Слайд 2Basic terminology
Biologically active substances (BAS) are substances that affect biological processes in

humans and animals.
Pharmaceutical substance is a drug in the form of one or more active substances with pharmacological activity, regardless of the nature of their origin, which is intended for the production, manufacture of drugs and determines their effectiveness.
Active, or pharmacologically active substances are biologically active substances that provide therapeutic value of the drug. They can change the state and functions of the body, exhibit prophylactic, diagnostic or therapeutic effect. They can be used in the form of substances in the manufacture of finished drugs.
Excipients are substances of inorganic or organic origin, used in the production or manufacture of drugs to give them the necessary physical and chemical properties.
Слайд 3Basic terminology
Medicinal raw materials - a set of natural and artificial materials

and substances used for production of medicines.
Drugs - substances or their mixtures of natural, semi-synthetic or biotechnological origin, which are used for the prevention, diagnosis and treatment of diseases or to change the state and functions of the human body.
Medicinal product is a drug in a certain dosage form.
Слайд 4Basic terminology
Phytopreparation is a medicinal product of plant origin in a particular

dosage form.
Galenic preparations are medicinal products of plant origin in the form of tinctures or extracts.
Novogalenic preparations are maximum extracts of herbal products, cleared of ballast substances, which contain the whole complex of BAS.
The term "herbal preparations" is used to describe a mixture of some types of crushed (less often whole) herbs, sometimes with an admixture of mineral salts, essential oils, etc., which can be used for therapeutic purposes.
Слайд 5Basic terminology
Standardization is the establishment of authenticity, quality and other indicators in
accordance with the requirements of the standard.
A normative document (ND) is a document which sets out the requirements for the quality, products of primary processing (briquettes, collections, fat and essential oils), phytopreparations and other medicines, as well as methods of analysis of the relevant products.
Слайд 6Basic terminology
State Pharmacopoeia is an official guide for pharmacists; a collection that

regulates the quality of medicines, indicating methods of manufacturing, rules of prescription, the highest doses, storage rules, etc.; it may also contain the texts of regulations concerning the circulation of medicines, other information and reference materials.
Pharmacopoeial article - normative document, regulating the quality of medicinal raw material, drug or standard sample and including the appropriate methods of analysis.
Pharmacopoeial article of the enterprise - a normative document, regulating the quality of raw materials or drugs and including the appropriate methods of analysis.
Слайд 7Basic terminology
Russian Federation State Standard (GOST) - a document defining the regulatory

requirements for raw materials, products, drugs, manufacturing processes, which regulates the methods of determining the quality of products and the conditions necessary for their preservation.
Standard is a regulatory document for general and multiple use, which establishes the rules, requirements, general principles or characteristics to achieve an optimal level of order in a particular area.
Слайд 8Basic terminology
Technical specifications (TS) is a normative document that establishes the requirements

for specific products and regulates the relationship between the manufacturer and the consumer of the product.
Industry standards are standards that set out additional technical conditions for the production and delivery of products.
Слайд 9Dosage forms
Dosage forms are drugs that have certain physical and chemical properties

