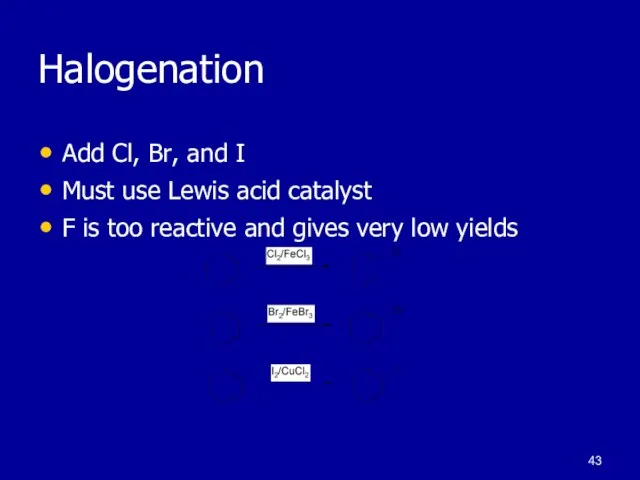
and gives very low yields
Слайд 44Biological Halogenation
Accomplished during biosynthesis of
thyroxine
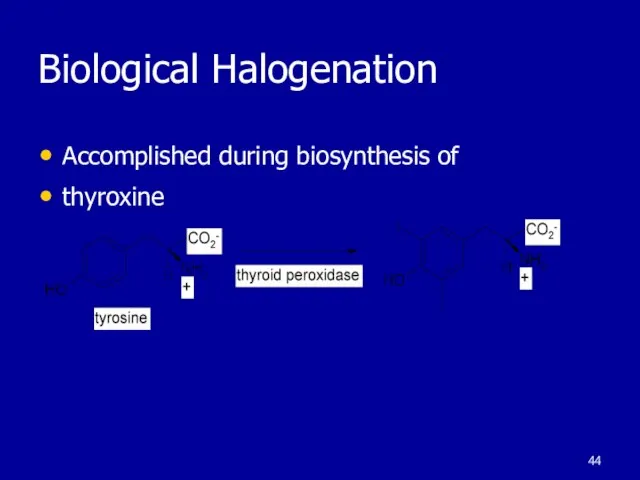
Слайд 45Aromatic Nitration
The combination of nitric acid and sulfuric acid produces NO2+ (nitronium
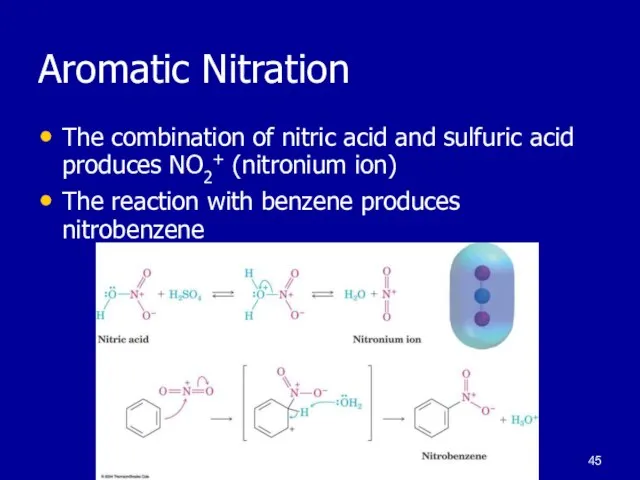
ion)
The reaction with benzene produces nitrobenzene
Слайд 46Nitrobenzenes: Precursors to Anilines
Nitric acid destroys alkenes through [O]
In sulfuric acid reacts
![Nitrobenzenes: Precursors to Anilines Nitric acid destroys alkenes through [O] In sulfuric](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/377883/slide-45.jpg)
with benzene giving nitrobenzene
Nitrobenzene may be reduced to aniline
Aniline useful precursors to many industrially important organic compounds
Слайд 48Aromatic Dyes
William Henry Perkin
Age 17 (1856)
Undergraduate student in medicine
Reacted aniline with potassium

dichromate
Tarry mess
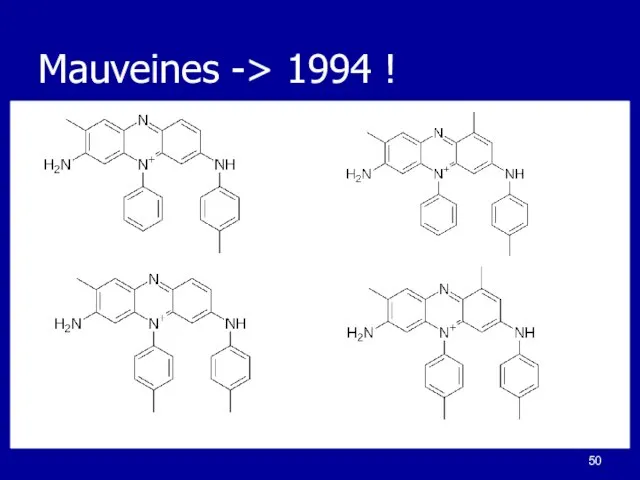
Слайд 49Aromatic Dyes
Isolated
Mauve - a purple color
Dyed white cloth
Patented material and process
First chemical

