Содержание
- 2. Last Updated:* © LMS SEGi education group Chapter Overview Chapter 3 exposed you with the different
- 3. Last Updated:* © LMS SEGi education group To expose the students to different types of metal
- 4. Last Updated:* © LMS SEGi education group Learning Outcomes At the end of the lesson, students
- 5. Last Updated:* © LMS SEGi education group INTRODUCTION TO FATIGUE Definition: The effect on metal of
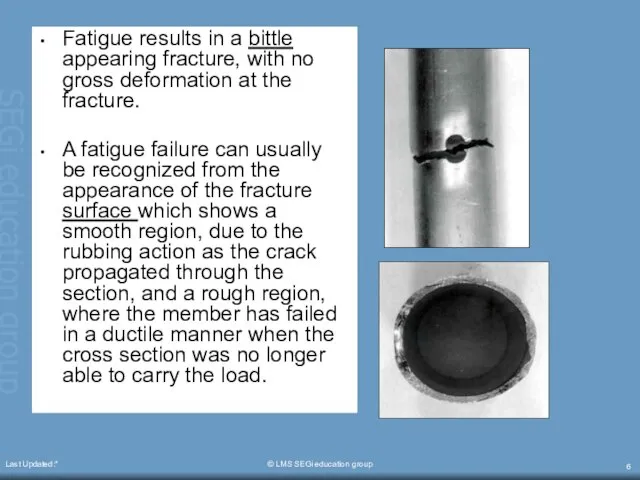
- 6. Last Updated:* © LMS SEGi education group Fatigue results in a bittle appearing fracture, with no
- 7. Last Updated:* © LMS SEGi education group Three basic factors are necessary to cause fatigue failure.
- 8. Last Updated:* © LMS SEGi education group In addition, there are a host of other variables,
- 9. Last Updated:* © LMS SEGi education group METALLURGY The science that deals with procedures used in
- 10. Last Updated:* © LMS SEGi education group Residual Stresses Definition: Stresses that remain in material or
- 11. Last Updated:* © LMS SEGi education group Fatigue Design Guideline (minimize stress concentrations) Consider all types
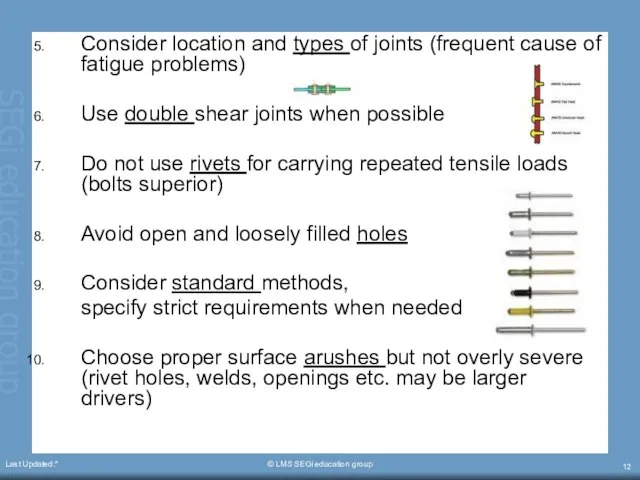
- 12. Last Updated:* © LMS SEGi education group Consider location and types of joints (frequent cause of
- 13. Last Updated:* © LMS SEGi education group Provide suitable protection against corrosion Avoid metal piahrg plating
- 14. Last Updated:* © LMS SEGi education group INTRODUCTION TO CORROSION Corrosion is chemically induced damage to