and provide optimal therapeutic effect.
Classification of dosage forms by aggregate state.
II. Classification of dosage forms depending on the method of application or method of dosing.
III. Classification of dosage forms depending on the method of administration into the body.
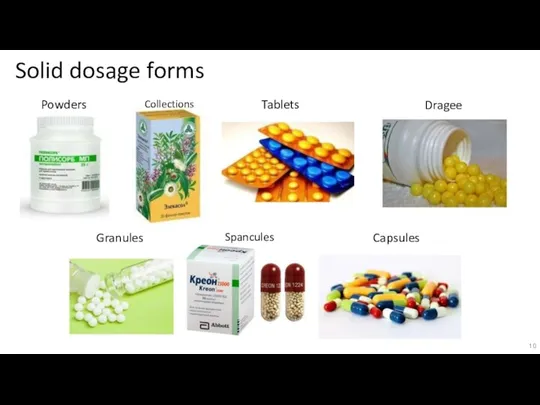
Слайд 10Solid dosage forms
Powders
Collections
Tablets
Dragee
Granules
Spancules
Capsules
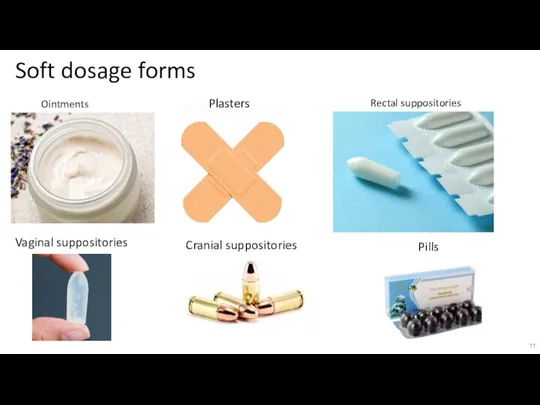
Слайд 11Soft dosage forms
Ointments
Plasters
Rectal suppositories
Vaginal suppositories
Pills
Cranial suppositories
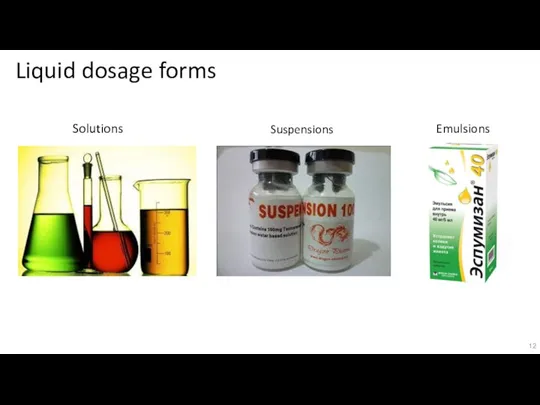
Слайд 12Liquid dosage forms
Solutions
Suspensions
Emulsions











 Университет Я. Э. Пуркине Факультет естественных наук Основан 4 ноября 2005 года. Цель факультета - подготовка бакалавров, магистров и
Университет Я. Э. Пуркине Факультет естественных наук Основан 4 ноября 2005 года. Цель факультета - подготовка бакалавров, магистров и Элементы и параметры карьеров
Элементы и параметры карьеров Проверочная работа5 класс
Проверочная работа5 класс Деньги. Банки. Банковская система
Деньги. Банки. Банковская система Социальная работа в пенитенциарных учреждениях Российской Федерации
Социальная работа в пенитенциарных учреждениях Российской Федерации Антисциентизм всовременном мире
Антисциентизм всовременном мире Художественный образ
Художественный образ Ostern in Deutschland
Ostern in Deutschland Сильнейший Учитель Отаку
Сильнейший Учитель Отаку  Исследование пакгаузов Центрального здания Всероссийской художественно-промышленной выставки 1896 года в Нижнем Новгороде
Исследование пакгаузов Центрального здания Всероссийской художественно-промышленной выставки 1896 года в Нижнем Новгороде Применение различных способов оплаты в системе обязательного медицинского страхования
Применение различных способов оплаты в системе обязательного медицинского страхования Pit Nip Pesel
Pit Nip Pesel Основные тактики волейбола
Основные тактики волейбола Проектная деятельность в учебном процессе
Проектная деятельность в учебном процессе Внешняя политика России в 1801-12 годах
Внешняя политика России в 1801-12 годах China teacher
China teacher Мы талантливы
Мы талантливы CSSЯзык описания представлений
CSSЯзык описания представлений Рыночная экономика. КОНКУРЕНЦИЯ
Рыночная экономика. КОНКУРЕНЦИЯ РЕЧЬ И КУЛЬТУРА:ЖАРГОНИЗМЫ В РЕЧИ ШКОЛЬНИКОВ. «Умён ты или глуп, Велик ты или мал, Не знаем мы, пока Ты слова не сказал!» Персидски
РЕЧЬ И КУЛЬТУРА:ЖАРГОНИЗМЫ В РЕЧИ ШКОЛЬНИКОВ. «Умён ты или глуп, Велик ты или мал, Не знаем мы, пока Ты слова не сказал!» Персидски International Mother Language Day is celebrated on 21 February every year
International Mother Language Day is celebrated on 21 February every year Карл Раймунд Поппер
Карл Раймунд Поппер Презентация на тему Делимость чисел 6 класс
Презентация на тему Делимость чисел 6 класс Правила пользования газом и газовым оборудованием
Правила пользования газом и газовым оборудованием Гуляев Степан Иванович
Гуляев Степан Иванович editdocument16648092075845 (1)
editdocument16648092075845 (1) Аграрная реформа Столыпина
Аграрная реформа Столыпина Черезмерное использовние ИКТ в образовательном процессе
Черезмерное использовние ИКТ в образовательном процессе