company
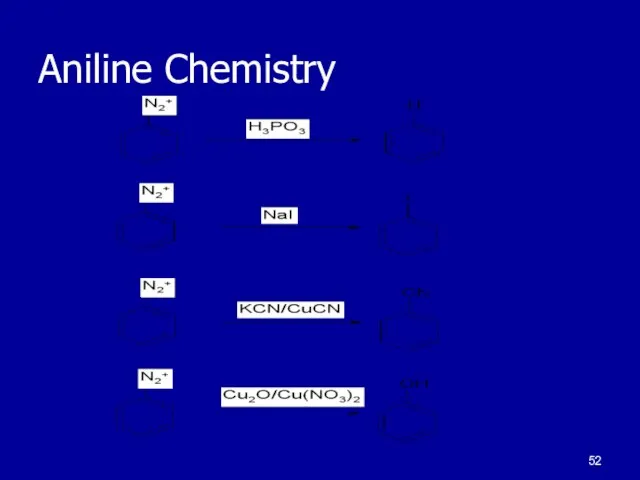
Слайд 51Some Aniline Chemistry
Anilines readily react with nitrous acid
Diazonium salts
Coupling reaction giving
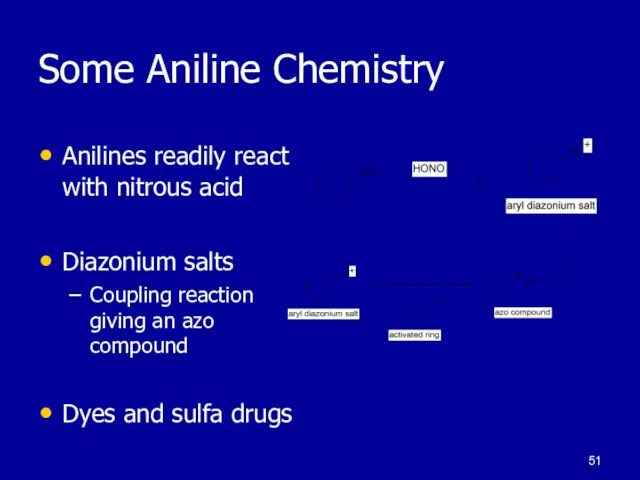
an azo compound
Dyes and sulfa drugs
Слайд 53How do we make sulfuric acid?
H2SO4 – least expensive manufactured chemical
S (mined

pure) + O2 SO3
SO3 + H2O H2SO4
Continue adding SO3 gives
Fuming sulfuric acid: H2SO4/ SO3
Слайд 54Aromatic Sulfonation
Substitution of H by SO3 (sulfonation)
Reaction with a mixture of sulfuric
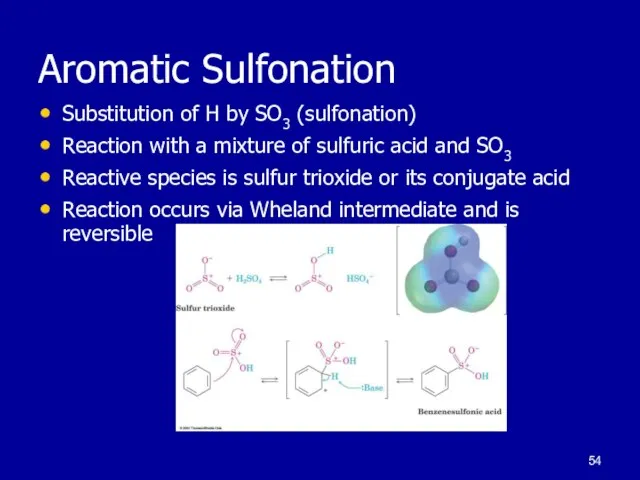
acid and SO3
Reactive species is sulfur trioxide or its conjugate acid
Reaction occurs via Wheland intermediate and is reversible
Слайд 55Benzene Sulfonic Acid
Manufacture of Ion Exchange Resins
Water softening
Water purification
Environmental restoration (removal of

