Содержание
- 2. Announcement Required Project Meeting for all Physics students: Wednesday, 1st October, 2014 @ 4 pm Senate
- 3. 1. Definitions Oxford English Dictionary: Physics: ‘Science dealing with properties and interactions of matter and energy’;
- 4. Objectives of Physics To find the limited number of fundamental laws that govern natural phenomena; To
- 5. Classical Physics Includes principles developed mainly before 1900; Mechanics: major developments by Newton, and continuing throughout
- 6. Modern Physics Deals with phenomena that could not be explained by Classical Physics; Special Relativity: explains
- 7. 2. Measurements and Units Physics is quantitative: define variables, symbols or physical quantities, and their units;
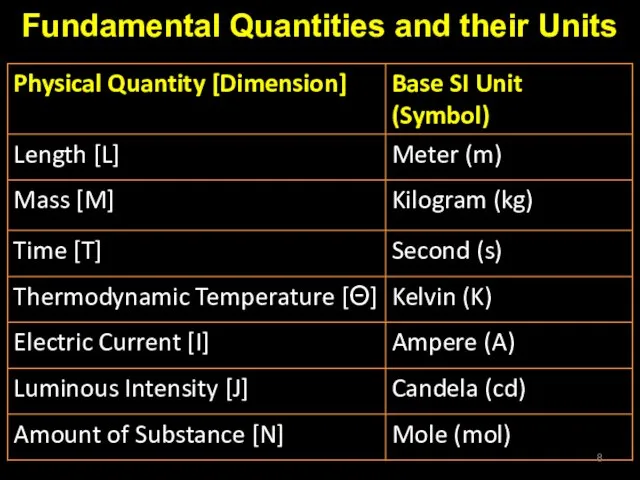
- 8. Fundamental Quantities and their Units
- 9. Base units All Physical quantities can be expressed in one of the seven base units; The
- 10. The most recent definition of the second is based on the high precision of atomic clocks
- 11. Example 1. Base and Derived Units Find the Newton (N) in terms of the base units.
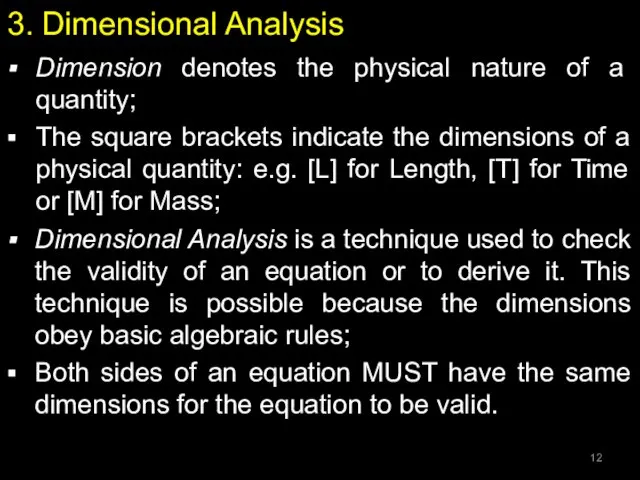
- 12. 3. Dimensional Analysis Dimension denotes the physical nature of a quantity; The square brackets indicate the
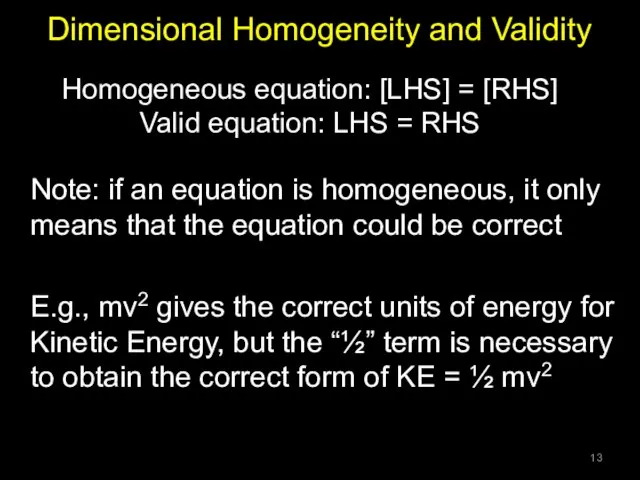
- 13. Dimensional Homogeneity and Validity Note: if an equation is homogeneous, it only means that the equation
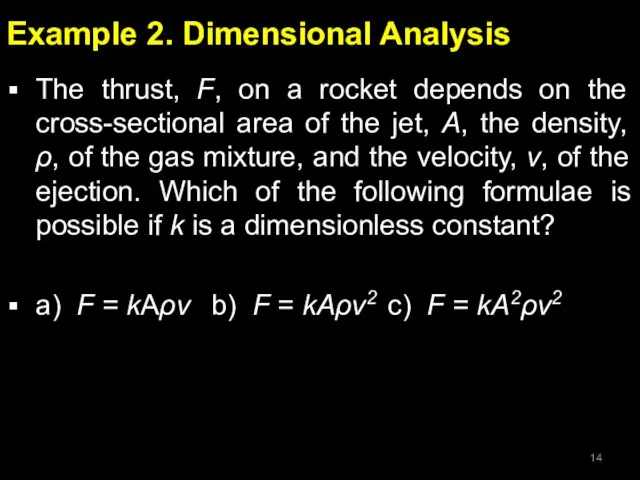
- 14. Example 2. Dimensional Analysis The thrust, F, on a rocket depends on the cross-sectional area of
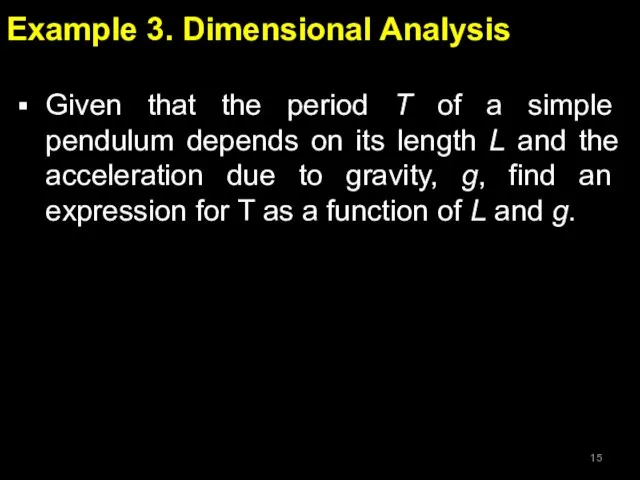
- 15. Example 3. Dimensional Analysis Given that the period T of a simple pendulum depends on its
