Содержание
- 2. PRESERVATIVE REDUCTION With Increasing Regulatory Framework EVERY STEP OF THE WAY The main regulatory framework for
- 3. ANNEX V - PRESERVATIVES Allowable & Unallowable Governs the use of list of allowable preservatives in
- 4. EVERY STEP OF THE WAY 5 THE PROBLEM WITH PRESERVATIVES… Dwindling options Preservatives that are not
- 5. the old methods of dirty manufacturing are no longer acceptable
- 6. Cosmetic manufacturers must be concerned about the safety of their ingredients while ensuring their product is
- 7. KEEPING PACE WITH REGULATORY EXPECTATIONS …and responding to the pressures of business EVERY STEP OF THE
- 8. ANTIMICROBIAL ACTIVITY BEGINS IN PRODUCTION Not with Formulation EVERY STEP OF THE WAY Antimicrobial activity is
- 9. EVERY STEP OF THE WAY Is unknown or uncontrolled microbial contamination during production making you preservative-dependent?
- 10. ACHIEVING A STATE OF CLEAN-BY-DESIGN Reducing Risk by Proactive Quality EVERY STEP OF THE WAY Manufacturers
- 11. ADDITIONAL GUIDANCE EVERY STEP OF THE WAY Guidance can be found in: ISO 22716:2007 - Cosmetics
- 12. EVERY STEP OF THE WAY Raw materials, in-process samples, and final product testing should be implemented
- 13. ALTERNATIVE MICROBIAL DETECTION METHODS Rapid microbial detection technology allows companies to quickly and accurately determine whether
- 14. EVERY STEP OF THE WAY Just being rapid isn’t enough. Your RMM needs to be the
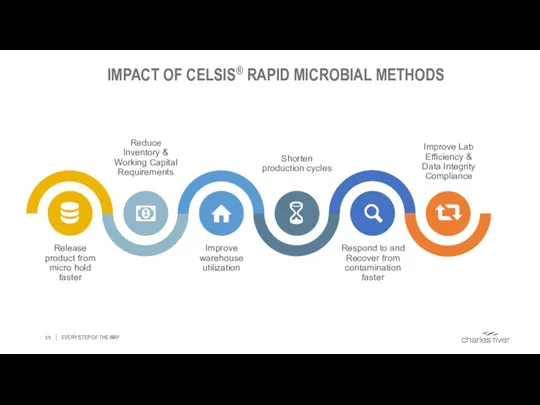
- 15. IMPACT OF CELSIS® RAPID MICROBIAL METHODS EVERY STEP OF THE WAY Release product from micro hold
- 17. EVERY STEP OF THE WAY BUILT FOR SIMPLICITY Celsis® Rapid Microbial Detection Instruments Easy integration Into