- 15. Last Updated:* © LMS SEGi education group WHY METALS CORRODE? Metals corrode because we use them
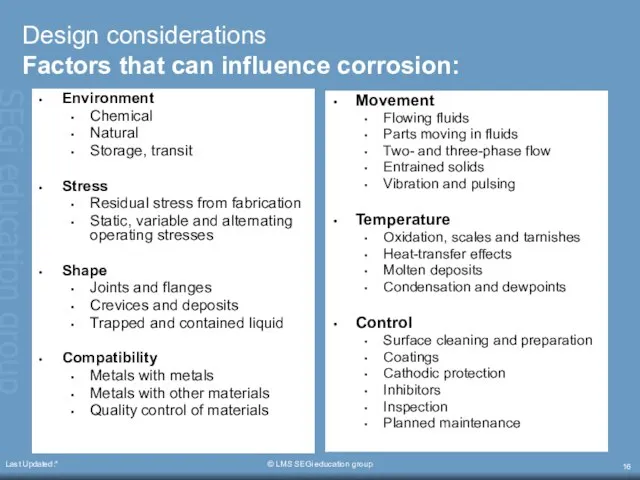
- 16. Last Updated:* © LMS SEGi education group Design considerations Factors that can influence corrosion: Environment Chemical
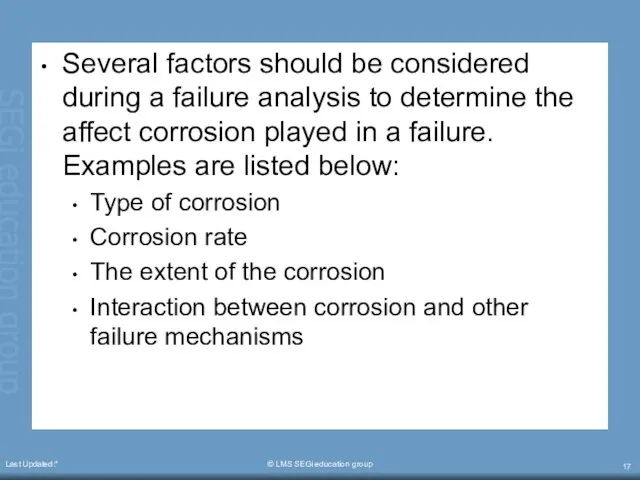
- 17. Last Updated:* © LMS SEGi education group Several factors should be considered during a failure analysis
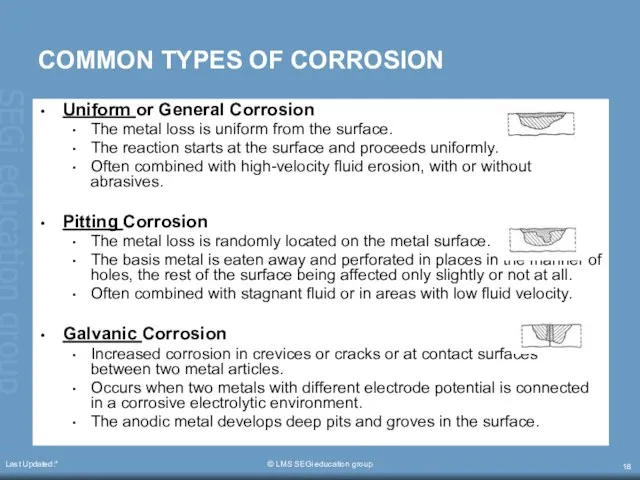
- 18. Last Updated:* © LMS SEGi education group COMMON TYPES OF CORROSION Uniform or General Corrosion The
- 19. Last Updated:* © LMS SEGi education group *Crevice Corrosion Occurs at places with gaskets, bolts and
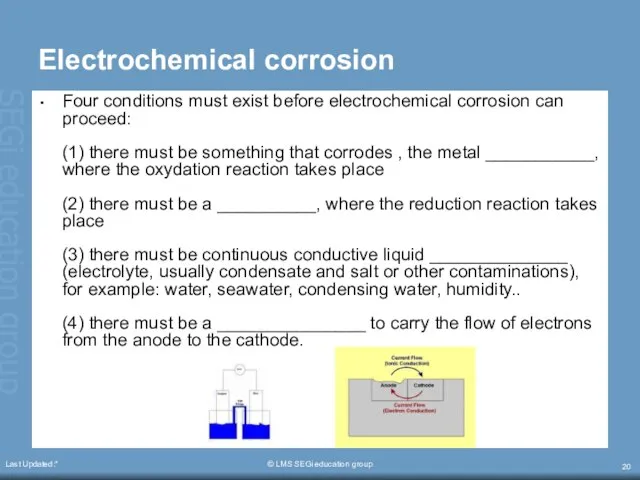
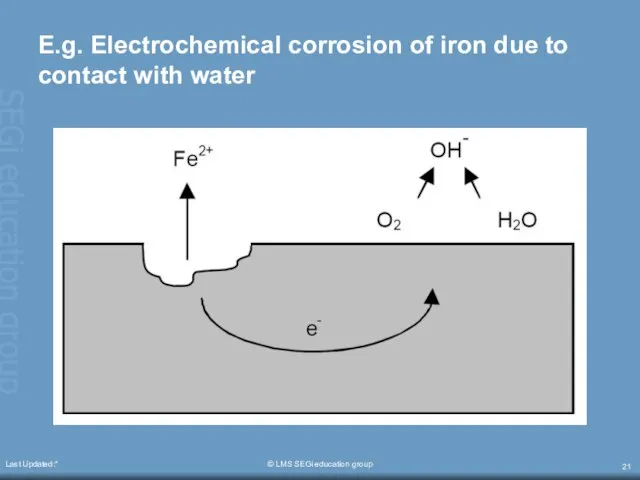
- 20. Last Updated:* © LMS SEGi education group Electrochemical corrosion Four conditions must exist before electrochemical corrosion
- 21. Last Updated:* © LMS SEGi education group E.g. Electrochemical corrosion of iron due to contact with
- 22. Last Updated:* © LMS SEGi education group This conductor is usually in the form of metal-to-metal
- 23. Last Updated:* © LMS SEGi education group Corrosion is a normal, natural process. Corrosion can seldom
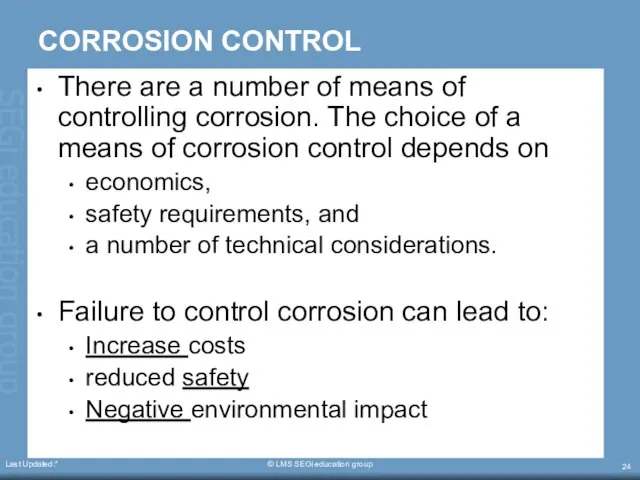
- 24. Last Updated:* © LMS SEGi education group CORROSION CONTROL There are a number of means of
- 25. Last Updated:* © LMS SEGi education group CORROSION CONTROL Protective _____________, Can be metallic, such as
- 26. Last Updated:* © LMS SEGi education group INTRODUCTION TO CREEP When a material is subjected to
