Содержание
- 2. Main objectives: 1. Find out basics of convection, conduction and radiation 2. Understand main principles of
- 3. Basics of Heat Transfer Heat is the form of energy that can be transferred from one
- 4. Heat Transfer Fig. 1. In the early nineteenth century, heat was thought to be an invisible
- 5. Heat Transfer Fig. 2. We are normally interested in how long it takes for the hot
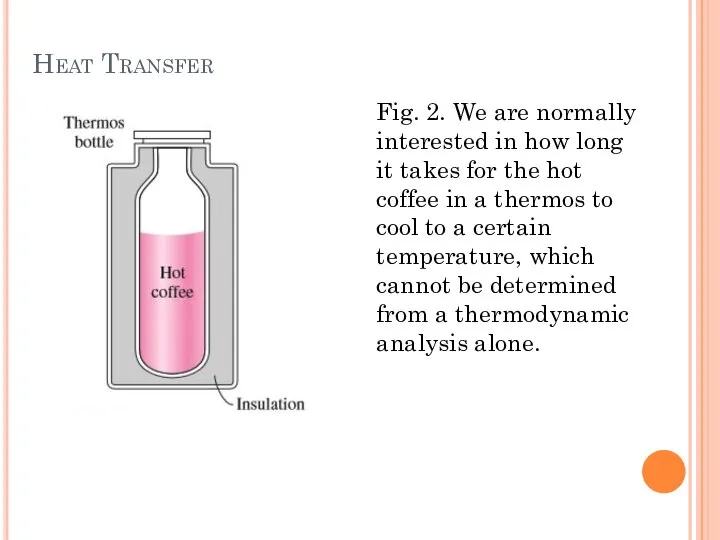
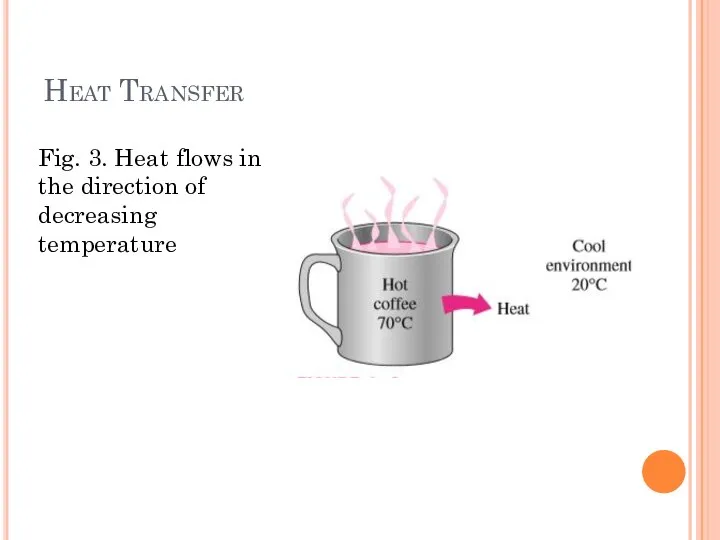
- 6. Heat Transfer Fig. 3. Heat flows in the direction of decreasing temperature
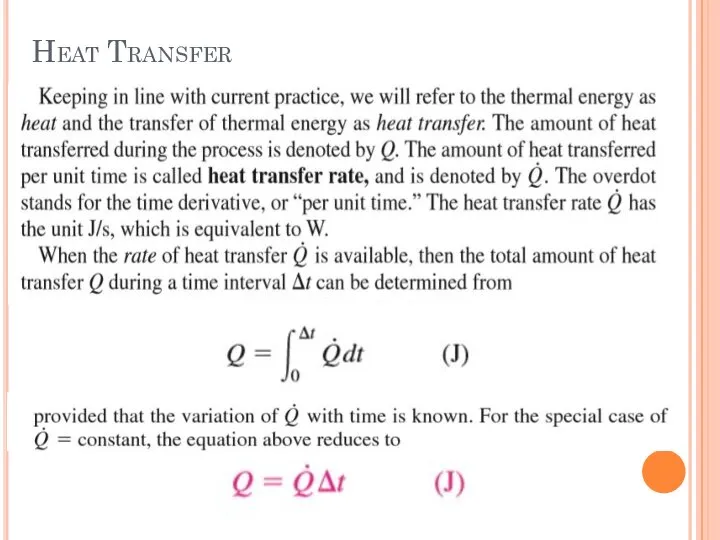
- 7. Heat Transfer
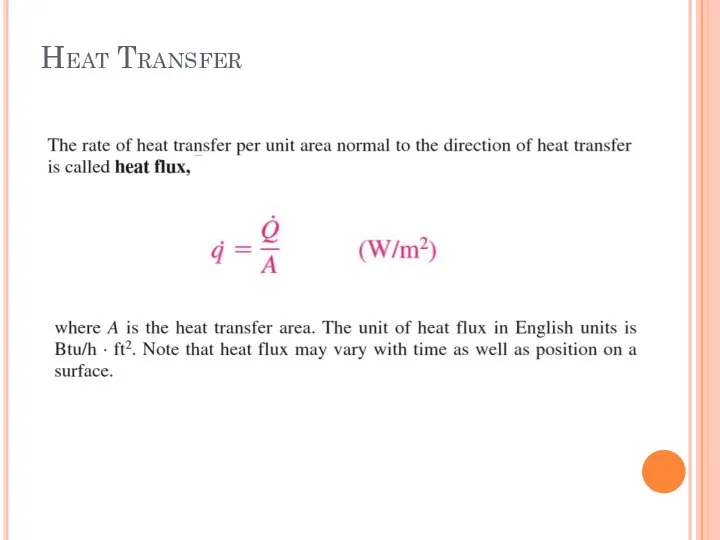
- 8. Heat Transfer
