Содержание
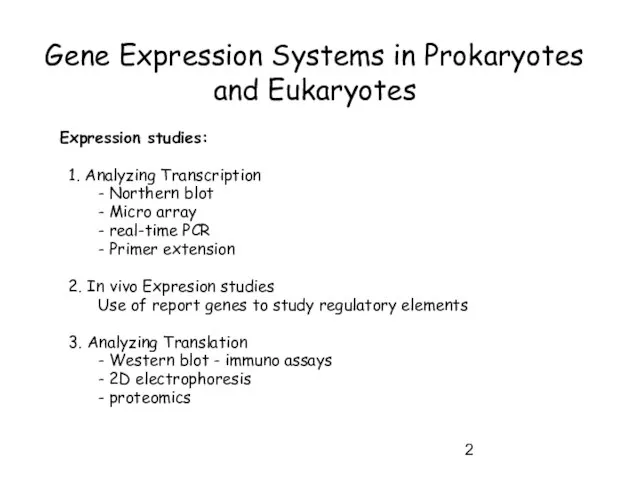
- 2. Gene Expression Systems in Prokaryotes and Eukaryotes Expression studies: 1. Analyzing Transcription - Northern blot -
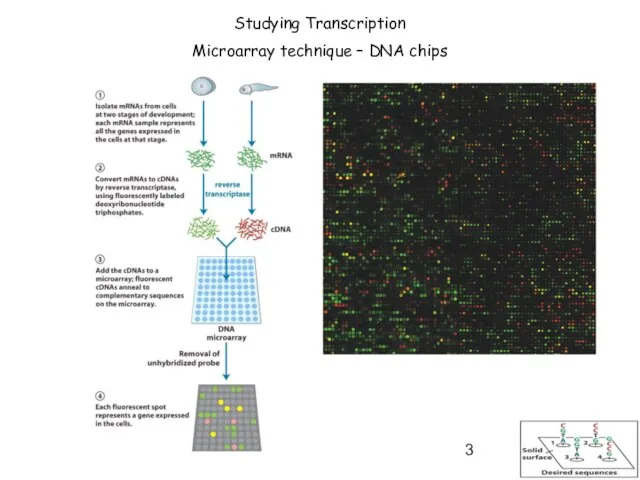
- 3. Studying Transcription Microarray technique – DNA chips
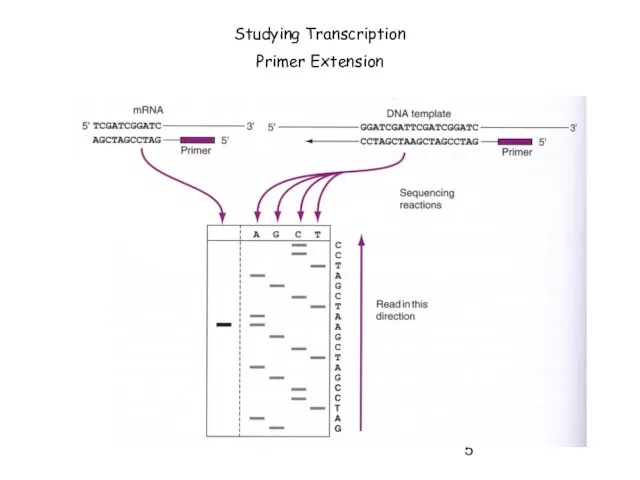
- 5. Studying Transcription Primer Extension
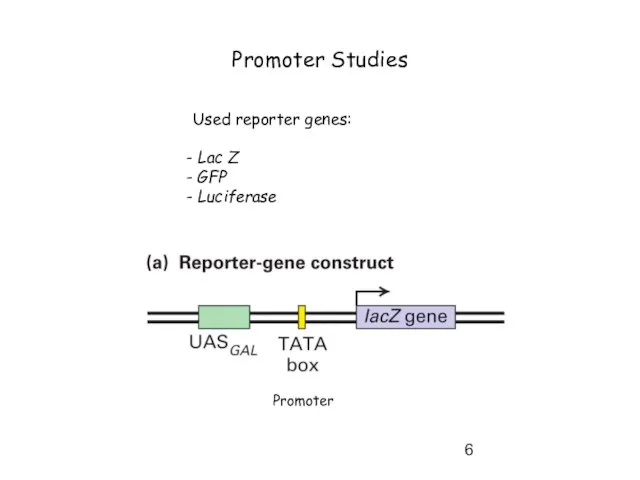
- 6. Promoter Studies Used reporter genes: Lac Z GFP Luciferase Promoter
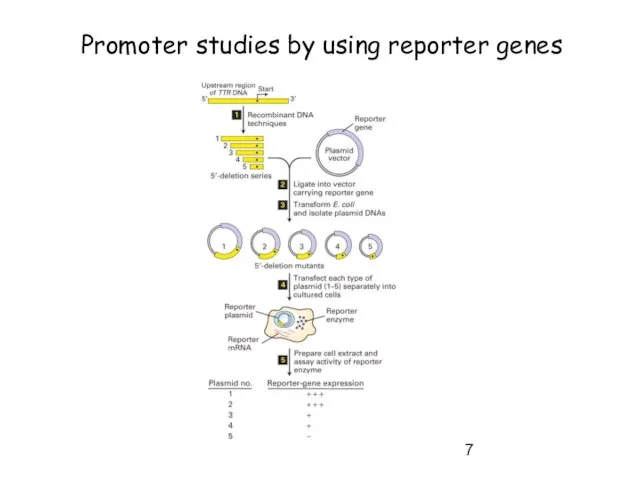
- 7. Promoter studies by using reporter genes
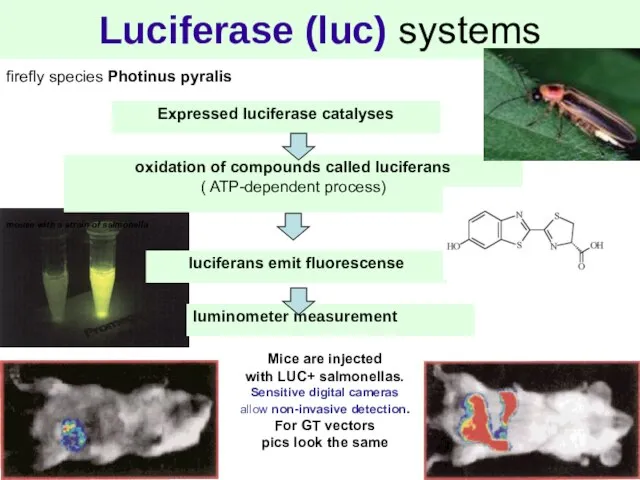
- 8. Luciferase (luc) systems firefly species Photinus pyralis oxidation of compounds called luciferans ( ATP-dependent process) luciferans
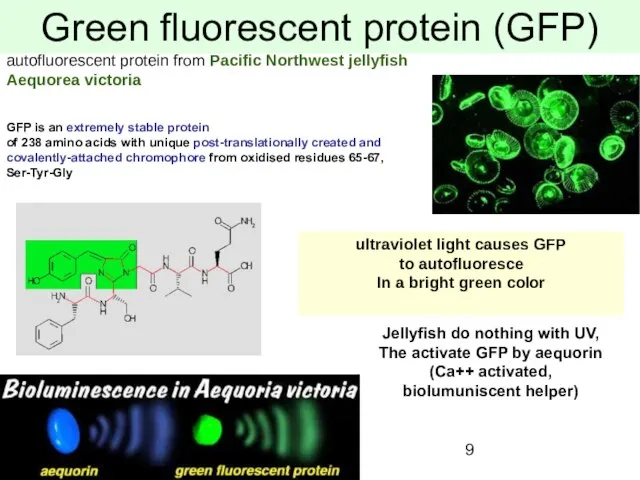
- 9. Green fluorescent protein (GFP) autofluorescent protein from Pacific Northwest jellyfish Aequorea victoria ultraviolet light causes GFP
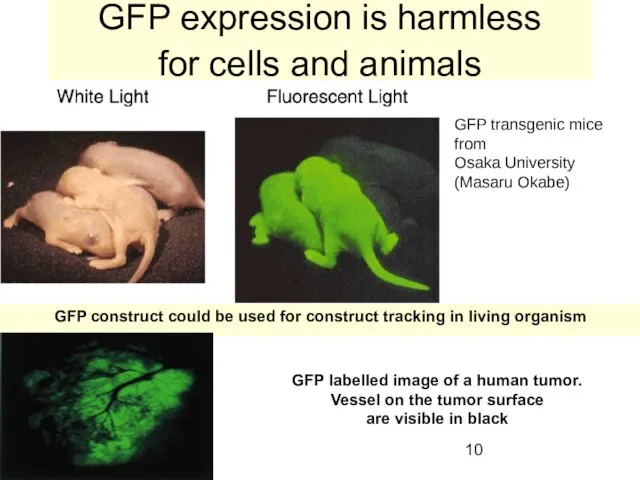
- 10. GFP expression is harmless for cells and animals GFP transgenic mice from Osaka University (Masaru Okabe)
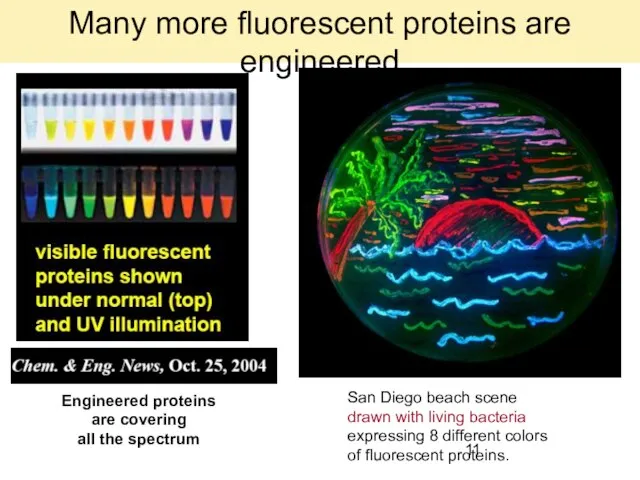
- 11. Engineered proteins are covering all the spectrum San Diego beach scene drawn with living bacteria expressing
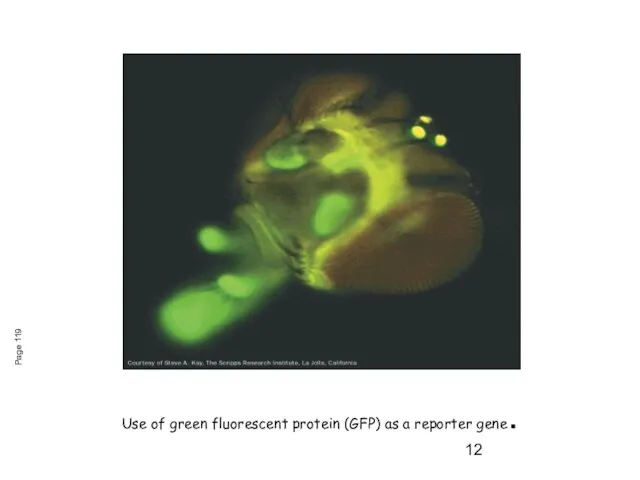
- 12. Use of green fluorescent protein (GFP) as a reporter gene. Page 119
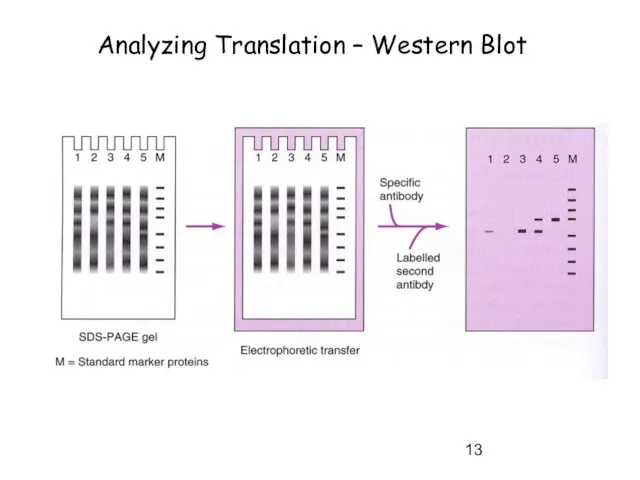
- 13. Analyzing Translation – Western Blot
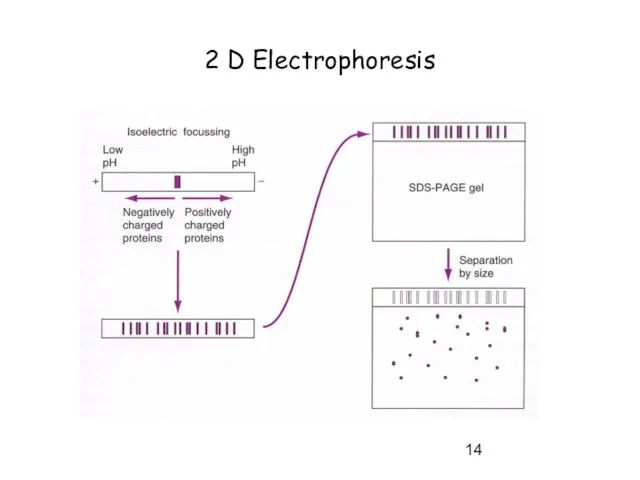
