Содержание
- 2. Please feel free to use and share some or all of these slides in your noncommercial
- 3. CheckMate 274 Exploratory Analysis: Background Phase III CheckMate 274 trial evaluated adjuvant nivolumab vs placebo in
- 4. CheckMate 274 Exploratory Analysis: Study Design1 Patient Population CheckMate 274 (n = 699) Minimum follow-up: 11.0
- 5. CheckMate 274 Exploratory Analysis: IFN-γ Signature, CD4 Gene Expression Predict Nivolumab Efficacy In IFN-γ gene signature
- 6. CheckMate 274 Exploratory Analysis: CD8 and TMB Prognostic of Improved DFS Necchi. ESMO 2022. Abstr 1737MO.
- 7. CheckMate 274 Exploratory Analysis: Investigators’ Conclusions Positive association found for DFS and biomarkers of preexisting antitumor
- 9. Скачать презентацию
Слайд 2Please feel free to use and share some or all of these
Please feel free to use and share some or all of these

When using our slides, please retain the source attribution:
These slides may not be published, posted online, or used in commercial presentations without permission. Please contact [email protected] for details
About These Slides
Слайд 3CheckMate 274 Exploratory Analysis: Background
Phase III CheckMate 274 trial evaluated adjuvant nivolumab
CheckMate 274 Exploratory Analysis: Background
Phase III CheckMate 274 trial evaluated adjuvant nivolumab

Trial met both primary endpoints of improving DFS in ITT (HR: 0.70; P <.001) and PD-L1 ≥1% (HR: 0.55; P <.001) patient populations
Adjuvant treatment with nivolumab is now approved for patients with high-risk muscle-invasive urothelial carcinoma following radical resection2
Clinical trial data from immunotherapy studies in urothelial carcinoma suggest predictive association with PD-L1, TMB, immune infiltration (CD8 and CD4), and activation signatures (eg, IFN-γ)3-7
This exploratory biomarker analysis assessed clinical association of pretreatment tumor and immune features with DFS in patients with muscle-invasive urothelial carcinoma receiving adjuvant nivolumab in CheckMate 274 trial8
1. Bajorin. NEJM. 2021;384:2102. 2. Nivolumab PI. 3. Wang. Nature Commun. 2018;9:3503.
4. van Dijk. Nat Med. 2020;26:1839. 5. Zheng. Front Genet. 2021;12:764184.
6. Sharma. Lancet Oncol. 2017;18:312. 7. Galsky. Clin Can Res. 2020;26:5120. 8. Necchi. ESMO 2022. Abstr 1737MO.
Слайд 4CheckMate 274 Exploratory Analysis: Study Design1
Patient Population
CheckMate 274 (n = 699)
Minimum follow-up:
CheckMate 274 Exploratory Analysis: Study Design1
Patient Population
CheckMate 274 (n = 699)
Minimum follow-up:
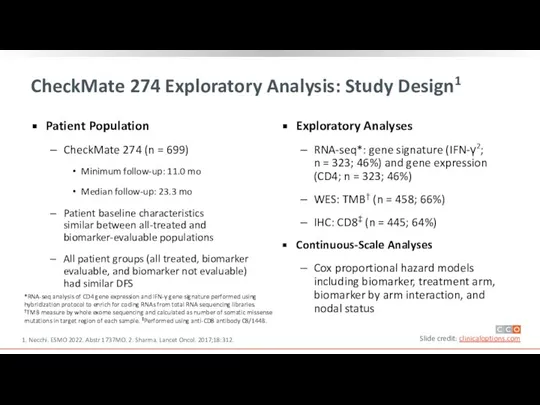
Median follow-up: 23.3 mo
Patient baseline characteristics similar between all-treated and biomarker-evaluable populations
All patient groups (all treated, biomarker evaluable, and biomarker not evaluable) had similar DFS
Exploratory Analyses
RNA-seq*: gene signature (IFN-γ2;
n = 323; 46%) and gene expression (CD4; n = 323; 46%)
WES: TMB† (n = 458; 66%)
IHC: CD8‡ (n = 445; 64%)
Continuous-Scale Analyses
Cox proportional hazard models including biomarker, treatment arm, biomarker by arm interaction, and nodal status
1. Necchi. ESMO 2022. Abstr 1737MO. 2. Sharma. Lancet Oncol. 2017;18:312.
*RNA-seq analysis of CD4 gene expression and IFN-γ gene signature performed using hybridization protocol to enrich for coding RNAs from total RNA sequencing libraries.
†TMB measure by whole exome sequencing and calculated as number of somatic missense mutations in target region of each sample. ‡Performed using anti-CD8 antibody C8/144B.
Слайд 5CheckMate 274 Exploratory Analysis: IFN-γ Signature, CD4 Gene Expression Predict Nivolumab Efficacy
In
CheckMate 274 Exploratory Analysis: IFN-γ Signature, CD4 Gene Expression Predict Nivolumab Efficacy
In

IFN-γ gene signature score and DFS were positively associated (P <.001)
IFN-γ gene signature score varied with treatment effect (P = .013)
Higher IFN-γ gene signature score was associated with improved DFS with nivolumab but not placebo
In CD4 gene expression tertiles comparisons (n = 323)
CD4 gene expression and DFS were positively associated (P = .001)
CD4 gene expression varied with treatment effect (P <.001)
Higher CD4 gene expression was associated with improved DFS with nivolumab but not placebo
Necchi. ESMO 2022. Abstr 1737MO.
Слайд 6CheckMate 274 Exploratory Analysis:
CD8 and TMB Prognostic of Improved DFS
Necchi. ESMO
CheckMate 274 Exploratory Analysis:
CD8 and TMB Prognostic of Improved DFS
Necchi. ESMO