toxic metal ions)
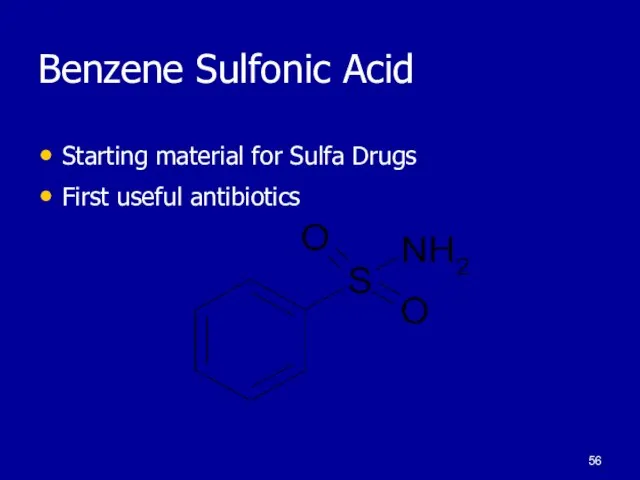
Слайд 56Benzene Sulfonic Acid
Starting material for Sulfa Drugs
First useful antibiotics
Слайд 57Hydroxylation
Direct hydroxylation is difficult in lab
Indirect method uses sulfonic acid

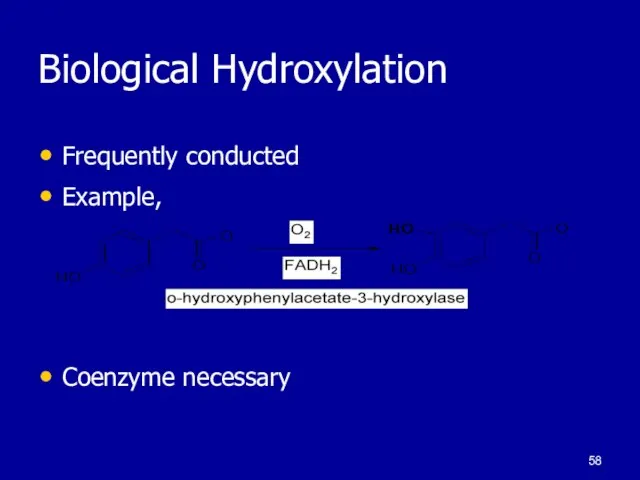
Слайд 58Biological Hydroxylation
Frequently conducted
Example,
Coenzyme necessary
Слайд 59Alkylation of Aromatic Rings
The Friedel–Crafts Reaction
Aromatic substitution of a R+ for
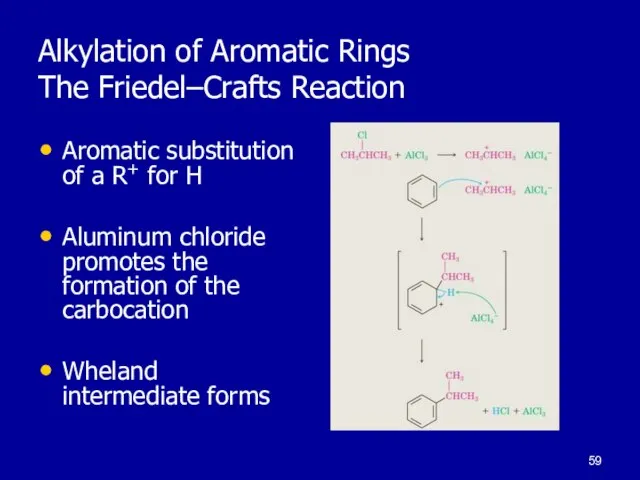
H
Aluminum chloride promotes the formation of the carbocation
Wheland intermediate forms
Слайд 60Limitations of the Friedel-Crafts Alkylation
Only alkyl halides can be used (F, Cl,
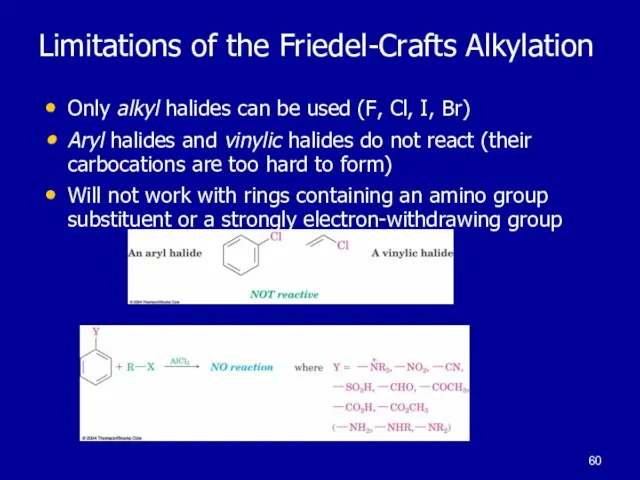
I, Br)
Aryl halides and vinylic halides do not react (their carbocations are too hard to form)
Will not work with rings containing an amino group substituent or a strongly electron-withdrawing group
Слайд 61Limitations
Multiple alkylations occur because the first alkyl group activates the ring
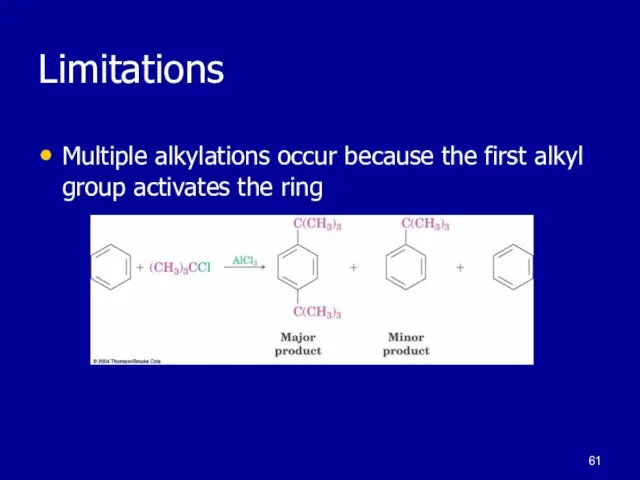
Слайд 62polyalkylation
The alkyl group activates the ring making the products more reactive that