- 14. 2 D Electrophoresis
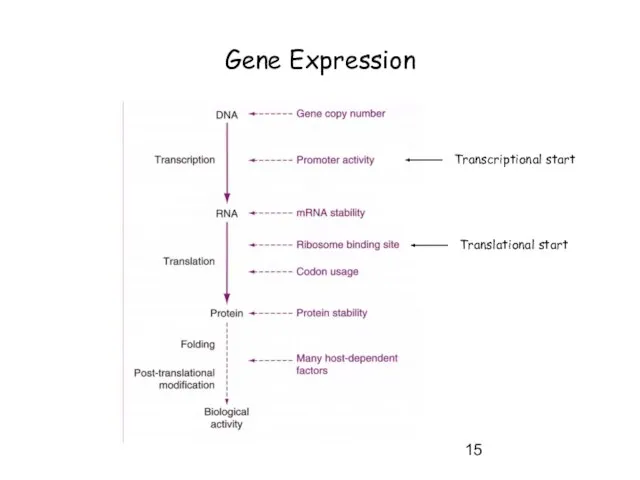
- 15. Gene Expression Transcriptional start Translational start
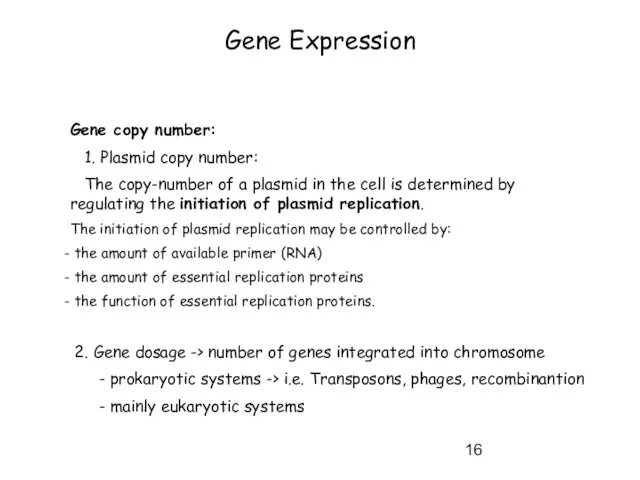
- 16. Gene Expression Gene copy number: 1. Plasmid copy number: The copy-number of a plasmid in the
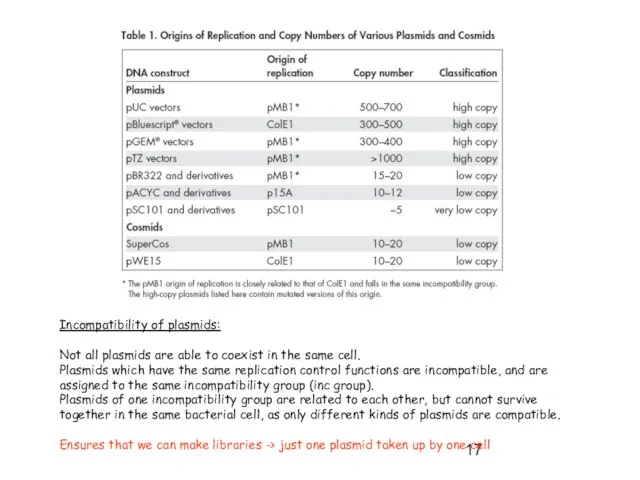
- 17. Incompatibility of plasmids: Not all plasmids are able to coexist in the same cell. Plasmids which
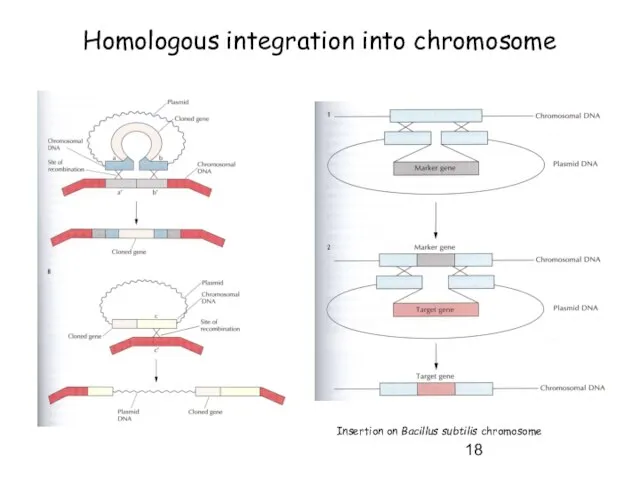
- 18. Homologous integration into chromosome Insertion on Bacillus subtilis chromosome
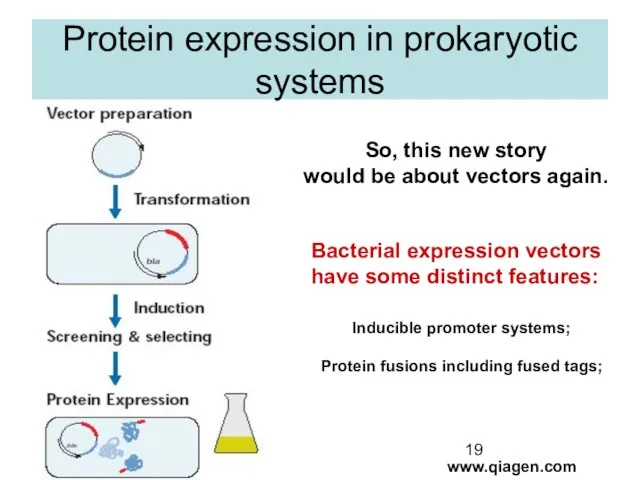
- 19. Protein expression in prokaryotic systems www.qiagen.com So, this new story would be about vectors again. Bacterial
- 20. General advices for one who wants to produce gene expression in prokaryotes 1. Do not forget
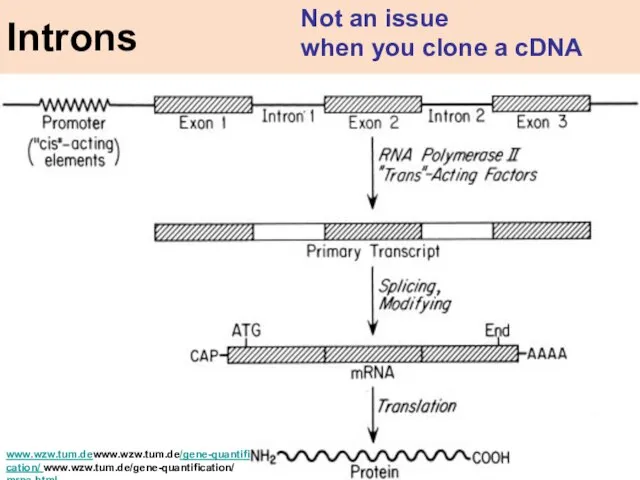
- 21. www.wzw.tum.dewww.wzw.tum.de/gene-quantification/ www.wzw.tum.de/gene-quantification/ mrna.html Introns Not an issue when you clone a cDNA
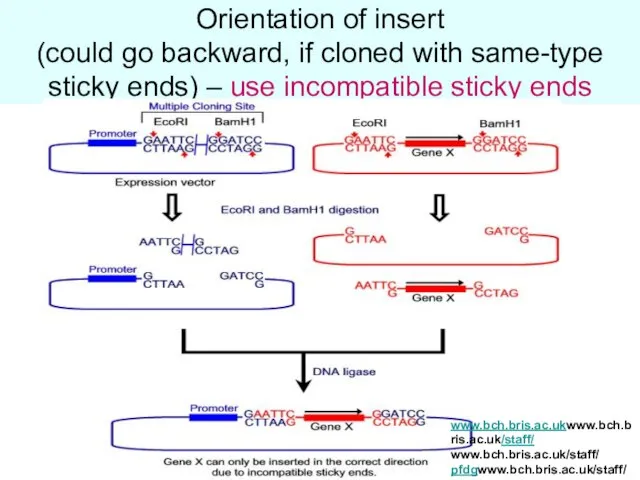
- 22. Orientation of insert (could go backward, if cloned with same-type sticky ends) – use incompatible sticky
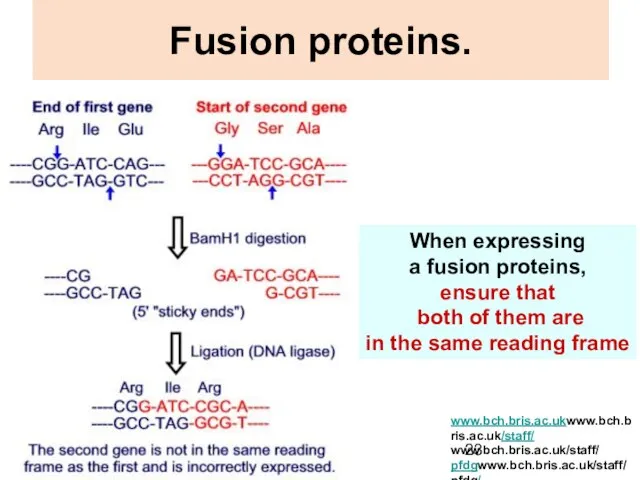
- 23. Fusion proteins. When expressing a fusion proteins, ensure that both of them are in the same
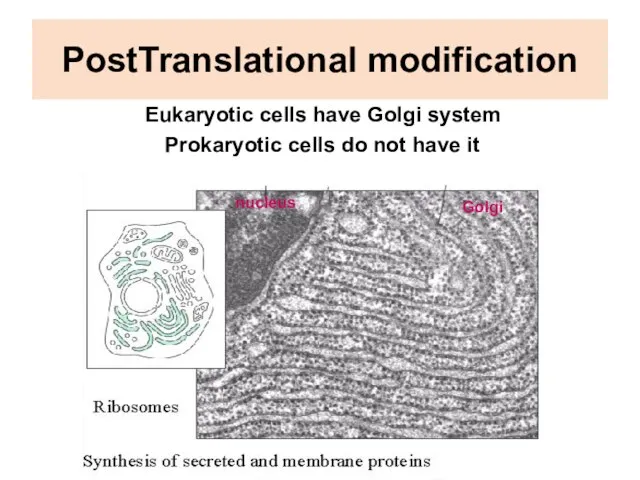
- 24. PostTranslational modification Eukaryotic cells have Golgi system Prokaryotic cells do not have it nucleus Golgi
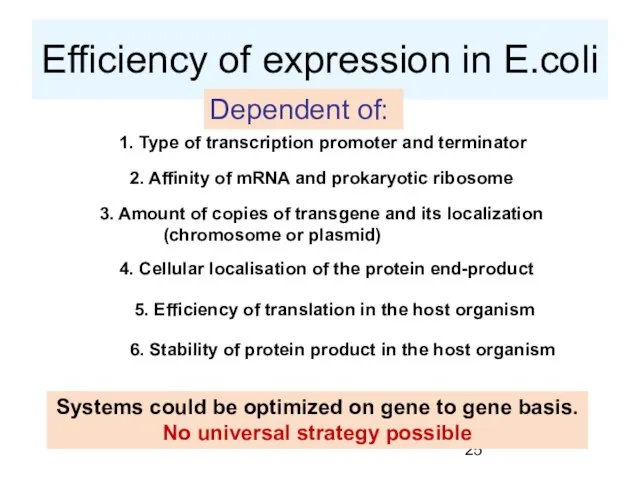
