Слайд 2SOLUTION AND SOLUBILITIES
TERMS
Solution: a homogeneous mixture containing particles the size of a

typical ion or covalent molecule. (0.1–2.0 nm in diameter)
Colloid: a homogeneous mixture containing particles with diameters in the range 2–500 nm
Suspensions are mixtures with even larger particles, but they are not considered true solutions because they separate upon standing.
Solute: the dissolved substance in a solution
Solvent: the major component in a solution
Слайд 3SOLUTION AND SOLUBILITIES
A solution is saturated when no additional solute can be

dissolved at a particular temperature
A Supersaturated solution can form when more than the equilibrium amount of solute is dissolved at an elevated temperature, and then the supersaturated solution is slowly cooled.
An Unsaturated solution is formed when more of the solute can dissolve in it at a particular temperature.
Слайд 4SOLUTION AND SOLUBILITIES
KINDS OF SOLUTIONS
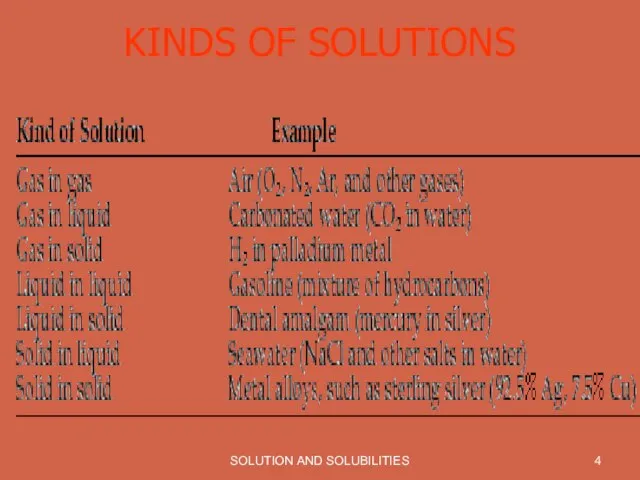
Слайд 6SOLUTION AND SOLUBILITIES
SOLUBILITY
The amount of solute per unit solvent required to form

a saturated solution is called the solute's Solubility.
When two liquids are completely soluble in each other they are said to be Miscible.
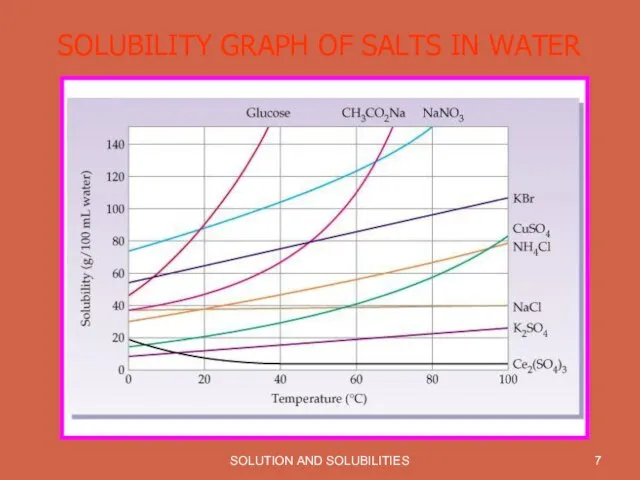
Solubility is effected by Temperature. With increase in temperature solubility of most of the substances increases.
Most gases become less soluble in water as the temperature increases.
Слайд 7SOLUTION AND SOLUBILITIES
SOLUBILITY GRAPH OF SALTS IN WATER
Слайд 8SOLUTION AND SOLUBILITIES
SOLUBILITY GRAPH OF GASES IN WATER
Pressure has little effect on
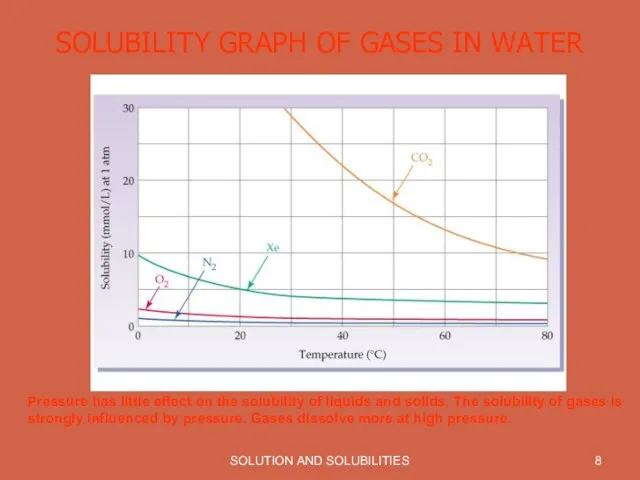
the solubility of liquids and solids. The solubility of gases is strongly influenced by pressure. Gases dissolve more at high pressure.
Слайд 9SOLUTION AND SOLUBILITIES
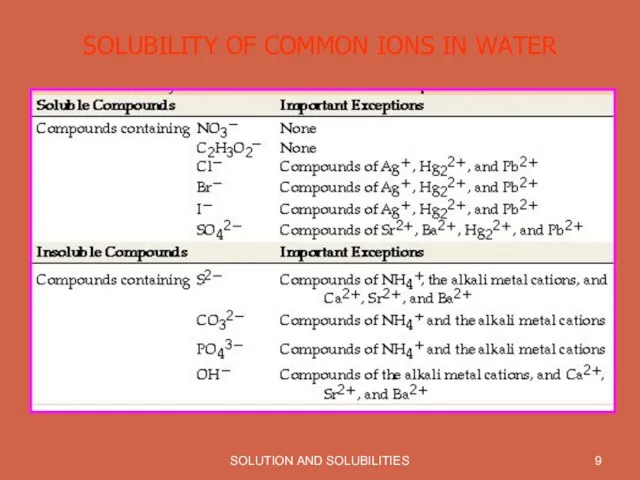
SOLUBILITY OF COMMON IONS IN WATER
Слайд 10SOLUTION AND SOLUBILITIES
DISSOLUTION OF SODIUM CHLORIDE IN WATER