the reactants leading to polyalkylation. Use of excess aromatic compound minimizes polyalkylation in the lab.
Слайд 63Limitations
Carbocation Rearrangements During Alkylation
Similar to those that occur during electrophilic additions
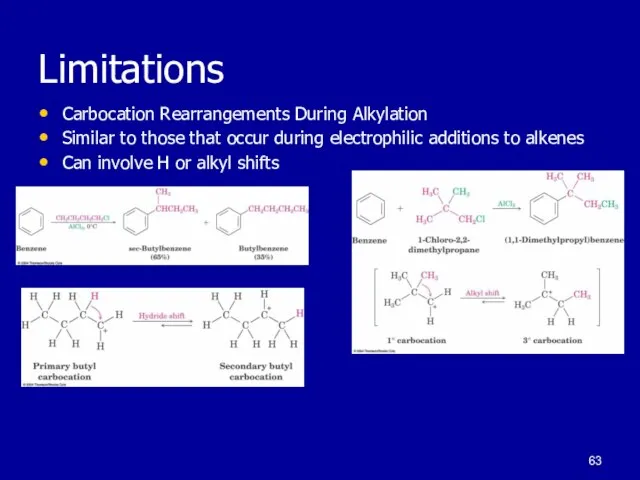
to alkenes
Can involve H or alkyl shifts
Слайд 64Related Reactions
Chloromethylation

Слайд 65Related Reaction
Acylation of Aromatic Rings
Reaction of an acid chloride (RCOCl) with
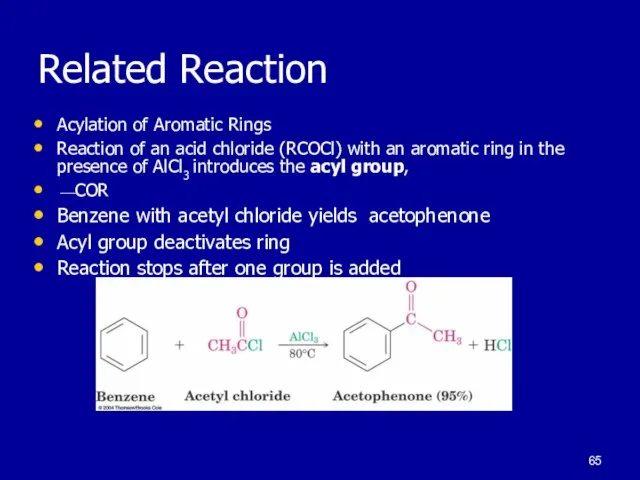
an aromatic ring in the presence of AlCl3 introduces the acyl group,
⎯COR
Benzene with acetyl chloride yields acetophenone
Acyl group deactivates ring
Reaction stops after one group is added
Слайд 66Biological Alkylations
Common reaction
No AlCl3 present
Utilizes an organodiphosphate
Dissociation is facilitated by Mg+2
Important reaction

in biosynthesis of Vitamin K1
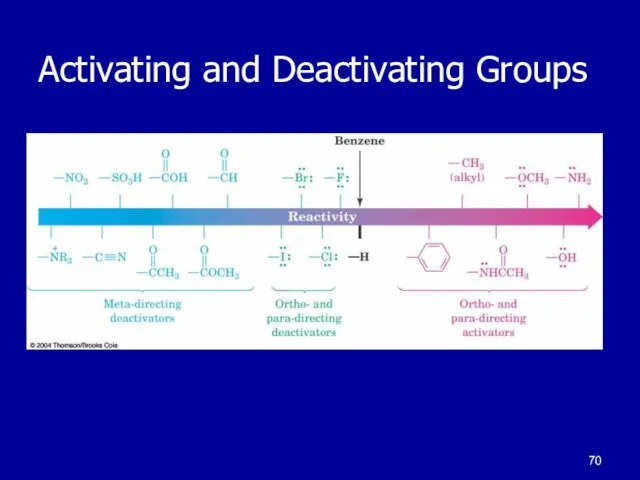
Слайд 67Ring Substitution Effects
Activation and deactivation of ring
Alkyl activates the ring
Acyl deactivates the