- 25. Efficiency of expression in E.coli Dependent of: 1. Type of transcription promoter and terminator 2. Affinity
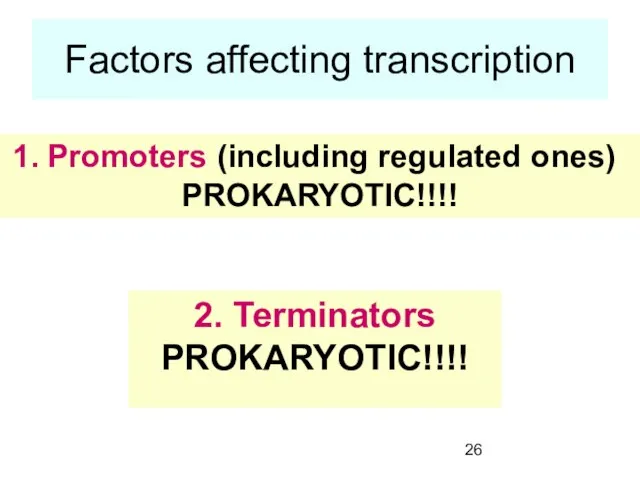
- 26. Factors affecting transcription Promoters (including regulated ones) PROKARYOTIC!!!! 2. Terminators PROKARYOTIC!!!!
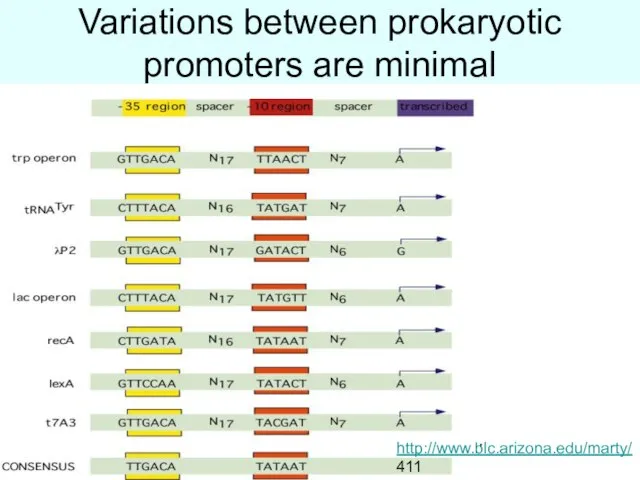
- 27. Variations between prokaryotic promoters are minimal http://www.blc.arizona.edu/marty/ 411
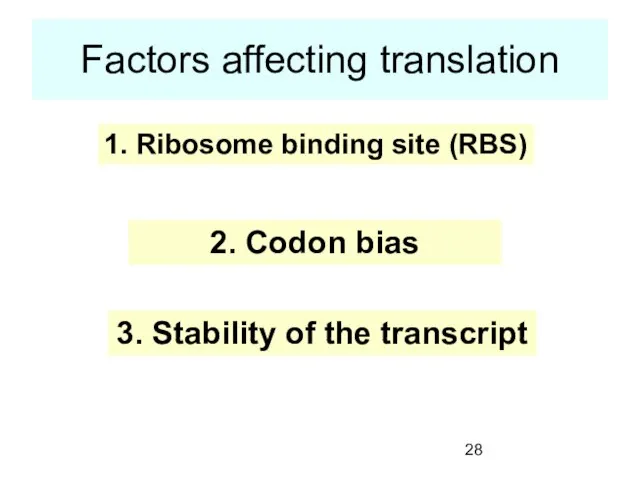
- 28. Factors affecting translation 1. Ribosome binding site (RBS) 2. Codon bias 3. Stability of the transcript
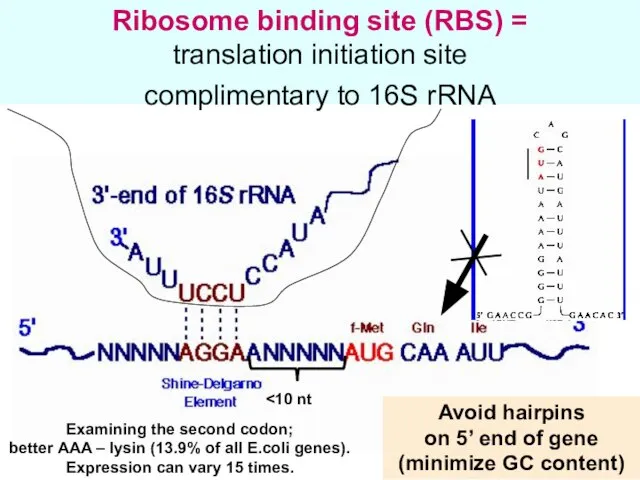
- 29. Ribosome binding site (RBS) = translation initiation site complimentary to 16S rRNA Avoid hairpins on 5’
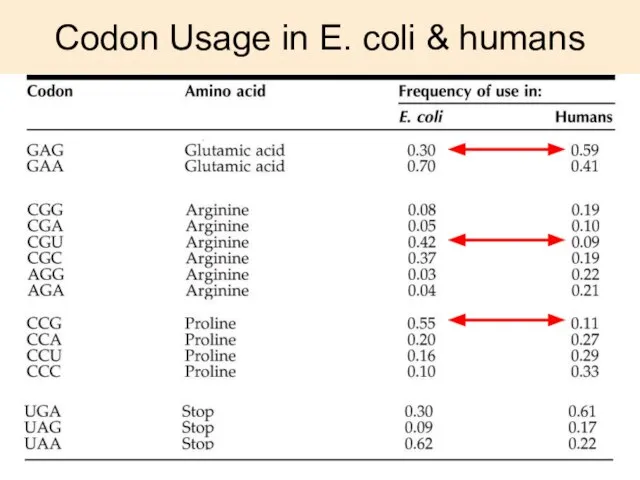
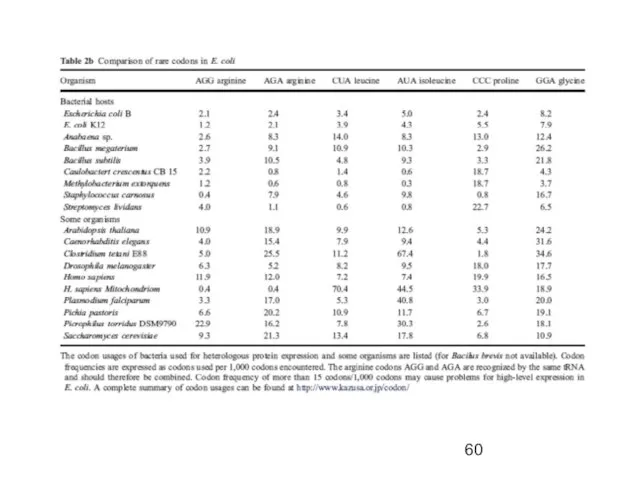
- 30. Codon Usage in E. coli & humans
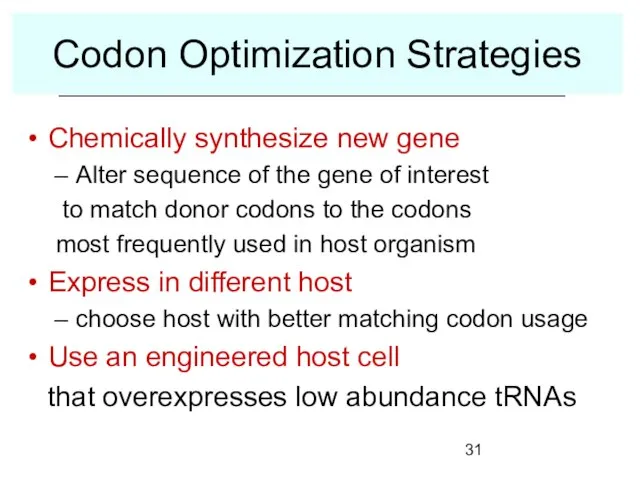
- 31. Codon Optimization Strategies Chemically synthesize new gene Alter sequence of the gene of interest to match
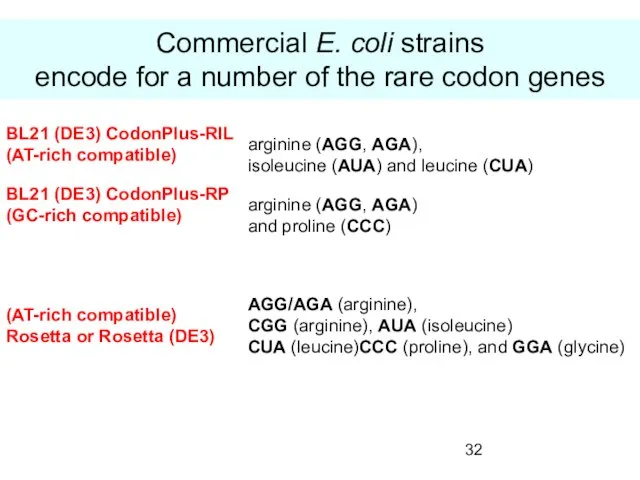
- 32. Commercial E. coli strains encode for a number of the rare codon genes
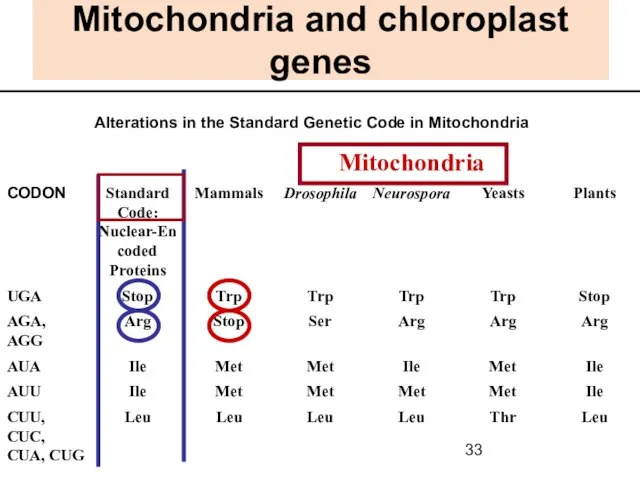
- 33. Mitochondria and chloroplast genes Alterations in the Standard Genetic Code in Mitochondria
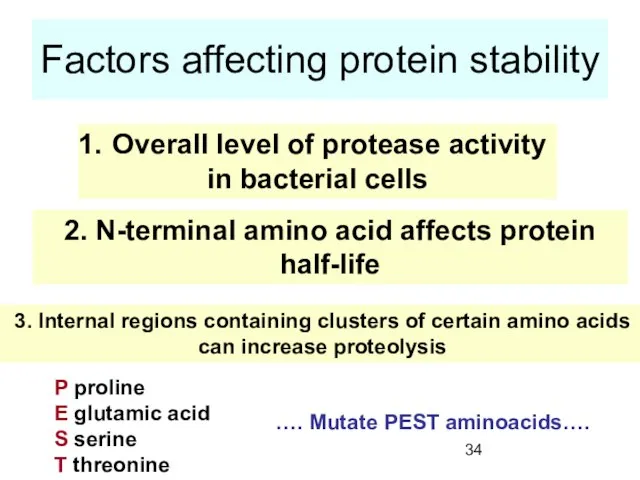
- 34. Factors affecting protein stability Overall level of protease activity in bacterial cells 2. N-terminal amino acid
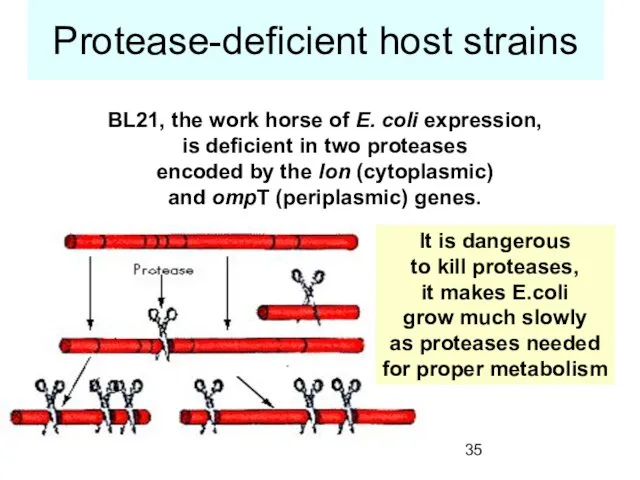