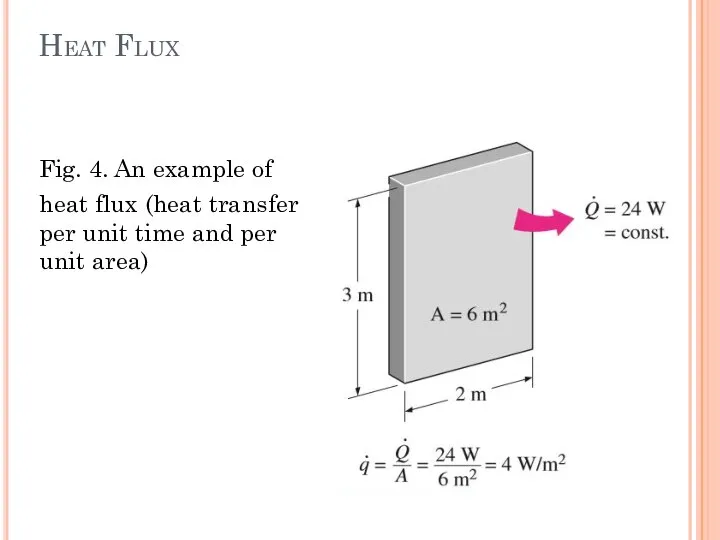
- 9. Heat Flux Fig. 4. An example of heat flux (heat transfer per unit time and per
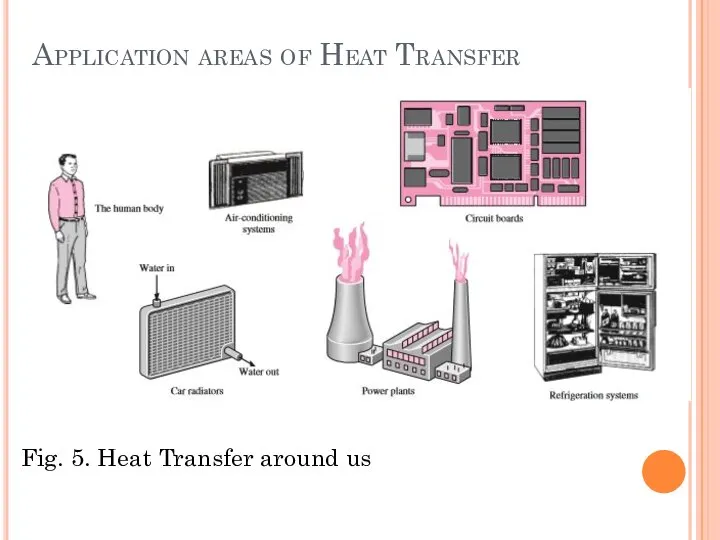
- 10. Application areas of Heat Transfer Heat transfer is commonly encountered in engineering systems and other aspects
- 11. Application areas of Heat Transfer Many ordinary household appliances are designed, in whole or in part,
- 12. Application areas of Heat Transfer Fig. 5. Heat Transfer around us
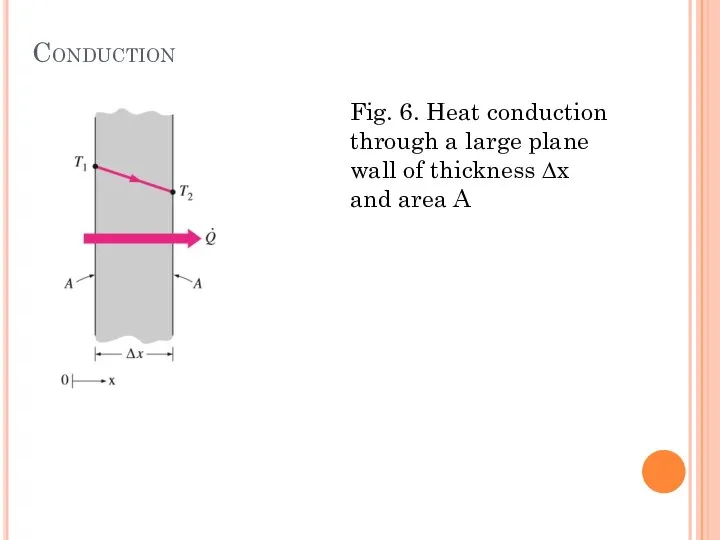
- 13. Conduction Conduction is the transfer of energy from the more energetic particles of a substance to
- 14. Conduction Fig. 6. Heat conduction through a large plane wall of thickness ∆x and area A