ring
Activating Groups
group promotes substitution faster than benzene
Deactivating Groups
group promotes substitution slower than benzene
Слайд 68Activating and Deactivating Groups
Activating groups
electron donating groups
stabilizes the carbocation intermediate
activates through induction

or resonance
Deactivating groups
electron withdrawing groups
destabilizes the carbocation intermediate
deactivates through induction or resonance
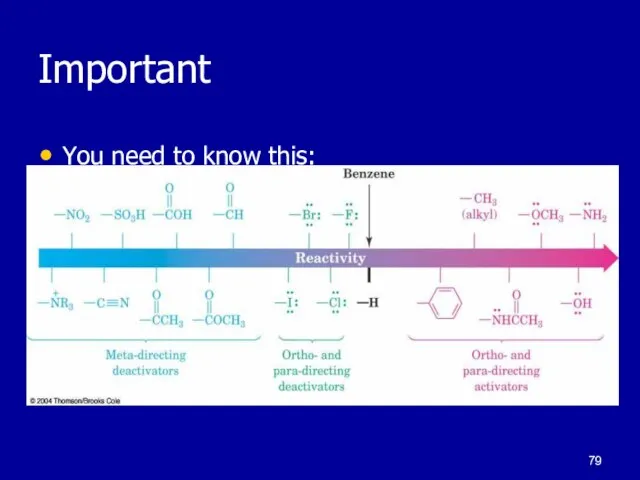
Слайд 69Common substituent groups and their effect on EAS:
-NH2, -NHR, -NR2
-OH
-OR
-NHCOCH3
-C6H5
-R
-H
-X
-CHO, -COR
-SO3H
-COOH, -COOR
-CN
-NR3+
-NO2
increasing
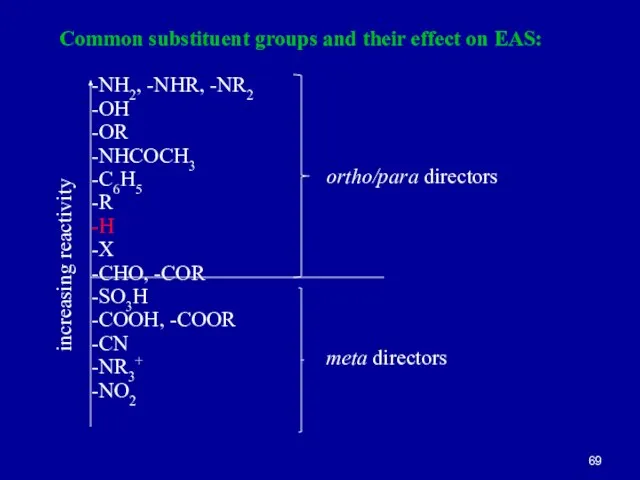
reactivity
ortho/para directors
meta directors
Слайд 70Activating and Deactivating Groups
Слайд 71Origins of Substituent Effects
Inductive effect - withdrawal or donation of electrons through
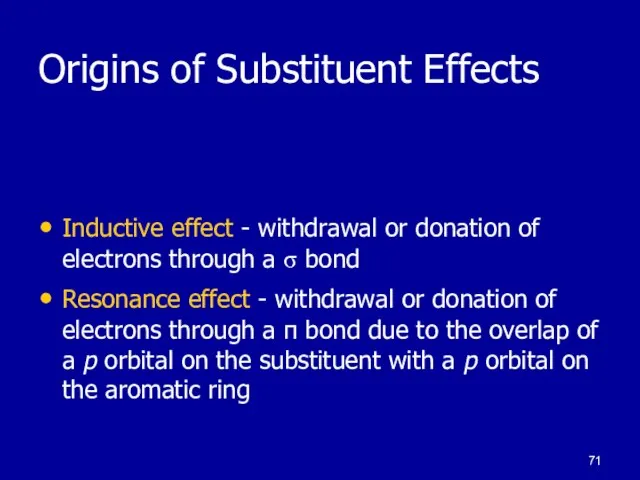
a σ bond
Resonance effect - withdrawal or donation of electrons through a π bond due to the overlap of a p orbital on the substituent with a p orbital on the aromatic ring
Слайд 72Inductive Effects
Controlled by electronegativity and the polarity of bonds in functional groups
Halogens,
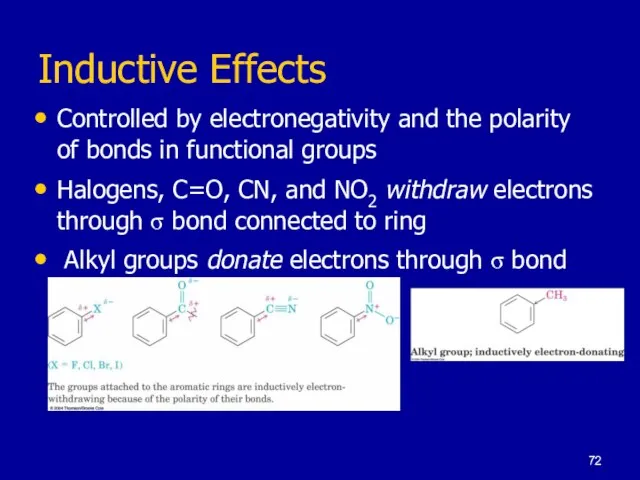
C=O, CN, and NO2 withdraw electrons through σ bond connected to ring
Alkyl groups donate electrons through σ bond
Слайд 73Resonance Effects: Electron Withdrawal
C=O, CN, NO2 substituents withdraw electrons from the aromatic
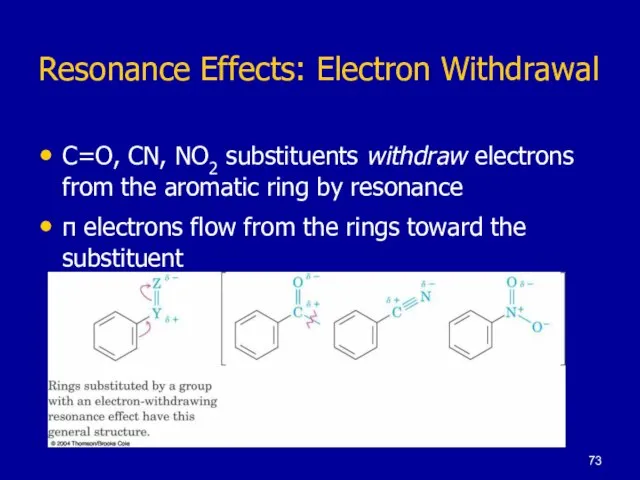
ring by resonance
π electrons flow from the rings toward the substituent
Слайд 74Resonance Effects: Electron Donation
Halogen, OH, alkoxyl (OR), and amino substituents donate electrons
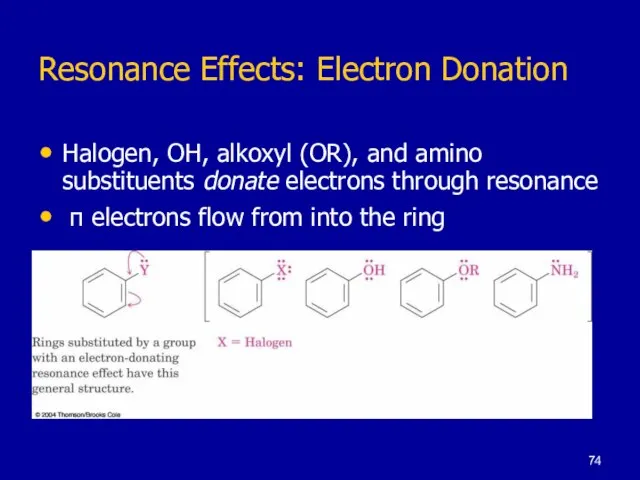
through resonance
π electrons flow from into the ring
Слайд 76Analysis of Data
Methoxy and Methyl
Activating
Ortho and para products
Nitro and Carbomethoxy
Deactivating
Meta product
Bromine
Deactivating
Ortho