- 35. Protease-deficient host strains BL21, the work horse of E. coli expression, is deficient in two proteases
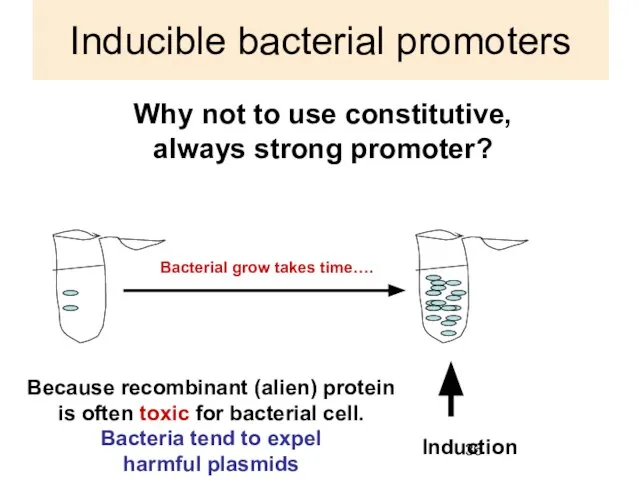
- 36. Inducible bacterial promoters Why not to use constitutive, always strong promoter? Induction Because recombinant (alien) protein
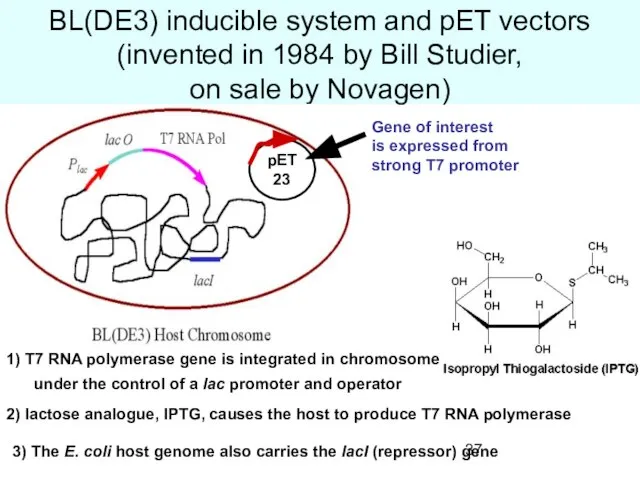
- 37. BL(DE3) inducible system and pET vectors (invented in 1984 by Bill Studier, on sale by Novagen)
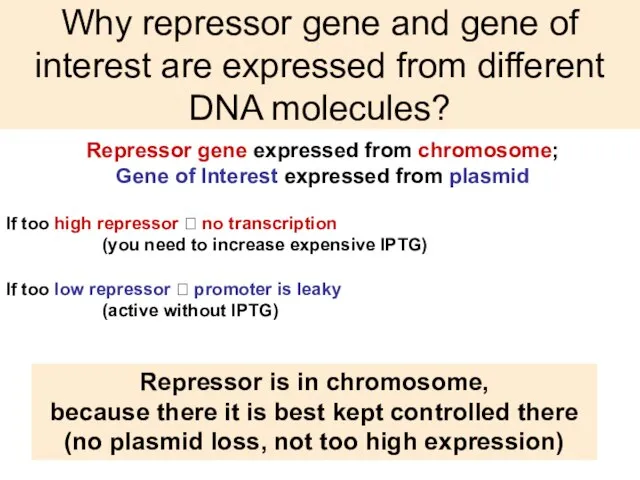
- 38. Why repressor gene and gene of interest are expressed from different DNA molecules? Repressor gene expressed
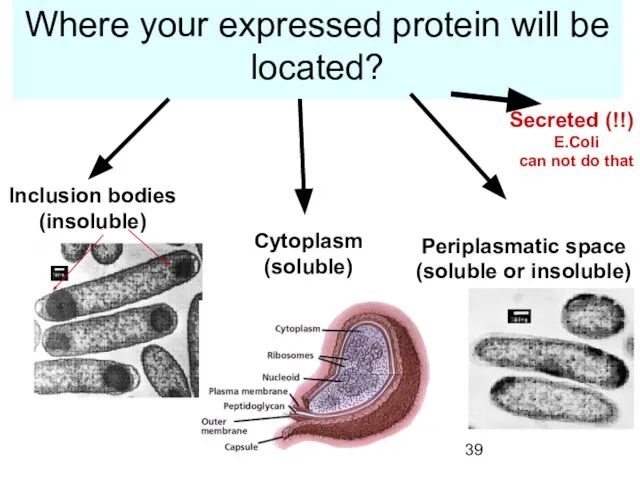
- 39. Where your expressed protein will be located? Inclusion bodies (insoluble) Cytoplasm (soluble) Periplasmatic space (soluble or
- 40. 1. Inclusion bodies (most common case) -- Inclusion bodies are formed through the accumulation of folding
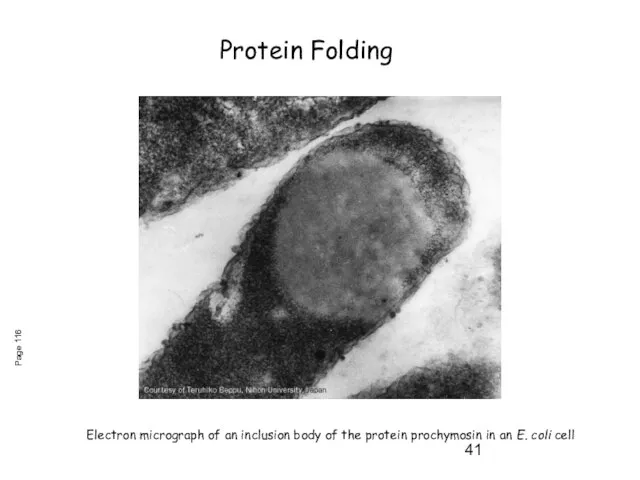
- 41. Electron micrograph of an inclusion body of the protein prochymosin in an E. coli cell Page
- 42. Good side of inclusion bodies inclusion bodies can be accumulated in the cytoplasm to much higher
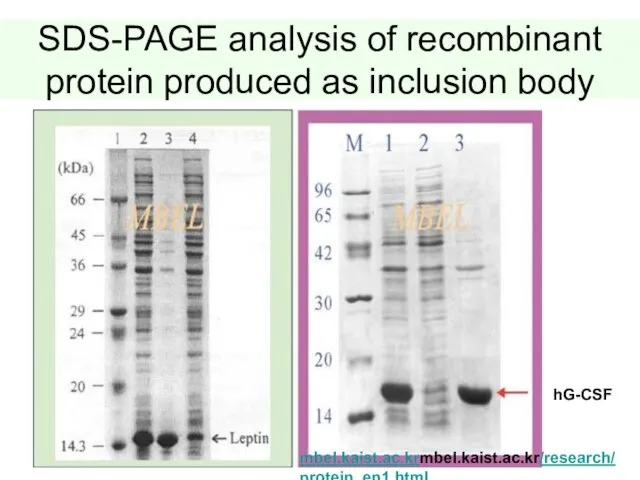
- 43. SDS-PAGE analysis of recombinant protein produced as inclusion body hG-CSF mbel.kaist.ac.krmbel.kaist.ac.kr/research/ protein_en1.html
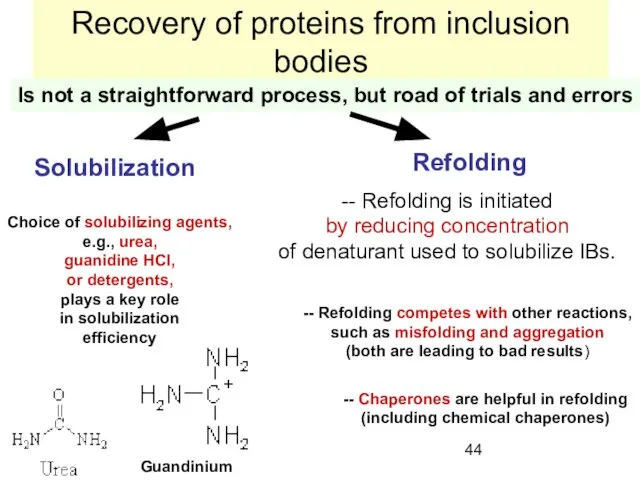
- 44. Recovery of proteins from inclusion bodies Is not a straightforward process, but road of trials and
- 45. Question of questions – how to purify your protein?