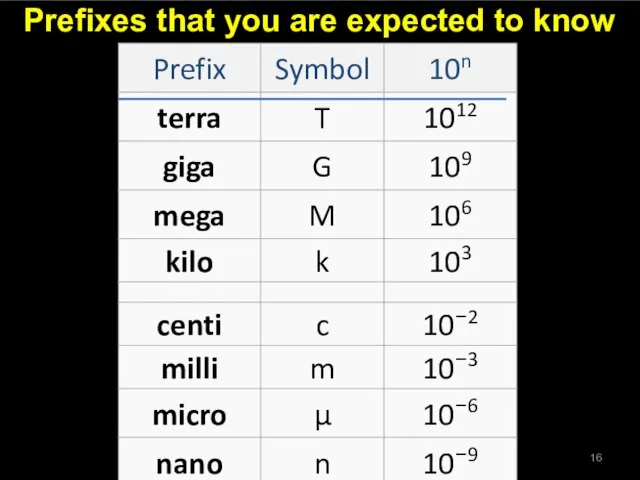
- 16. Prefixes that you are expected to know
- 17. 4. Uncertainty and Significant Figures Physical quantities cannot be measured with total accuracy and utmost confidence
- 18. Significant Figures Zeroes may or may not be significant; those used to locate the decimal point
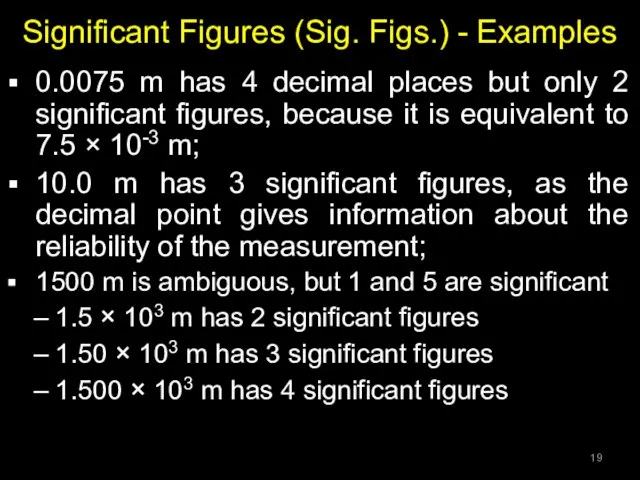
- 19. Significant Figures (Sig. Figs.) - Examples 0.0075 m has 4 decimal places but only 2 significant
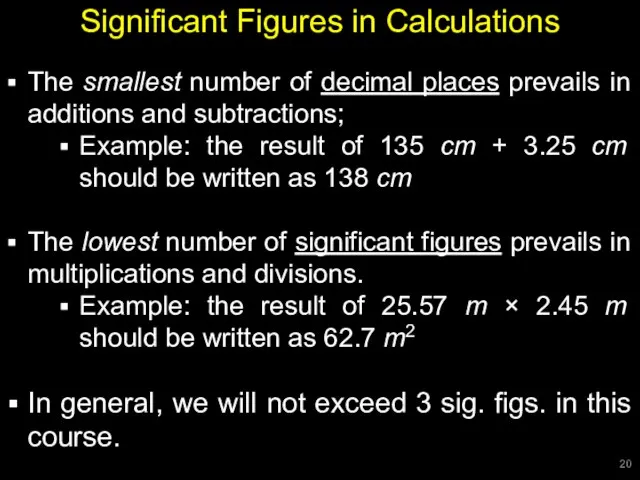
- 20. Significant Figures in Calculations The smallest number of decimal places prevails in additions and subtractions; Example:
- 21. Rounding Sig. Figs. Is the last dropped digit Drop it if it is If it is
- 22. Intermediate Steps General rule for calculations involving multiple steps (this course only!): the Sig. Fig. and
- 24. Скачать презентацию













 День работников пищевой промышленностиИтоги
День работников пищевой промышленностиИтоги Офицер тактического звена управления
Офицер тактического звена управления Презентация на тему Мир профессии The world of professions
Презентация на тему Мир профессии The world of professions  Правовая лексика
Правовая лексика Храм Рождества Христова и преподобного Сергея Радонежского
Храм Рождества Христова и преподобного Сергея Радонежского Презентация технология
Презентация технология Франция: долгий путь к единству
Франция: долгий путь к единству Радио RECORD
Радио RECORD Проектно-сметная документация
Проектно-сметная документация Подарочная коробка конфет Джокер
Подарочная коробка конфет Джокер Азбука плавания
Азбука плавания Архитектура России XVIII века
Архитектура России XVIII века Декоративное искусство