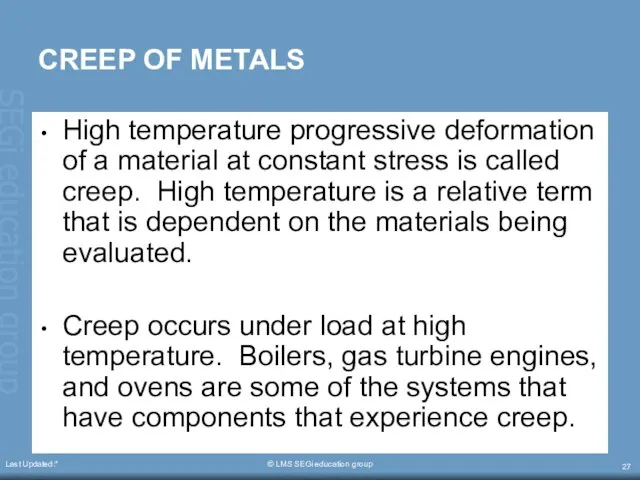
- 27. Last Updated:* © LMS SEGi education group CREEP OF METALS High temperature progressive deformation of a
- 28. Last Updated:* © LMS SEGi education group For a low melting point metal like lead, creep
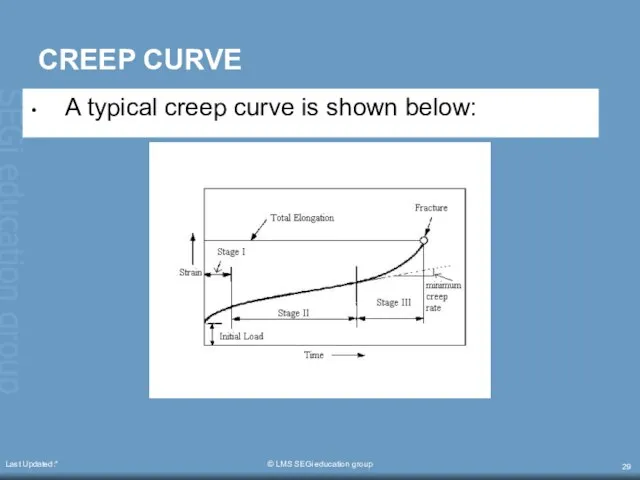
- 29. Last Updated:* © LMS SEGi education group CREEP CURVE A typical creep curve is shown below:
- 30. Last Updated:* © LMS SEGi education group Effect of High Temperature on Metals: ___________ strength. Greater
- 32. Скачать презентацию

















 Полимеразная цепная реакция — ПЦР
Полимеразная цепная реакция — ПЦР Великие математики
Великие математики Психология. Основная литература
Психология. Основная литература Дымковские игрушки
Дымковские игрушки ТЕМА УРОКА:«АППЛИКАЦИЯ НА ТКАНИСЧАСТЛИВОЕ ПЛАВАНЬЕ»
ТЕМА УРОКА:«АППЛИКАЦИЯ НА ТКАНИСЧАСТЛИВОЕ ПЛАВАНЬЕ» Первая и вторая сигнальные системы
Первая и вторая сигнальные системы 1
1 Раздел IV. Адаптивный спорт. Организация адаптивного спорта в Российской Федерации и за рубежом
Раздел IV. Адаптивный спорт. Организация адаптивного спорта в Российской Федерации и за рубежом «Проблемы законодательного обеспечения создания благоприятных социально-экономических условий жизни населения муниципальных
«Проблемы законодательного обеспечения создания благоприятных социально-экономических условий жизни населения муниципальных  Краснодар «Маленький Париж»
Краснодар «Маленький Париж» Достоевский в Петербурге
Достоевский в Петербурге Перфоманс
Перфоманс Культура России эпохи Петра Великого