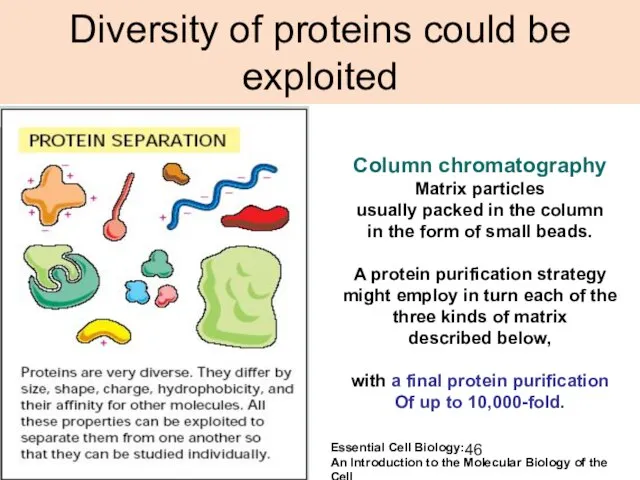
- 46. Diversity of proteins could be exploited Column chromatography Matrix particles usually packed in the column in
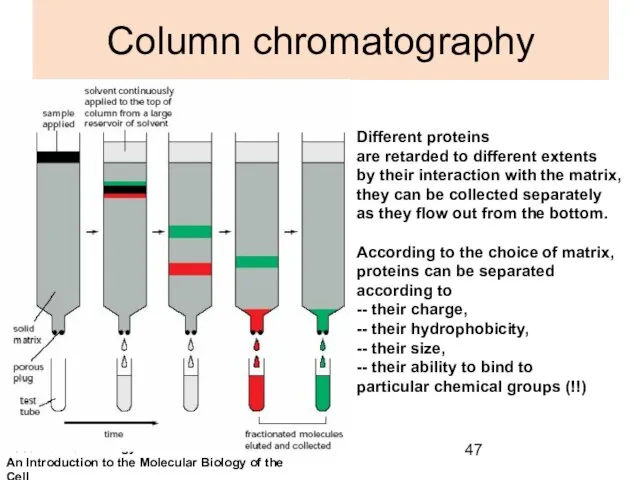
- 47. Column chromatography Different proteins are retarded to different extents by their interaction with the matrix, they
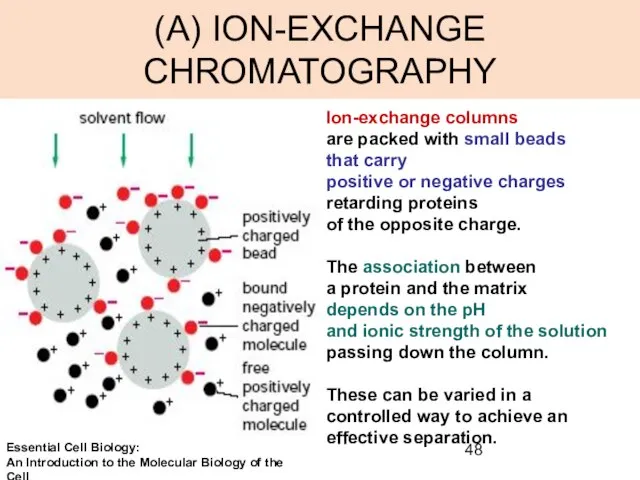
- 48. (A) ION-EXCHANGE CHROMATOGRAPHY Ion-exchange columns are packed with small beads that carry positive or negative charges
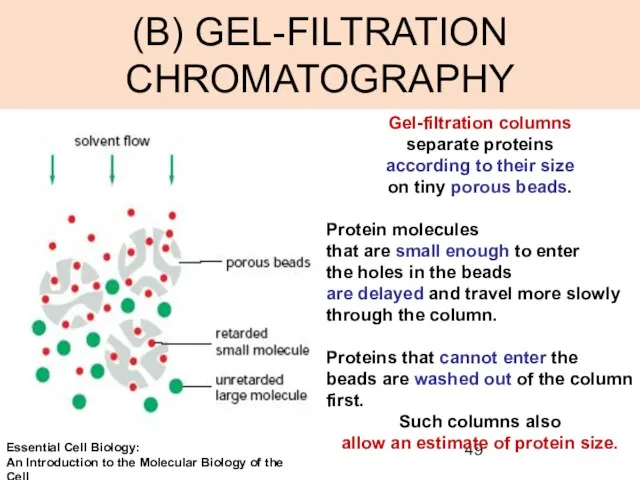
- 49. (B) GEL-FILTRATION CHROMATOGRAPHY Gel-filtration columns separate proteins according to their size on tiny porous beads. Protein
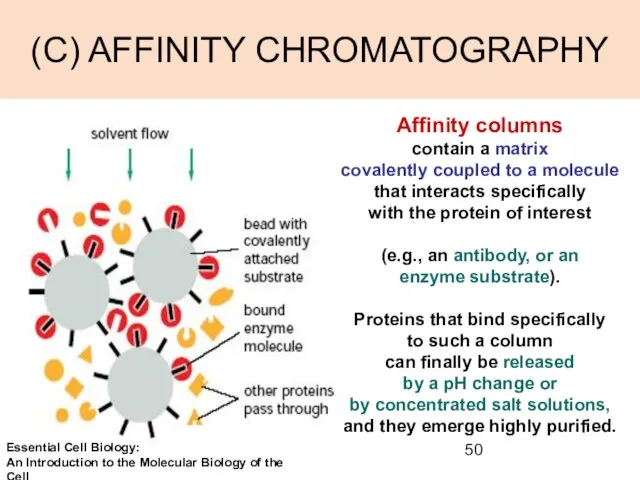
- 50. (C) AFFINITY CHROMATOGRAPHY Affinity columns contain a matrix covalently coupled to a molecule that interacts specifically
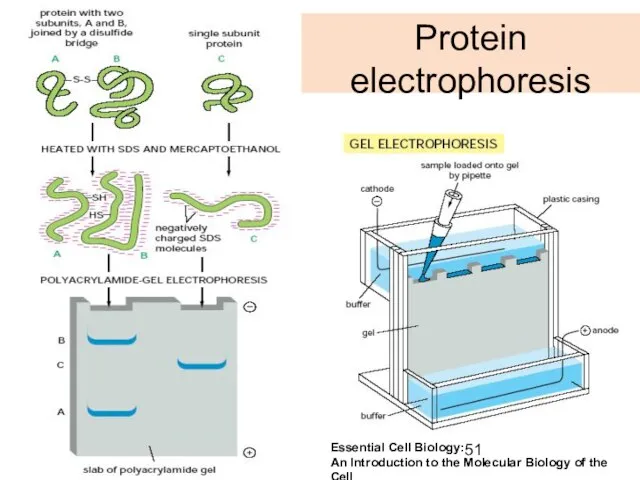
- 51. Protein electrophoresis Essential Cell Biology: An Introduction to the Molecular Biology of the Cell
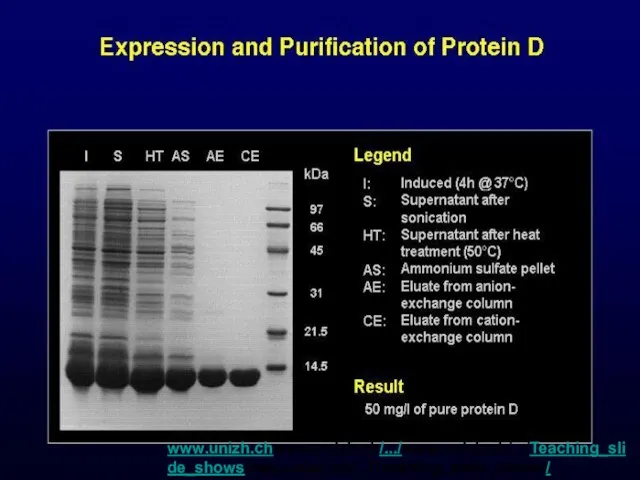
- 52. www.unizh.chwww.unizh.ch/.../www.unizh.ch/.../Teaching_slide_showswww.unizh.ch/.../Teaching_slide_shows/ Lambda/sld015.htm www.unizh.chwww.unizh.ch/.../www.unizh.ch/.../Teaching_slide_showswww.unizh.ch/.../Teaching_slide_shows/ Lambda/sld015.htm
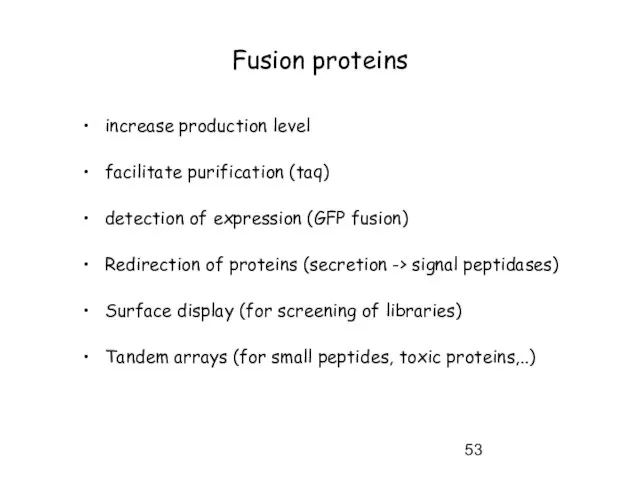
- 53. Fusion proteins increase production level facilitate purification (taq) detection of expression (GFP fusion) Redirection of proteins
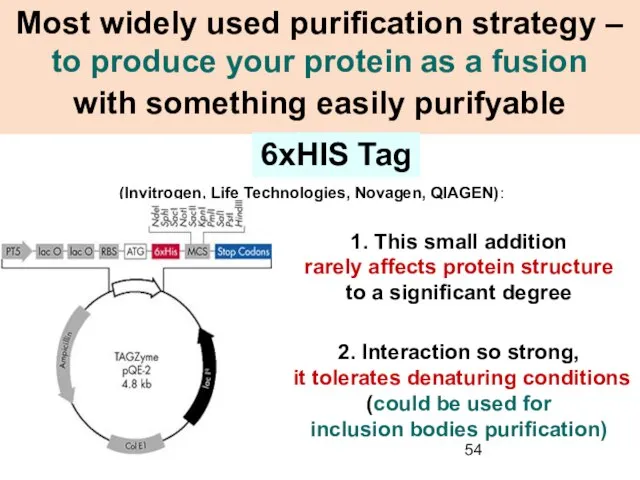