and para products
Слайд 77Ring Effects - Conclusions
Activating groups
Substitution is faster than for benzene
Groups direct substitution
to o/p positions
Deactivating Groups
Substitution is slower than for benzene
Groups direct substitution to m position
Halogens
Deactivate ring
Substitution is slower than for benzene
Groups direct substitution to o/p positions
Слайд 78Ring Effects – The Explanation
Activating groups donate electrons to the ring,
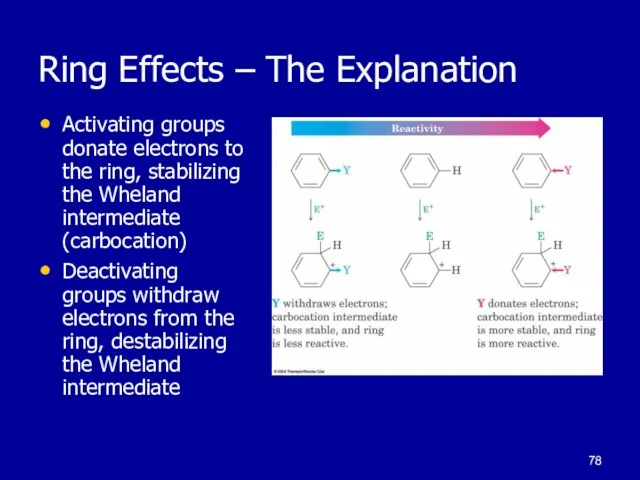
stabilizing the Wheland intermediate (carbocation)
Deactivating groups withdraw electrons from the ring, destabilizing the Wheland intermediate
Слайд 80Oxidation of Benzene
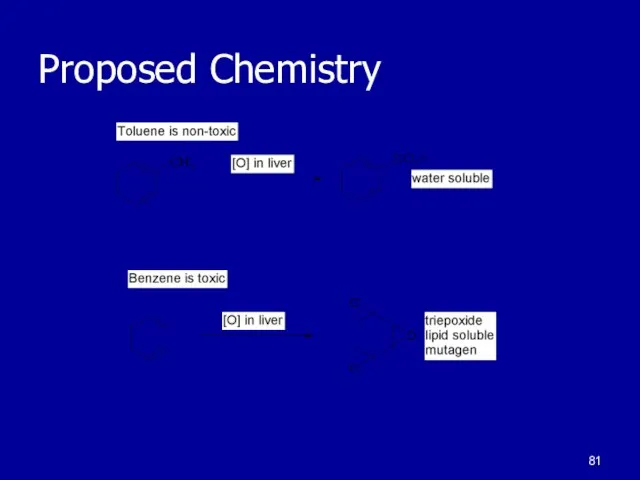
Toluene is readily oxidized by reagents
Benzene is inert to oxidizing