Декоративное искусство ProSpace (Казахстан)
ProSpace (Казахстан) Фредерик Франсуа Шопен
Фредерик Франсуа Шопен Архитектура Древнего Рима
Архитектура Древнего Рима 5. Создание и распад империи Карла Великого
5. Создание и распад империи Карла Великого Тема:«Нарушения опорно-двигательной системы учащихся начального и среднего звена.Динамика нарушений за 2005-2010 г. Причины»Выполн
Тема:«Нарушения опорно-двигательной системы учащихся начального и среднего звена.Динамика нарушений за 2005-2010 г. Причины»Выполн АО Самотлорнефтегаз
АО Самотлорнефтегаз Актуальные направления современной лингвистики
Актуальные направления современной лингвистики АДАПТАЦИЯ ФИЛЬТРА КАЛМАНАДЛЯ ИСПОЛЬЗОВАНИЯ С ЛОКАЛЬНОЙИ ГЛОБАЛЬНОЙ СИСТЕМОЙ НАВИГАЦИИ
АДАПТАЦИЯ ФИЛЬТРА КАЛМАНАДЛЯ ИСПОЛЬЗОВАНИЯ С ЛОКАЛЬНОЙИ ГЛОБАЛЬНОЙ СИСТЕМОЙ НАВИГАЦИИ Конструирование железобетонных балок без предварительного напряжения арматуры
Конструирование железобетонных балок без предварительного напряжения арматуры Презентация на тему Джеймс Кук
Презентация на тему Джеймс Кук Click to edit Master title style Click to edit Master subtitle style
Click to edit Master title style Click to edit Master subtitle style  Миф о гильгамеше
Миф о гильгамеше phpi3rdQv_p.35.-YUzhnaya-Amerika.-Klimat.-Vnutrennie-vody
phpi3rdQv_p.35.-YUzhnaya-Amerika.-Klimat.-Vnutrennie-vody Семинар-совещание «Актуальные задачи развития и законодательного регулирования энергетики в Российской Федерации»
Семинар-совещание «Актуальные задачи развития и законодательного регулирования энергетики в Российской Федерации» Индивидуальный тур по наблюдению за дикими животными
Индивидуальный тур по наблюдению за дикими животными