- 54. Most widely used purification strategy – to produce your protein as a fusion with something easily
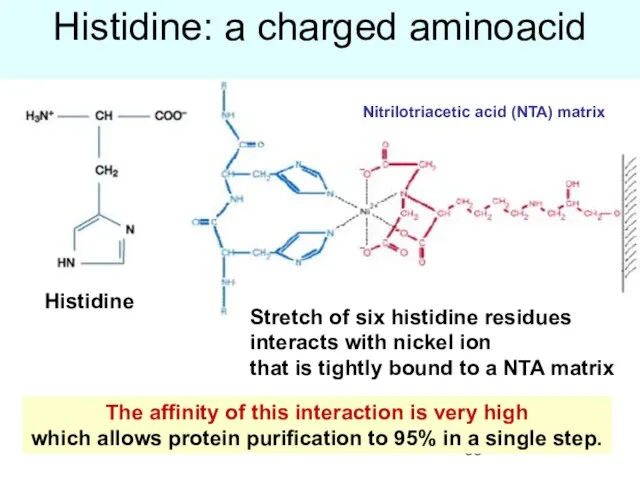
- 55. Histidine: a charged aminoacid The affinity of this interaction is very high which allows protein purification
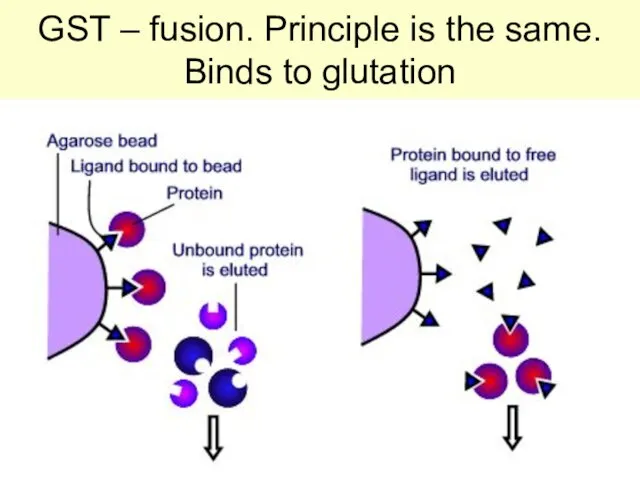
- 56. GST – fusion. Principle is the same. Binds to glutation
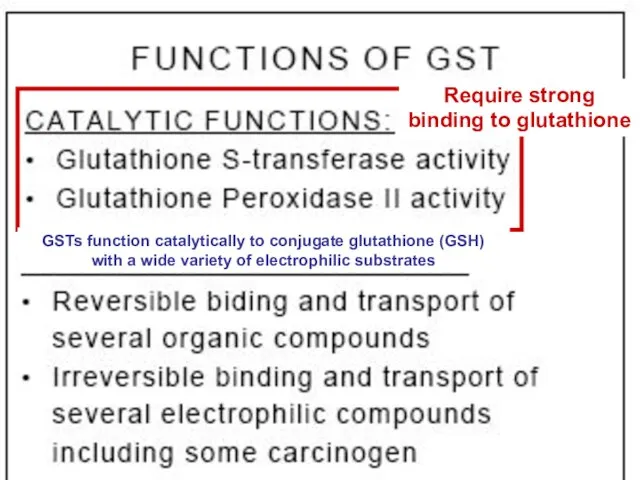
- 57. Require strong binding to glutathione Require strong binding to glutathione GSTs function catalytically to conjugate glutathione
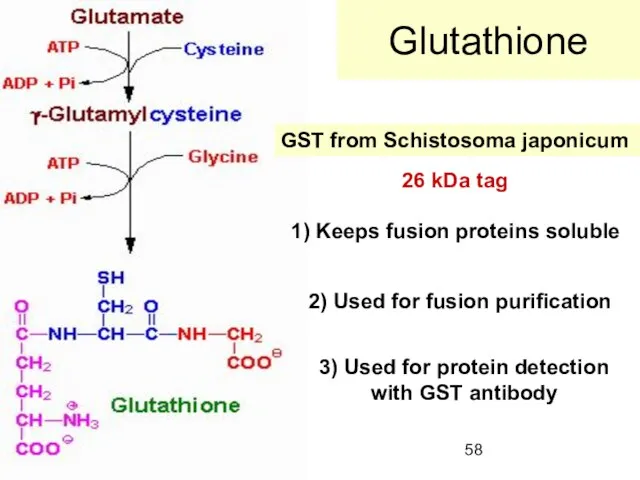
- 58. Glutathione GST from Schistosoma japonicum 1) Keeps fusion proteins soluble 2) Used for fusion purification 3)
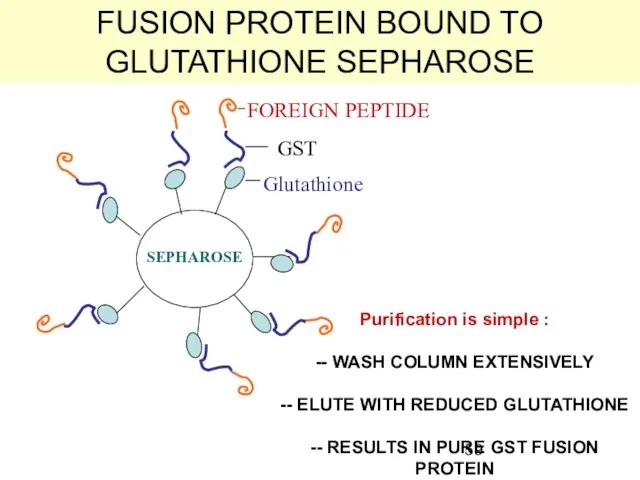
- 59. FUSION PROTEIN BOUND TO GLUTATHIONE SEPHAROSE Glutathione GST FOREIGN PEPTIDE SEPHAROSE Purification is simple : --
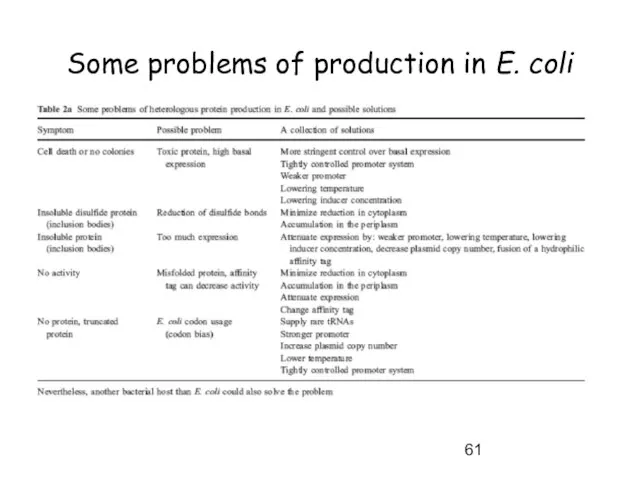
- 61. Some problems of production in E. coli
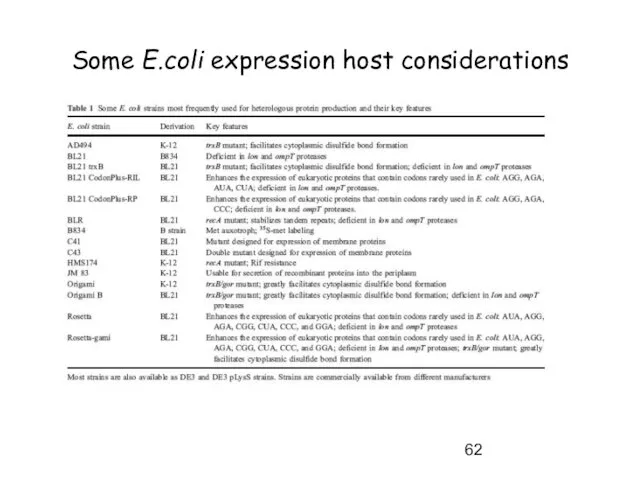
- 62. Some E.coli expression host considerations
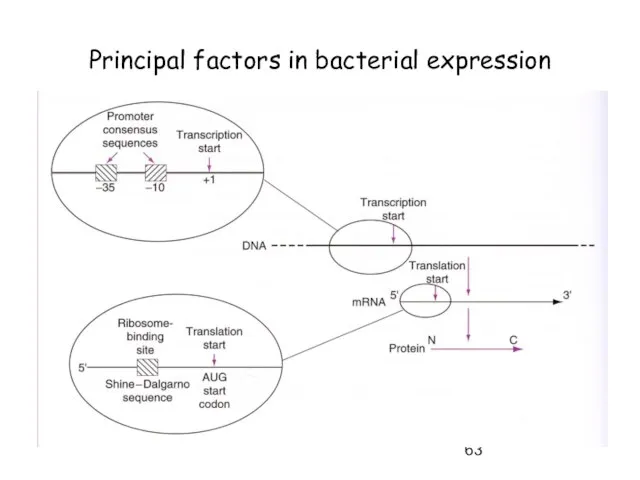
- 63. Principal factors in bacterial expression
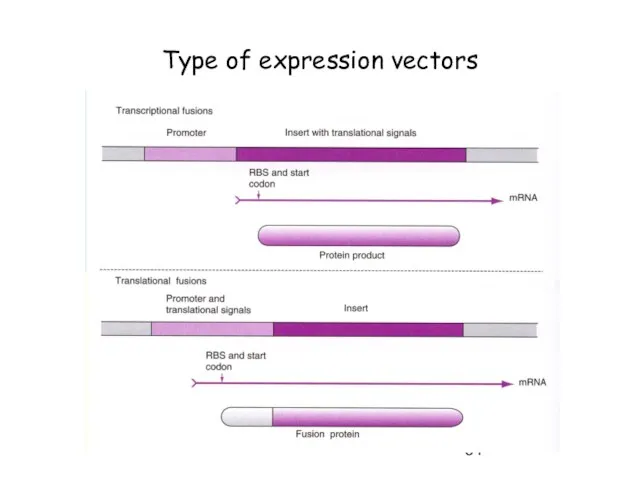
- 64. Type of expression vectors
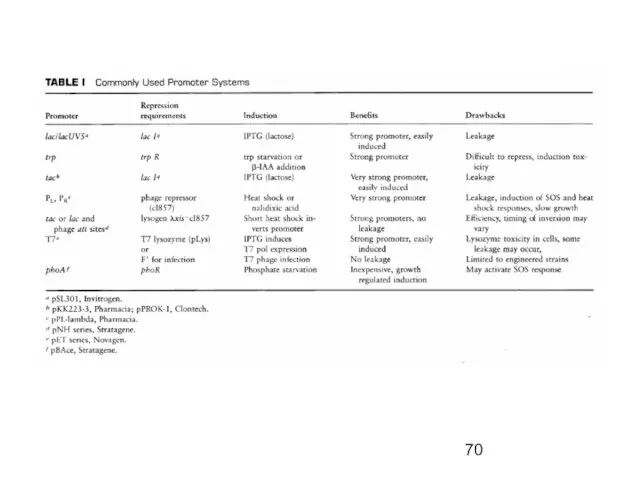
- 65. Initiation of Transcription Promoters for Expression in Prokaryotes In Escherichia coli - Lac system - plac
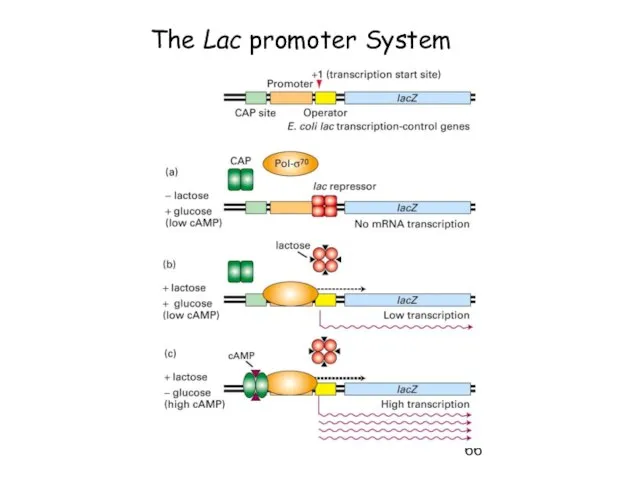
- 66. The Lac promoter System
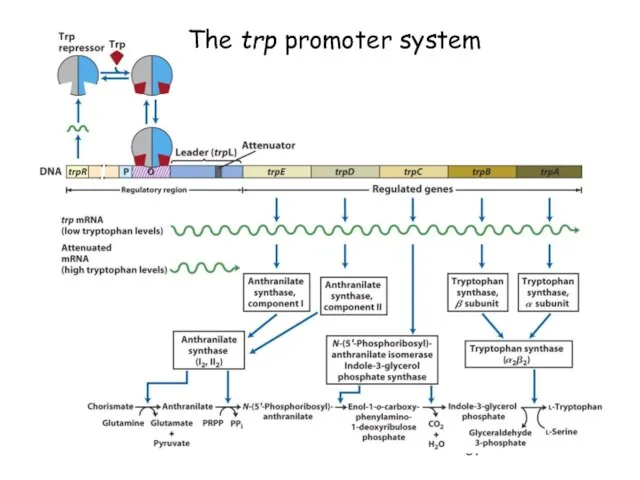
- 67. The trp promoter system