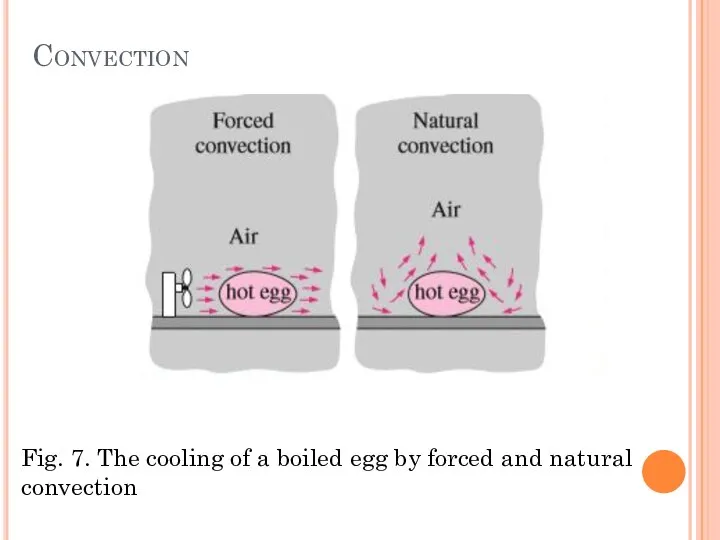
- 15. Convection Convection is the mode of energy transfer between a solid surface and the adjacent liquid
- 16. Convection Convection is called forced convection if the fluid is forced to flow over the surface
- 17. Convection Fig. 7. The cooling of a boiled egg by forced and natural convection
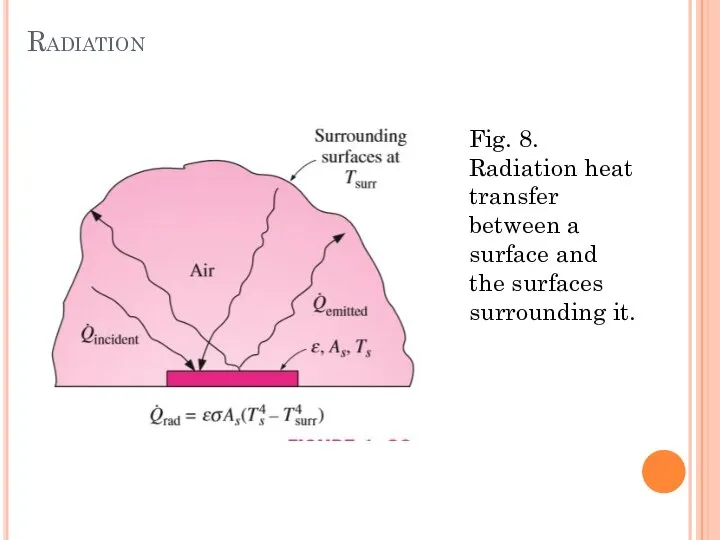
- 18. Radiation Radiation is the energy emitted by matter in the form of electromagnetic waves (or photons)
- 19. Radiation Fig. 8. Radiation heat transfer between a surface and the surfaces surrounding it.
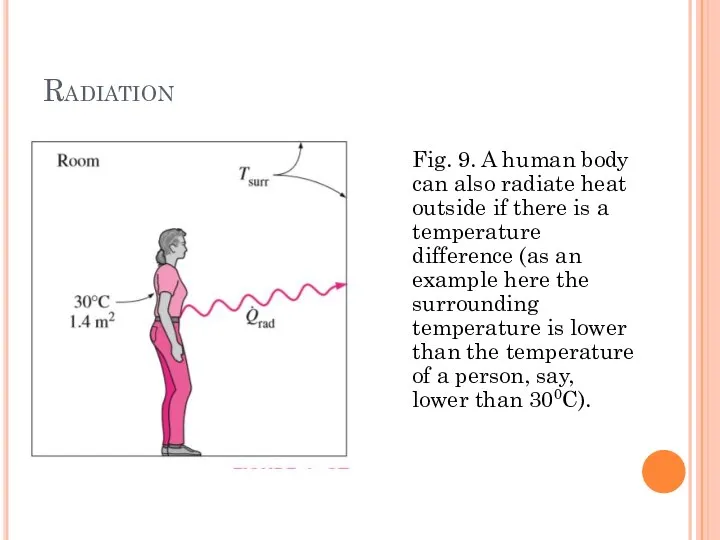