Культура России эпохи Петра Великого ЯМАДА Хидэюки
ЯМАДА Хидэюки Банк внешней торговли (Внешторгбанк)
Банк внешней торговли (Внешторгбанк)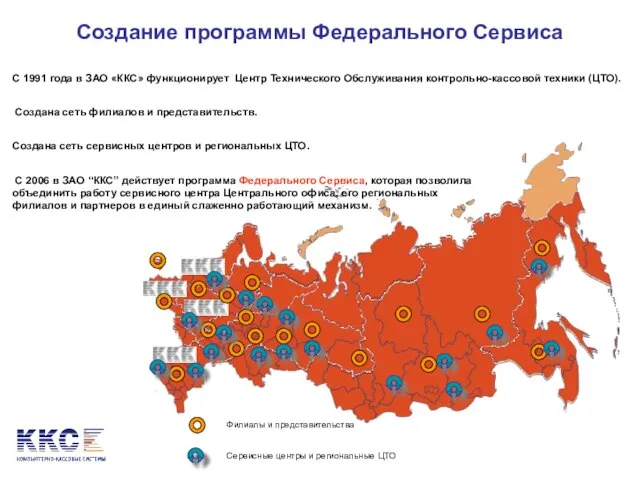 Создание программы Федерального Сервиса
Создание программы Федерального Сервиса Бег как вид физической культуры
Бег как вид физической культуры Устойчивость к магнитному полю промышленной частоты
Устойчивость к магнитному полю промышленной частоты О планах по ремонту автомобильных дорог общего пользования местного значения Хвойнинского муниципального округа в 2022 году
О планах по ремонту автомобильных дорог общего пользования местного значения Хвойнинского муниципального округа в 2022 году Процедуры и функции. Модули
Процедуры и функции. Модули Актеры Саратова
Актеры Саратова Система персонифицированного учета для здравоохранения на базе решения SAP ERP 2005 ЦЕЛЬ Создание типового решени
Система персонифицированного учета для здравоохранения на базе решения SAP ERP 2005 ЦЕЛЬ Создание типового решени ПРЕЗЕНТАЦИЯ
ПРЕЗЕНТАЦИЯ Профессиональные модули. Лекция 8
Профессиональные модули. Лекция 8 Создание проектов в интернет. Разработка сайтов. Лекция 1.
Создание проектов в интернет. Разработка сайтов. Лекция 1. Товарищество с ограниченной ответственностью Bakzhol NS
Товарищество с ограниченной ответственностью Bakzhol NS Welcome to Russia
Welcome to Russia  Конституционное право - ведущая отрасль в правовой системе Российской Федерации. Лекция 1
Конституционное право - ведущая отрасль в правовой системе Российской Федерации. Лекция 1