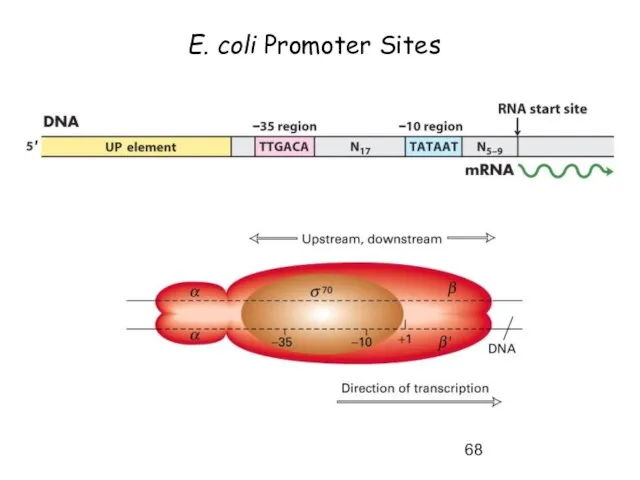
- 68. E. coli Promoter Sites
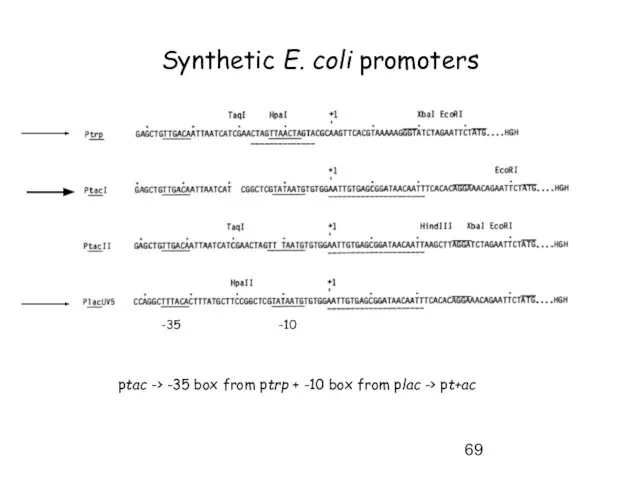
- 69. Synthetic E. coli promoters -35 -10 ptac -> -35 box from ptrp + -10 box from
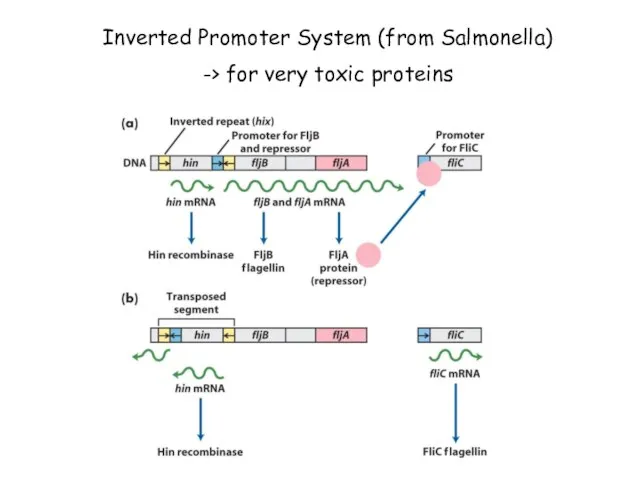
- 71. Inverted Promoter System (from Salmonella) -> for very toxic proteins
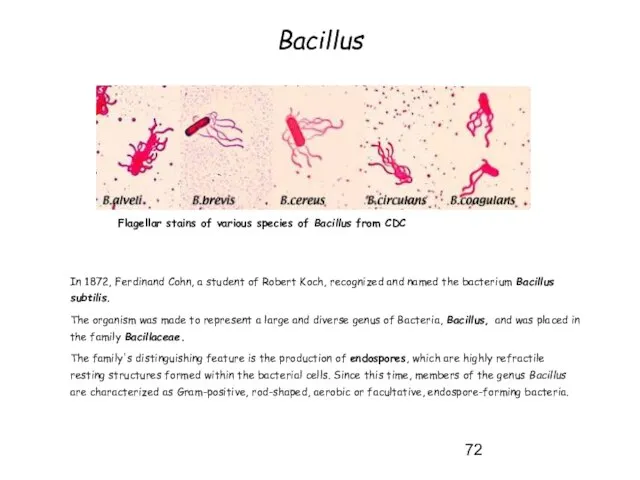
- 72. Bacillus In 1872, Ferdinand Cohn, a student of Robert Koch, recognized and named the bacterium Bacillus
- 73. Bacillus Antibiotic Producers: B. brevis (e.g. gramicidin, tyrothricin), B. cereus (e.g. cerexin, zwittermicin), B. circulans (e.g.
- 74. Bacillus Bacillus strains used as production organisms: - B. subtilis - B. brevis - B. licheniformis
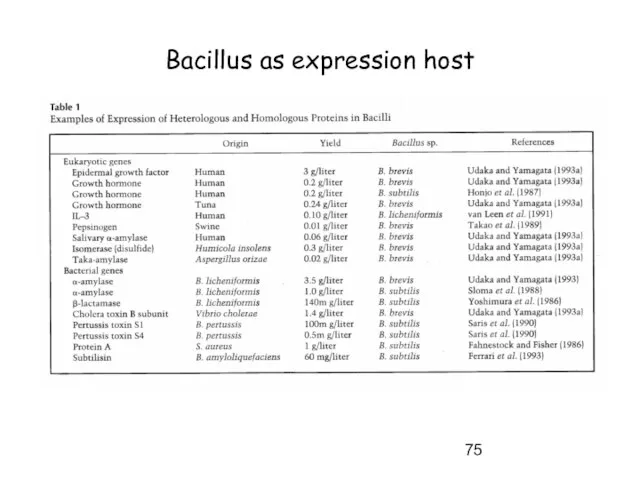
- 75. Bacillus as expression host
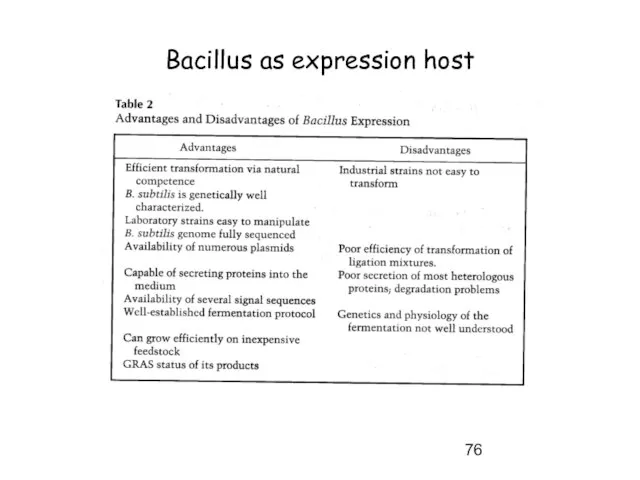
- 76. Bacillus as expression host
- 77. Products produced in Prokaryotic Systems Restriction Endonucleases -> produced in E. coli L- Ascorbic Acid (Vitamin
- 78. Expression in Eukaryotic Systems Yeast - Saccharomyces cerevisiae (baker’s yeast) - Pichia pastoris Insect Cells –
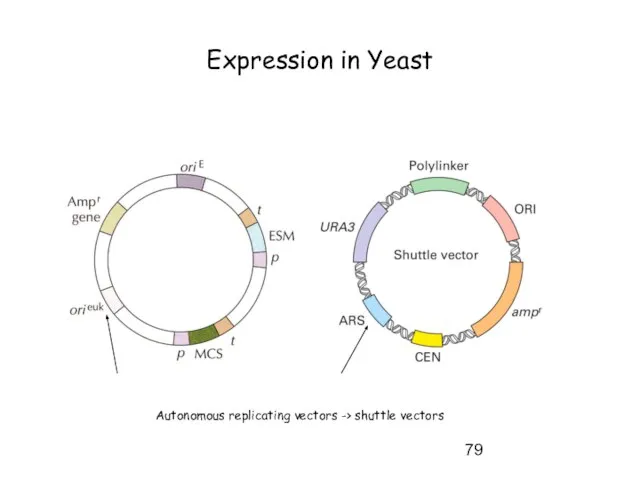
- 79. Expression in Yeast Autonomous replicating vectors -> shuttle vectors
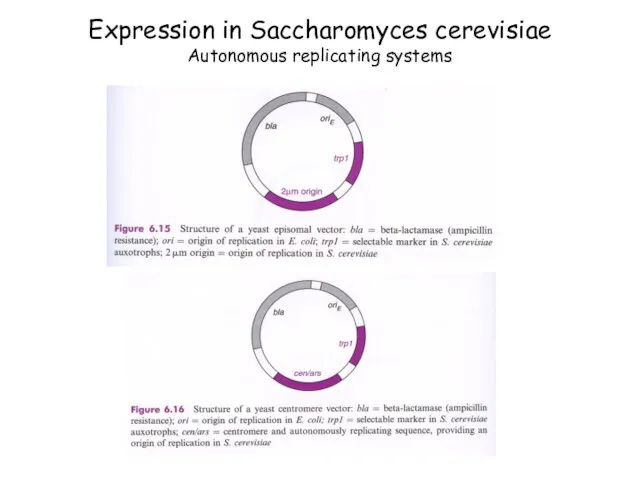
- 80. Expression in Saccharomyces cerevisiae Autonomous replicating systems
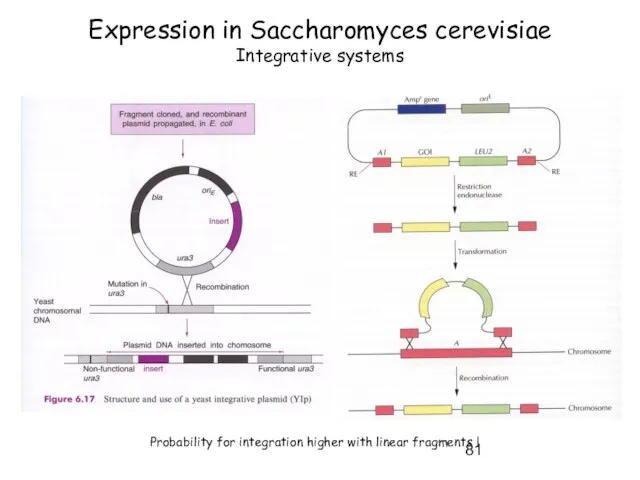
- 81. Expression in Saccharomyces cerevisiae Integrative systems Probability for integration higher with linear fragments !