- 20. Radiation Fig. 9. A human body can also radiate heat outside if there is a temperature
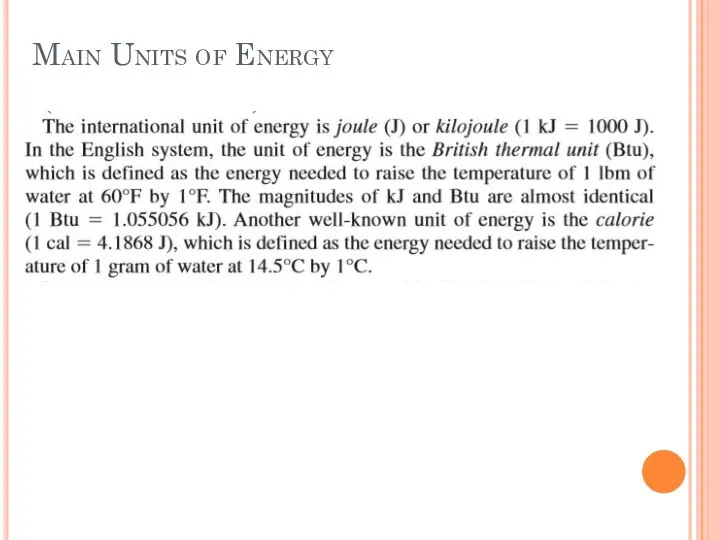
- 21. Main Units of Energy
- 22. British Thermal Unit The British thermal unit (BTU or Btu) is a traditional unit of work
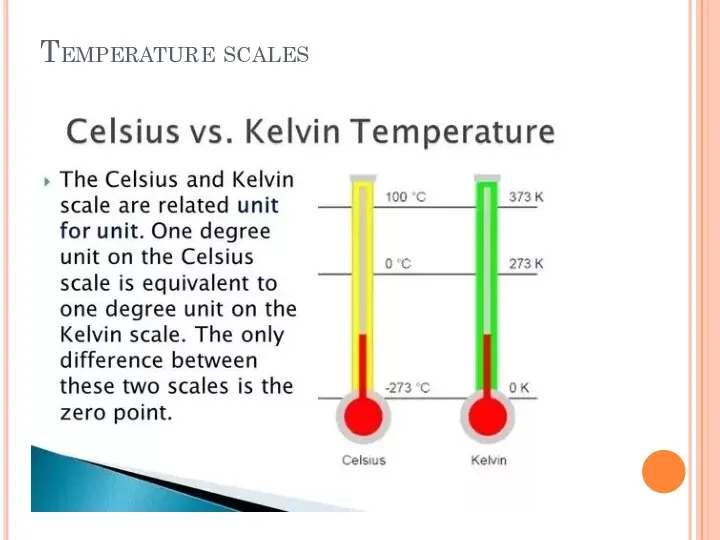
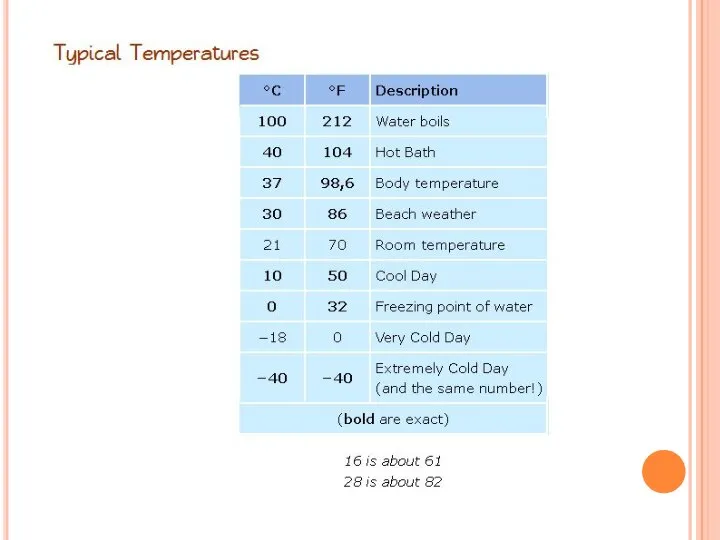
- 23. Temperature scales
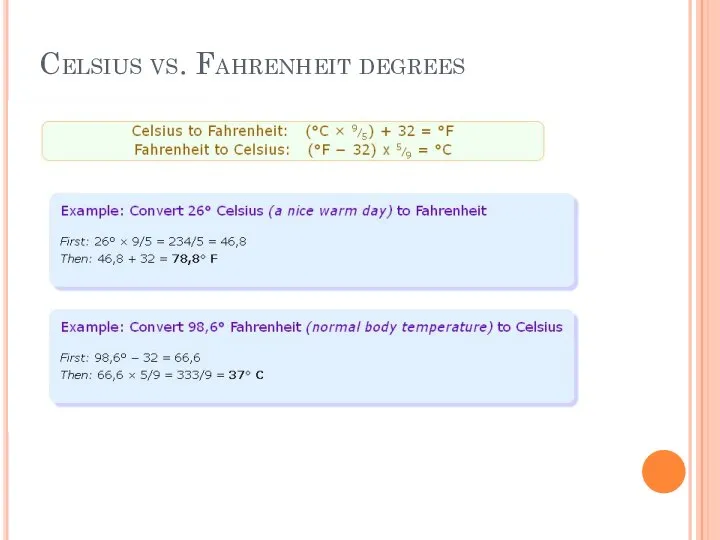
- 24. Celsius vs. Fahrenheit degrees
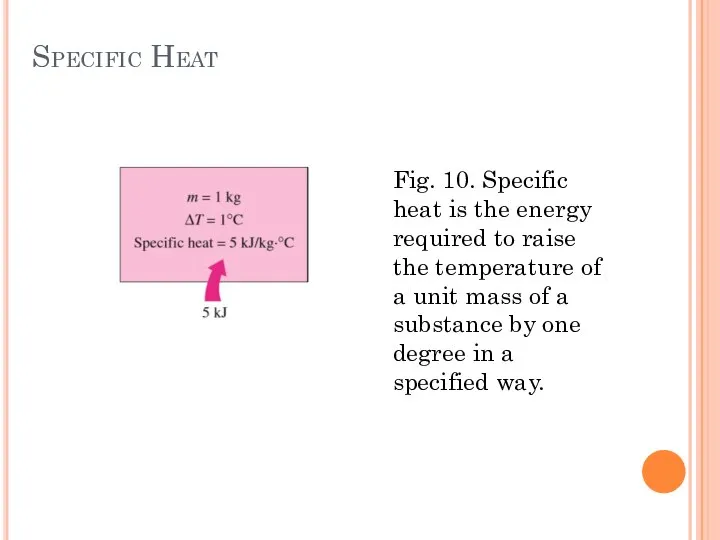
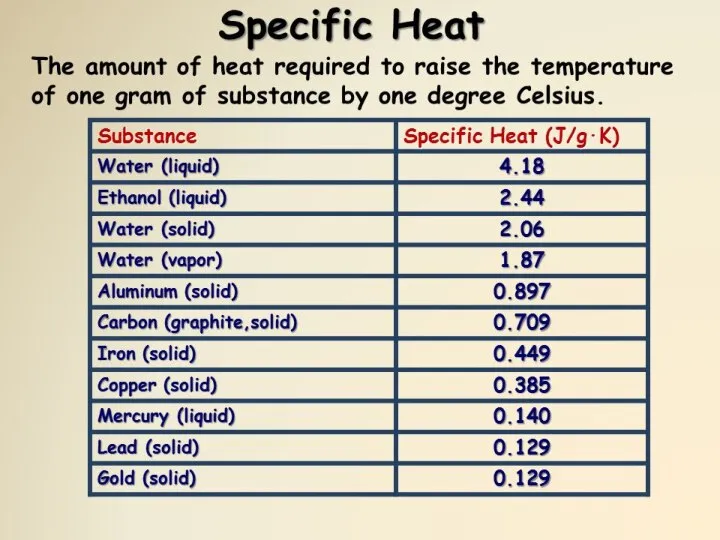
- 26. Specific heats of Gases, Liquids and Solids Specific heat is defined as the energy required to
- 27. Specific Heat Fig. 10. Specific heat is the energy required to raise the temperature of a