 Ресторан Золотой дракон
Ресторан Золотой дракон Конфлікти в закладі дошкільної освіти
Конфлікти в закладі дошкільної освіти Игра Пирамида
Игра Пирамида Как человек открывал Землю
Как человек открывал Землю Занимательные клеточки
Занимательные клеточки Компьютерные вирусы
Компьютерные вирусы FN1_LessonOne
FN1_LessonOne Цилиндр и конус
Цилиндр и конус Основы работы профБюро факультета
Основы работы профБюро факультета Педагогический советГОУ СОШ № 547 протокол № 1 от 30.08.2010 г.
Педагогический советГОУ СОШ № 547 протокол № 1 от 30.08.2010 г. Сборник артикуляционных упражнений
Сборник артикуляционных упражнений Темперамент. Для учащихся 5 класса
Темперамент. Для учащихся 5 класса Инжиниринговый центр Краснодарского края
Инжиниринговый центр Краснодарского края Древний Китай
Древний Китай Гимнастика
Гимнастика Методология проекта. Культура Японии
Методология проекта. Культура Японии Окказионализмы в детском словотворчестве
Окказионализмы в детском словотворчестве История развития пожарного дела в России
История развития пожарного дела в России Звук К
Звук К RoomTour show. Экскурсия по домам медийных личностей
RoomTour show. Экскурсия по домам медийных личностей Внеплановая Чёрная Пятница теперь в М.Видео
Внеплановая Чёрная Пятница теперь в М.Видео Формирование ключевых компетенций средствами межпредметной интеграции и использованием регионального компонента
Формирование ключевых компетенций средствами межпредметной интеграции и использованием регионального компонента Исследование уровня интернет-зависимости уучащихся
Исследование уровня интернет-зависимости уучащихся Этика, культура делового общения
Этика, культура делового общения Сон Иосифа
Сон Иосифа Что такое техника?
Что такое техника? Перспективы развития информационных технологий в библиотечной сфере Хабаровского края
Перспективы развития информационных технологий в библиотечной сфере Хабаровского края Наркотизм и национальная безопасность. Тест
Наркотизм и национальная безопасность. Тест