agents
Benzene is toxic to humans
Benzene is a suspected carcinogen
Cytochrom P
strong oxidant in Liver
Primary detoxification process used
Слайд 82Biological Oxidations of Side Chains
Biosynthesis of norepinephrine
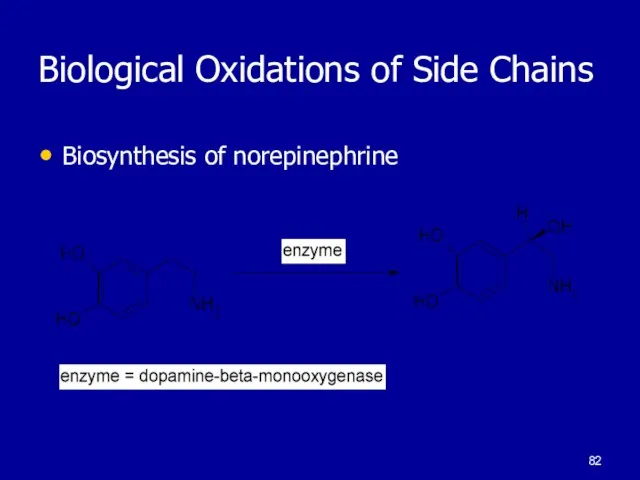
Слайд 83Oxidation of Aromatic Compounds
Alkyl side chains can be oxidized to ⎯CO2H
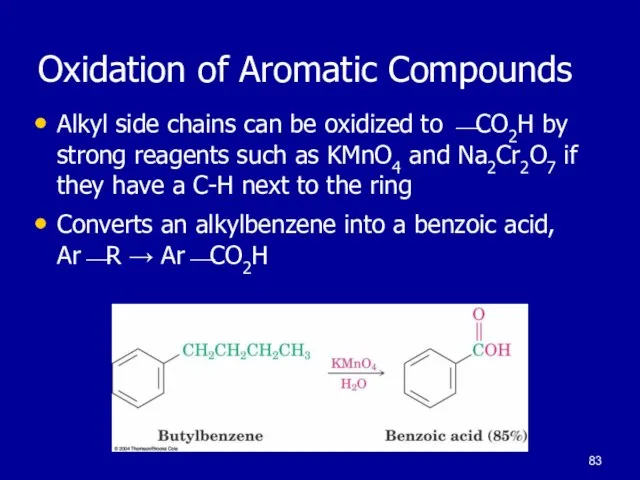
by strong reagents such as KMnO4 and Na2Cr2O7 if they have a C-H next to the ring
Converts an alkylbenzene into a benzoic acid, Ar⎯R → Ar⎯CO2H
Слайд 84Bromination of Alkylbenzene Side Chains
Reaction of an alkylbenzene with N-bromo-succinimide (NBS)
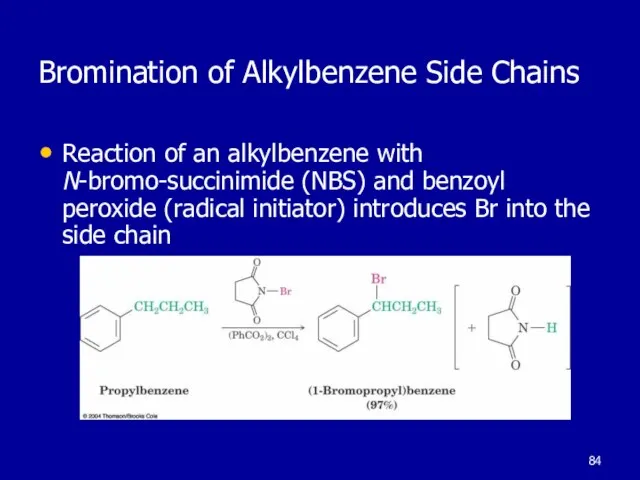
and benzoyl peroxide (radical initiator) introduces Br into the side chain
Слайд 85Reduction of Aromatic Compounds
Aromatic rings are inert to catalytic hydrogenation under