In CD8 IHC score tertiles comparisons (n = 445)
CD8 digital IHC score and DFS were positively associated (P <.001)
Treatment effect did not appear to vary with CD8 IHC score (P = .153)
Higher CD8 infiltration associated with improved DFS for both nivolumab and placebo
Strong positive correlation was identified between IFN-γ gene signature and CD8 digital IHC score (r = 0.80)
In TMB score tertiles* comparisons (n = 458)
TMB score and DFS were positively associated (P <.001)
Higher TMB score was associated with improved DFS with nivolumab, but evidence that it differed with placebo is limited (P = .081)
Trend for higher IFN-γ signature,
CD4 gene expression, and CD8 digital IHC, but not TMB biomarker distribution, with PD-L1 ≥1% status
*TMB tertiles: low (<74 mutations), medium (≥74 and <172), and high (≥ 172).
Слайд 7CheckMate 274 Exploratory Analysis:
Investigators’ Conclusions
Positive association found for DFS and biomarkers
CheckMate 274 Exploratory Analysis:
Investigators’ Conclusions
Positive association found for DFS and biomarkers

Gene signature for IFN-γ and gene expression for CD4 found predictive of clinical benefit with adjuvant nivolumab2
CD8 infiltration was observed to be prognostic of DFS2
Efficacy with nivolumab was positively associated with TMB and DFS2
Investigators concluded that these results reinforce mechanism for benefit with immunotherapy2
Validation of prior findings in metastatic urothelial carcinoma to adjuvant setting
Additional research needed to confirm utility of these findings for clinical trial design and informing treatment decisions in real world
1. Chen. Nature. 2017;541:321. 2. Necchi. ESMO 2022. Abstr 1737MO.
 СОЦИАЛЬНЫЙ ПРОЕКТ «Там на ухоженных дорожках»
СОЦИАЛЬНЫЙ ПРОЕКТ «Там на ухоженных дорожках» Развитие креативности по Альберту Эйнштейну
Развитие креативности по Альберту Эйнштейну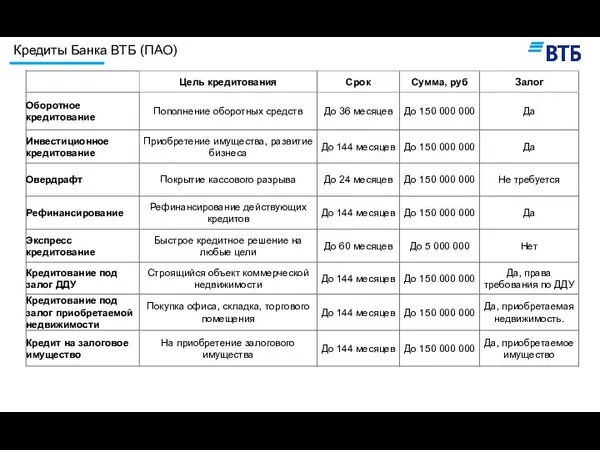 Кредиты банка ВТБ (ПАО)
Кредиты банка ВТБ (ПАО) Что же делать ?
Что же делать ? Права человека
Права человека Проблема готовности к школьному обучению в психолого – педагогических исследованиях .
Проблема готовности к школьному обучению в психолого – педагогических исследованиях . Псалмы 46-54
Псалмы 46-54 ЛР1
ЛР1 Мероприятия и промоушены. Август 2021
Мероприятия и промоушены. Август 2021 Про кошек и собак (2 класс)
Про кошек и собак (2 класс) РАЗГОВОР О ПРАВИЛЬНОМ ПИТАНИИ
РАЗГОВОР О ПРАВИЛЬНОМ ПИТАНИИ Информационно-техническое обеспечение рынка ценных бумаг
Информационно-техническое обеспечение рынка ценных бумаг Информация об областном конкурсе научных работ по проблемам развития агропромышленного комплекса.
Информация об областном конкурсе научных работ по проблемам развития агропромышленного комплекса. Карев Групп
Карев Групп 20170420_afrika.microsoft_powerpoint
20170420_afrika.microsoft_powerpoint Devoirs 2
Devoirs 2 Последствия после применения допинга (исследовательский проект )
Последствия после применения допинга (исследовательский проект ) Маркетинговое исследование авторынка
Маркетинговое исследование авторынка Уважаемые Десятиклассники! Познакомьтесь с материалами данной презентации и поучаствуйте в тестировании. Ответы на вопросы – те
Уважаемые Десятиклассники! Познакомьтесь с материалами данной презентации и поучаствуйте в тестировании. Ответы на вопросы – те Real Estate Market Analysis Basic Principles
Real Estate Market Analysis Basic Principles Посмотри вокруг 2 класс
Посмотри вокруг 2 класс Читает академик АН СИ Иван Петров
Читает академик АН СИ Иван Петров Методички 1) ЭКГ 2) Схема истории болезни
Методички 1) ЭКГ 2) Схема истории болезни Тропы
Тропы СОЧИНЯЙ - КА
СОЧИНЯЙ - КА Толерантность в школе
Толерантность в школе Презентация на тему Барельеф Изобразительное искусство
Презентация на тему Барельеф Изобразительное искусство Карьера в СМИ
Карьера в СМИ