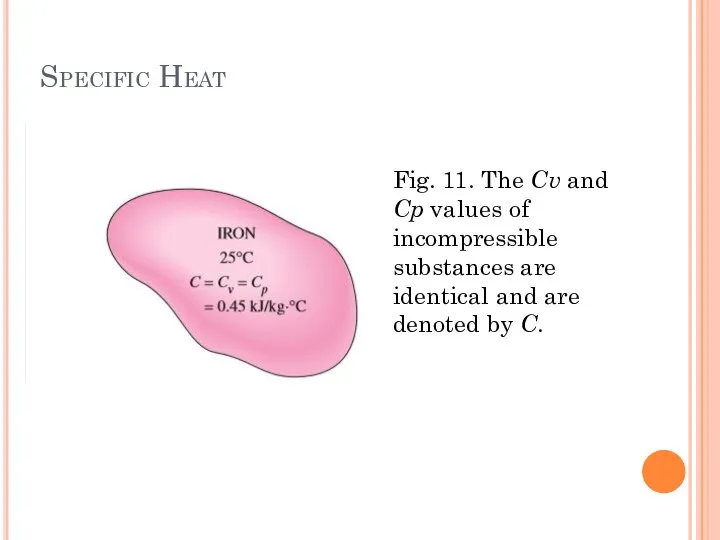
- 28. Specific Heat Fig. 11. The Cv and Cp values of incompressible substances are identical and are
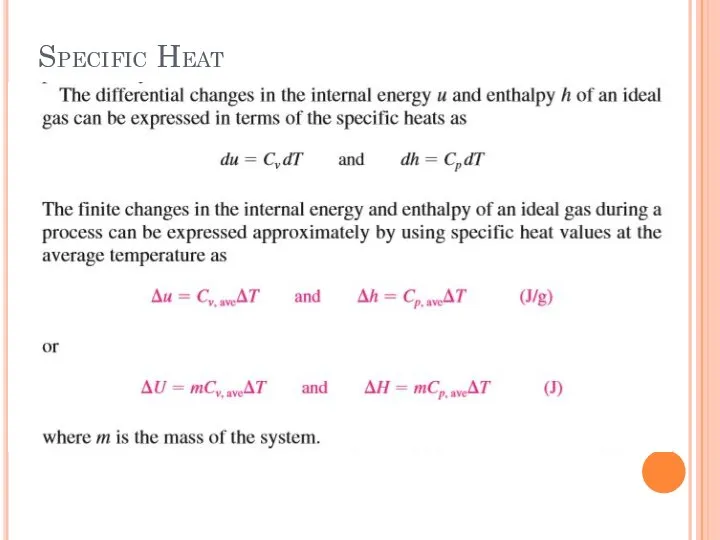
- 29. Specific Heat
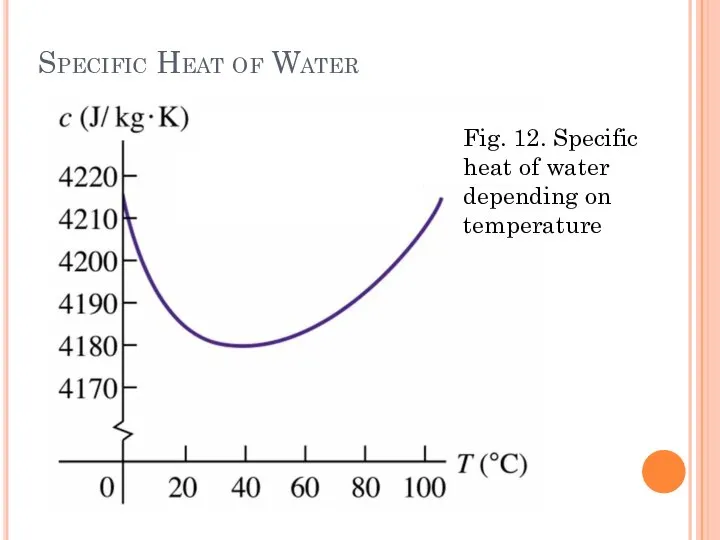
- 31. Specific Heat of Water Fig. 12. Specific heat of water depending on temperature
- 33. Скачать презентацию








 Биохимия мышц
Биохимия мышц вегетативная н.с
вегетативная н.с Представления о происхождении и развитии жизни на Земле
Представления о происхождении и развитии жизни на Земле Отряд Куриные
Отряд Куриные Презентация на тему Многообразие пресмыкающихся