conditions that reduce alkene double bonds
Can selectively reduce an alkene double bond in the presence of an aromatic ring
Reduction of an aromatic ring requires more powerful reducing conditions (high pressure or rhodium catalysts)
Слайд 86Reduction of Aromatic Compounds
Aromatic Rings can be reduced using Li or Na
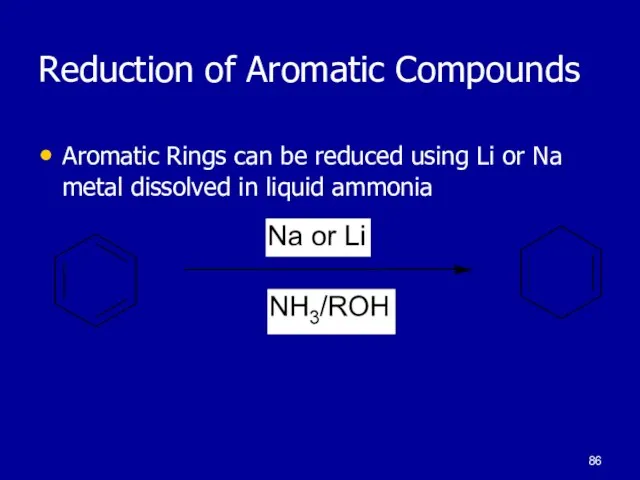
metal dissolved in liquid ammonia










































![Nitrobenzenes: Precursors to Anilines Nitric acid destroys alkenes through [O] In sulfuric](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/377883/slide-45.jpg)



































 Вплив соціально-економічних умов на формування особистості
Вплив соціально-економічних умов на формування особистості Банки в посткризисной экономике:модернизация ради прибыли
Банки в посткризисной экономике:модернизация ради прибыли Внедрение здоровьесберегающих технологий В.Ф. Базарного в практику работы учителя-дефектолога
Внедрение здоровьесберегающих технологий В.Ф. Базарного в практику работы учителя-дефектолога Задача линейного программирования и транспортная задача
Задача линейного программирования и транспортная задача  Авиационная травма
Авиационная травма Прощаемся с тёплым летом
Прощаемся с тёплым летом Поддержка программы Microsoft IT Academy
Поддержка программы Microsoft IT Academy Дополнительная общеразвивающая программа технической направленности самоделкины
Дополнительная общеразвивающая программа технической направленности самоделкины Взаимосвязь животных в природе
Взаимосвязь животных в природе Добывающие предприятия ОАО "Атомредметзолото" как естественные минерально-сырьевые центры по урану на территории России и предло
Добывающие предприятия ОАО "Атомредметзолото" как естественные минерально-сырьевые центры по урану на территории России и предло Мобильная фотография
Мобильная фотография История профсоюзного движения. Знаковые события
История профсоюзного движения. Знаковые события Трудовые правоотношения и заключение трудового договора
Трудовые правоотношения и заключение трудового договора Архитектурный облик Древней Руси
Архитектурный облик Древней Руси Презентация на тему Ввод информации с бумажных носителей
Презентация на тему Ввод информации с бумажных носителей Основное свойство дроби
Основное свойство дроби Бегун сегодня и завтра
Бегун сегодня и завтра Белый камень
Белый камень Гражданское право. Понятие и предмет гражданского права
Гражданское право. Понятие и предмет гражданского права business letter
business letter  Драматические образы в музыке
Драматические образы в музыке Кавказская война
Кавказская война Поворот
Поворот Семья в историческом интерьере
Семья в историческом интерьере Занимательная орфография
Занимательная орфография Понятие и виды социального предпринимательства
Понятие и виды социального предпринимательства Презентация на тему Породы древесины
Презентация на тему Породы древесины Общая характеристика производства по делам об административных правонарушениях. Доказательства и процесс доказывания. Тема №2
Общая характеристика производства по делам об административных правонарушениях. Доказательства и процесс доказывания. Тема №2