- 18. SAME FEATURES. DIFFERENT SIZES. Celsis® Rapid Microbial Detection Instruments EVERY STEP OF THE WAY the Celsis
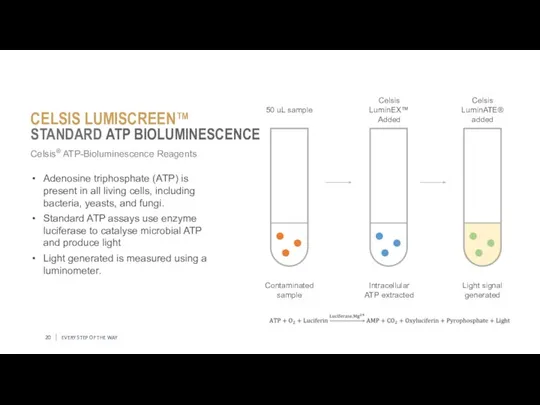
- 20. EVERY STEP OF THE WAY Celsis® ATP-Bioluminescence Reagents Adenosine triphosphate (ATP) is present in all living
- 21. EVERY STEP OF THE WAY Celsis® ATP-Bioluminescence Reagents Two-phase, proprietary enzyme reaction All living organisms also
- 22. EVERY STEP OF THE WAY ROBUST REAGENTS. RAPID RESULTS. Celsis® ATP-Bioluminescence Reagents A rapid microbial detection
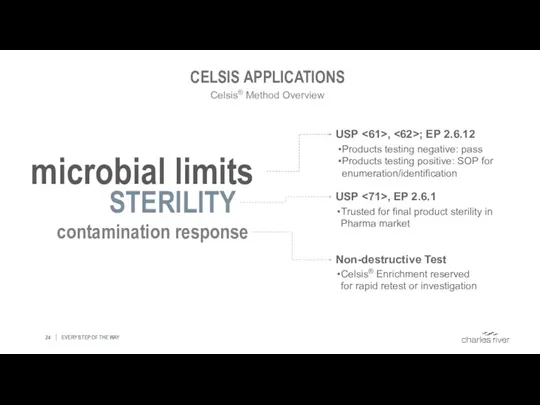
- 24. CELSIS APPLICATIONS EVERY STEP OF THE WAY Celsis® Method Overview microbial limits STERILITY contamination response
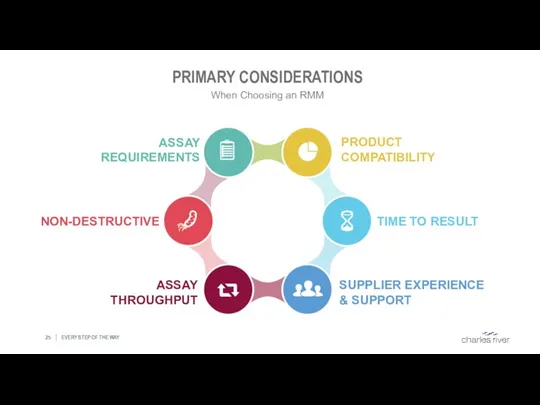
- 25. PRIMARY CONSIDERATIONS EVERY STEP OF THE WAY When Choosing an RMM SUPPLIER EXPERIENCE & SUPPORT TIME
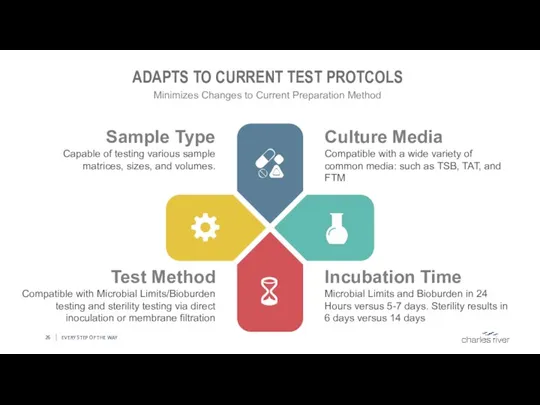
- 26. EVERY STEP OF THE WAY ADAPTS TO CURRENT TEST PROTCOLS Minimizes Changes to Current Preparation Method
- 27. FLEXIBLE PROTOCOL FOR BROAD PRODUCT SUITABILITY Celsis AMPiScreen® Method Overview EVERY STEP OF THE WAY W/
- 28. EVERY STEP OF THE WAY EXAMPLE PROTOCOL – MICRO LIMITS TESTING Celsis AMPiScreen® Method Overview Direct
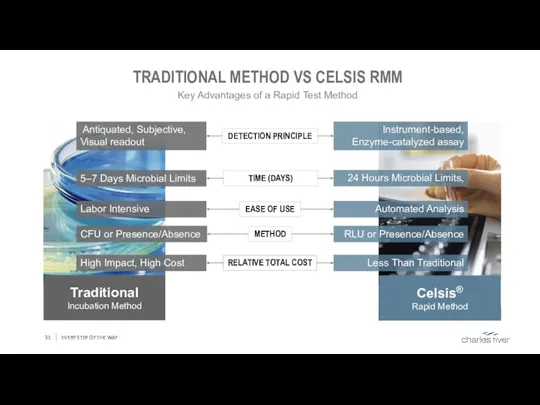
- 31. EVERY STEP OF THE WAY TRADITIONAL METHOD VS CELSIS RMM Traditional Incubation Method Antiquated, Subjective, Visual
- 32. DATA INTEGRITY EVERY STEP OF THE WAY Subjective evaluation in Tradtional Method Based on visual, human
- 33. IMPROVED TEST PERFORMANCE A Key Advantage to Rapid Testing Resulting in Improved Data Integrity position EVERY
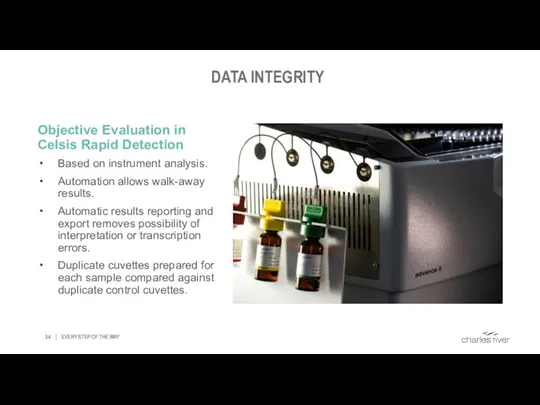
- 34. DATA INTEGRITY EVERY STEP OF THE WAY Objective Evaluation in Celsis Rapid Detection Based on instrument
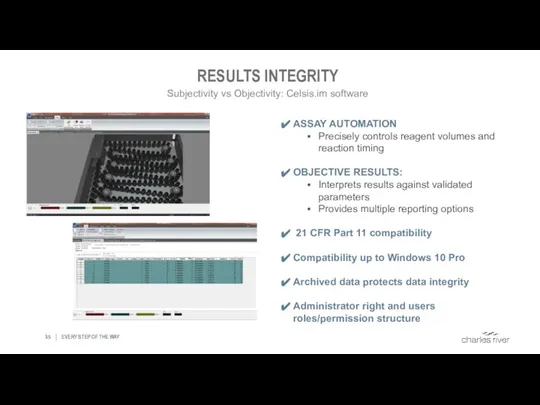