Презентация на тему Многообразие пресмыкающихся  Морской скат
Морской скат Хохлатый жаворонок - птица Красной книги
Хохлатый жаворонок - птица Красной книги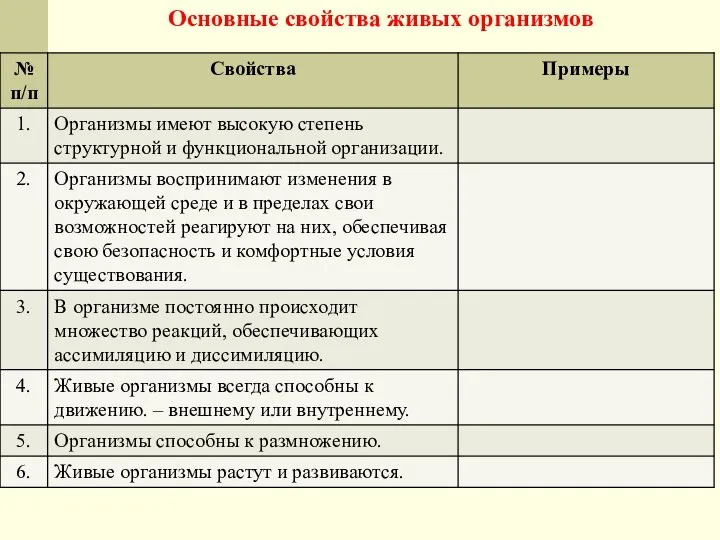 Основные свойства живых организмов
Основные свойства живых организмов Постэмбриональное развитие организмов
Постэмбриональное развитие организмов Топографическая и половая изменчивость высоты межпозвоночных дисков и тел позвонков поясничного отдела позвоночника
Топографическая и половая изменчивость высоты межпозвоночных дисков и тел позвонков поясничного отдела позвоночника Изучение искусственного отбора
Изучение искусственного отбора Хамелеон. 7 клас
Хамелеон. 7 клас Презентация на тему Признаки живых организмов