- 35. RESULTS INTEGRITY EVERY STEP OF THE WAY ASSAY AUTOMATION Precisely controls reagent volumes and reaction timing
- 37. YOUR SUCCESS IS OUR SUCCESS EVERY STEP OF THE WAY Industry Leading Expertise And Support
- 38. Reduced cost of manufacturing Reduced inventories Financial Savings Earlier response to contamination events Experienced global support
- 39. EVERY STEP OF THE WAY LEADING COMPANIES TRUSTING CELSIS® DETECTION Proven and In Use by Pharma
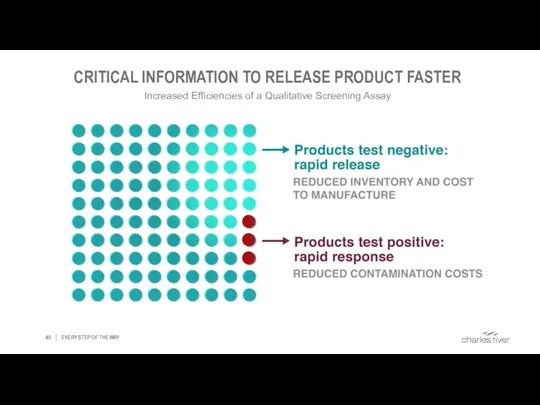
- 40. CRITICAL INFORMATION TO RELEASE PRODUCT FASTER EVERY STEP OF THE WAY Increased Efficiencies of a Qualitative
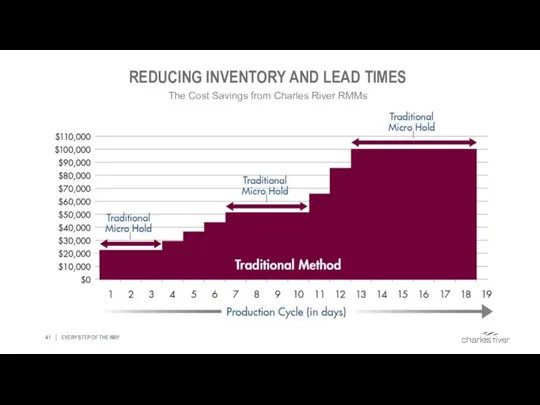
- 41. REDUCING INVENTORY AND LEAD TIMES EVERY STEP OF THE WAY The Cost Savings from Charles River
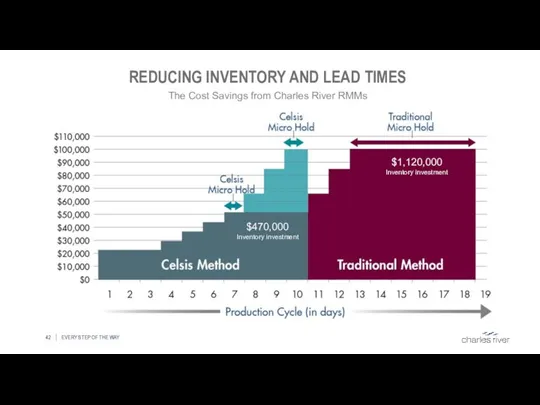
- 42. REDUCING INVENTORY AND LEAD TIMES EVERY STEP OF THE WAY The Cost Savings from Charles River
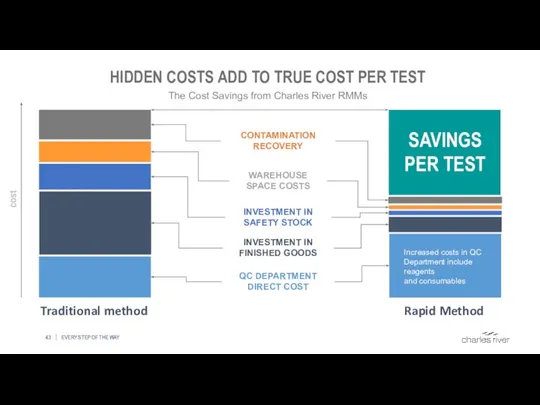
- 43. HIDDEN COSTS ADD TO TRUE COST PER TEST EVERY STEP OF THE WAY The Cost Savings
- 44. EVERY STEP OF THE WAY The Cost Savings from Charles River RMMs FINANCIAL IMPACT ASSESSMENT Using
- 45. SUMMARY AND NEXT STEPS EVERY STEP OF THE WAY NEXT STEPS Establish multi-function team and project
- 46. CONTACT US ENTER CONTACT INFO HERE Address: 251 Ballardvale Street Wilmington, MA 01887 Website: www.criver.com Email:
- 47. APPENDIX Additional Information
- 48. EVERY STEP OF THE WAY RMM TECHNOLOGY SELECTION Growth Based Methods Detectable signal is achieved after
- 49. EVERY STEP OF THE WAY Industry Leading Expertise And Support REGULATORY OVERVIEW Regulators do not pre-approve
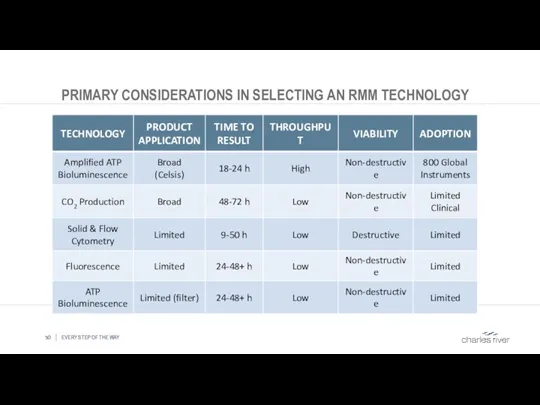
- 50. PRIMARY CONSIDERATIONS IN SELECTING AN RMM TECHNOLOGY EVERY STEP OF THE WAY