Презентация на тему Признаки живых организмов  Физиология ЦНС
Физиология ЦНС Семейство крестоцветных
Семейство крестоцветных Жизнь и физическая среда и адаптация организмов к ним
Жизнь и физическая среда и адаптация организмов к ним Нервные окончания
Нервные окончания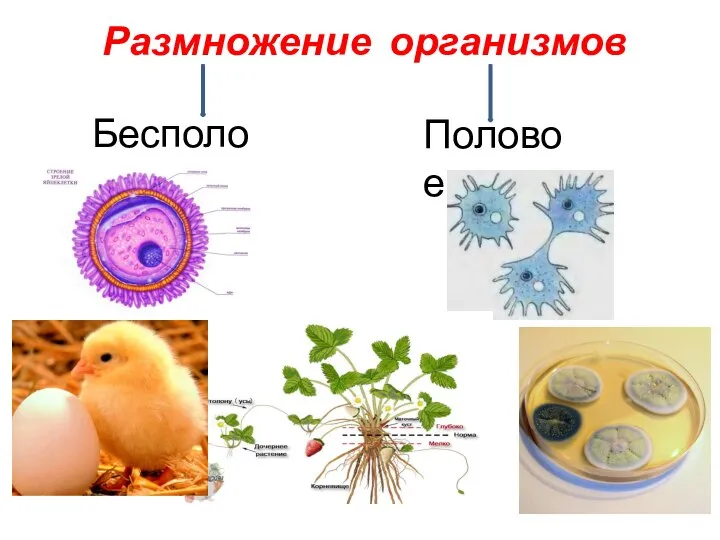 Бесполое и половое размножение организмов
Бесполое и половое размножение организмов Калориметрические методы анализа биомолекул
Калориметрические методы анализа биомолекул Презентация на тему Жабы и лягушки
Презентация на тему Жабы и лягушки  Кумкват. Характеристика кумквата
Кумкват. Характеристика кумквата Пищеварение в ротовой полости. Глотка и пищевод
Пищеварение в ротовой полости. Глотка и пищевод Неполное доминирование. Генотип и фенотип. Анализирующее скрещивание
Неполное доминирование. Генотип и фенотип. Анализирующее скрещивание Коллапс трахеи у йоркширского терьера
Коллапс трахеи у йоркширского терьера Общая характеристика гельминтов класса цестод. Цистицеркозы крупного рогатого скота и свиней, их медико-социальное значение
Общая характеристика гельминтов класса цестод. Цистицеркозы крупного рогатого скота и свиней, их медико-социальное значение Типы телосложения. Понятие конституции
Типы телосложения. Понятие конституции Амниоты. Класс пресмыкающиеся
Амниоты. Класс пресмыкающиеся Мозг и инстинкт
Мозг и инстинкт