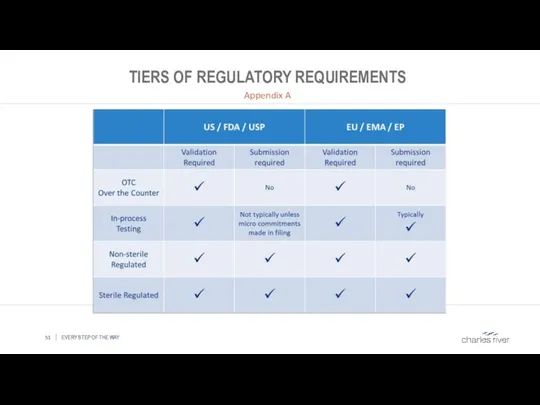
- 51. TIERS OF REGULATORY REQUIREMENTS Appendix A EVERY STEP OF THE WAY
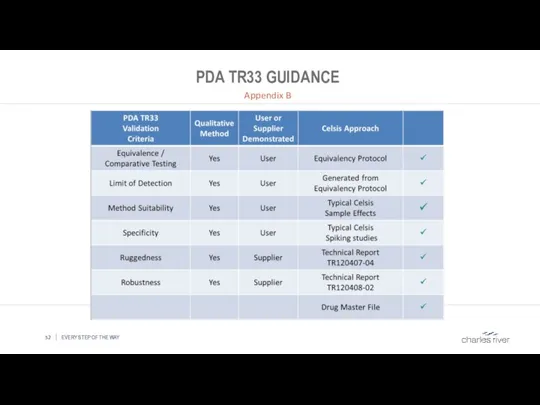
- 52. PDA TR33 GUIDANCE Appendix B EVERY STEP OF THE WAY
- 53. EVERY STEP OF THE WAY PROACTIVE QUALITY SYSTEMS REDUCE RISK What if you do find a
- 54. THE IMPORTANCE OF ACCURATE IDENTIFICATIONS & EM EVERY STEP OF THE WAY The first step in
- 55. EVERY STEP OF THE WAY WHY TREND ORGANISMS? When you have confidence in your ID results,
- 57. Скачать презентацию






































 Здоровье – богатство на все времена
Здоровье – богатство на все времена Навыки проведения интервью
Навыки проведения интервью Усиление железобетонных конструкций. Часть 2
Усиление железобетонных конструкций. Часть 2 Встречи на Методическом Олимпе. Формирование исследовательской культуры студентов
Встречи на Методическом Олимпе. Формирование исследовательской культуры студентов Имидж школьной библиотеки
Имидж школьной библиотеки Симметричное вырезание из бумаги (1 класс)
Симметричное вырезание из бумаги (1 класс) Новый Год и его особенности
Новый Год и его особенности Прежде, чем развивать бизнес, необходимо знать: есть ли рынки сбыта? Ювелирная промышленность: потенциал роста есть, и он заключае
Прежде, чем развивать бизнес, необходимо знать: есть ли рынки сбыта? Ювелирная промышленность: потенциал роста есть, и он заключае Ділові папери як засіб писемної професійної комунікації
Ділові папери як засіб писемної професійної комунікації Современный рынок женской кожаной обуви
Современный рынок женской кожаной обуви ДВНЗ Криворізький національний університет. Кафедра геодезії
ДВНЗ Криворізький національний університет. Кафедра геодезії Презентация на тему Кавказ
Презентация на тему Кавказ Презентация на тему Сокровища Земли под охраной человечества (4 класс)
Презентация на тему Сокровища Земли под охраной человечества (4 класс) Электрическая энергия, ее особенности и область применения
Электрическая энергия, ее особенности и область применения Таможенная процедура «Выпуск для внутреннего потребления» Подготовили: Студентки юридического факультета Учебной группы Ю-113б
Таможенная процедура «Выпуск для внутреннего потребления» Подготовили: Студентки юридического факультета Учебной группы Ю-113б  Экономическая оценка инвестиций. Производство свечей
Экономическая оценка инвестиций. Производство свечей ИССЛЕДОВАНИЕ ЗАВИСИМОСТИ ГРУЗОПОДЪЕМНОСТИ МОДЕЛИ МОНГОЛЬФЬЕРА ОТ ТЕМПЕРАТУРЫ ВНУТРЕННЕГО ВОЗДУХА 2010 г.
ИССЛЕДОВАНИЕ ЗАВИСИМОСТИ ГРУЗОПОДЪЕМНОСТИ МОДЕЛИ МОНГОЛЬФЬЕРА ОТ ТЕМПЕРАТУРЫ ВНУТРЕННЕГО ВОЗДУХА 2010 г. Беседа об этикете поведения детей и взрослых
Беседа об этикете поведения детей и взрослых Основы алгоритмизации
Основы алгоритмизации Барабанная энциклопедия
Барабанная энциклопедия Плетеные изделия из ивовой лозы
Плетеные изделия из ивовой лозы Экономическая теория
Экономическая теория Я держу в ладошках солнце. Загадки о правах
Я держу в ладошках солнце. Загадки о правах Подготовка детей к школе в условиях внедрения ФГОС
Подготовка детей к школе в условиях внедрения ФГОС Урок алгебры 10 класс Учитель математики Калита Н.А.
Урок алгебры 10 класс Учитель математики Калита Н.А. Риск - ориентированный надзор. Информационная открытость
Риск - ориентированный надзор. Информационная открытость Наша будущая специальность – Учет и аудит
Наша будущая специальность – Учет и аудит Презентация на тему Психологическое воздействие на аудиторию
Презентация на тему Психологическое воздействие на аудиторию