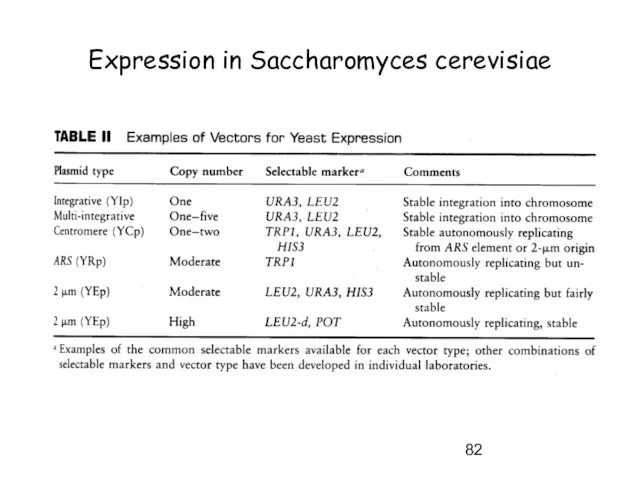
- 82. Expression in Saccharomyces cerevisiae
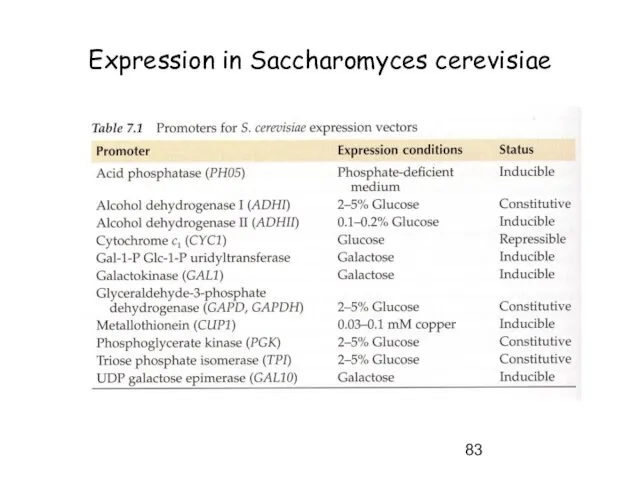
- 83. Expression in Saccharomyces cerevisiae
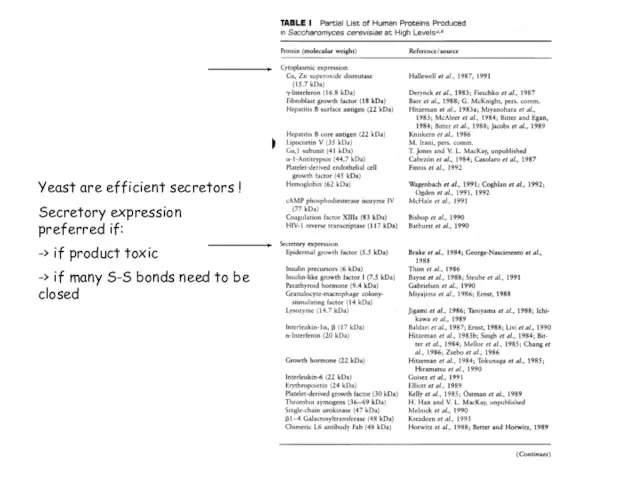
- 84. Yeast are efficient secretors ! Secretory expression preferred if: -> if product toxic -> if many
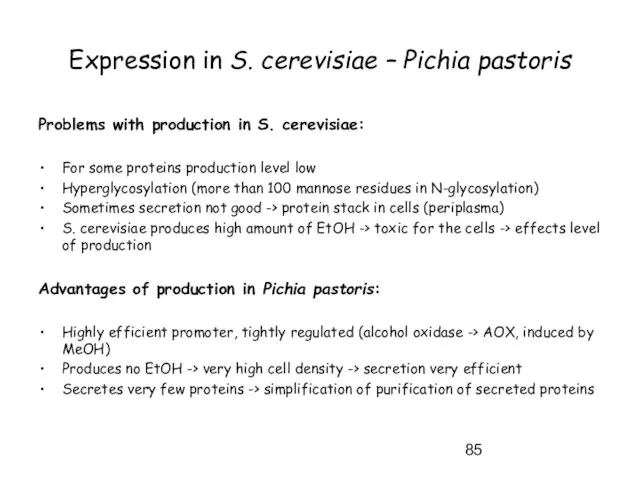
- 85. Expression in S. cerevisiae – Pichia pastoris Problems with production in S. cerevisiae: For some proteins
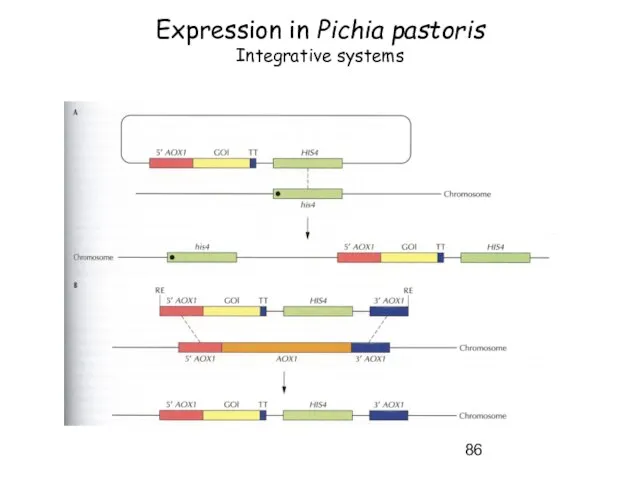
- 86. Expression in Pichia pastoris Integrative systems
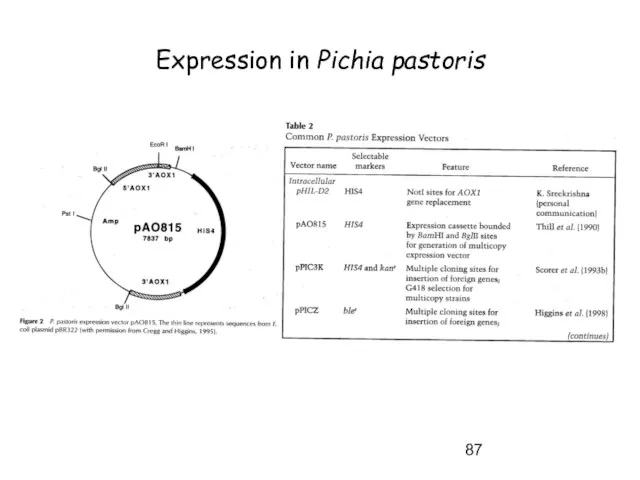
- 87. Expression in Pichia pastoris
- 88. Expression in Pichia pastoris
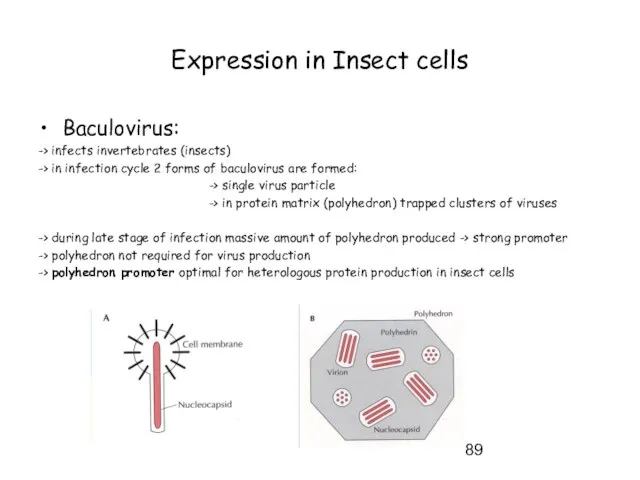
- 89. Expression in Insect cells Baculovirus: -> infects invertebrates (insects) -> in infection cycle 2 forms of
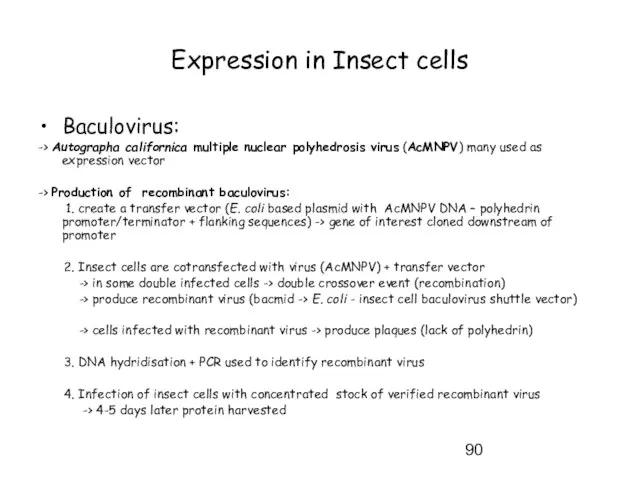
- 90. Expression in Insect cells Baculovirus: -> Autographa californica multiple nuclear polyhedrosis virus (AcMNPV) many used as
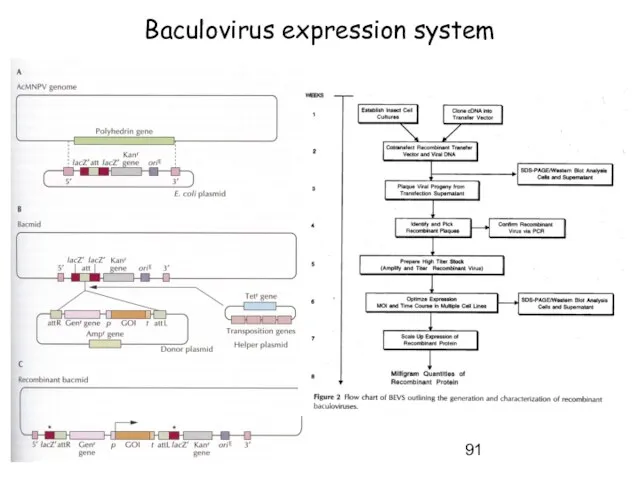
- 91. Baculovirus expression system
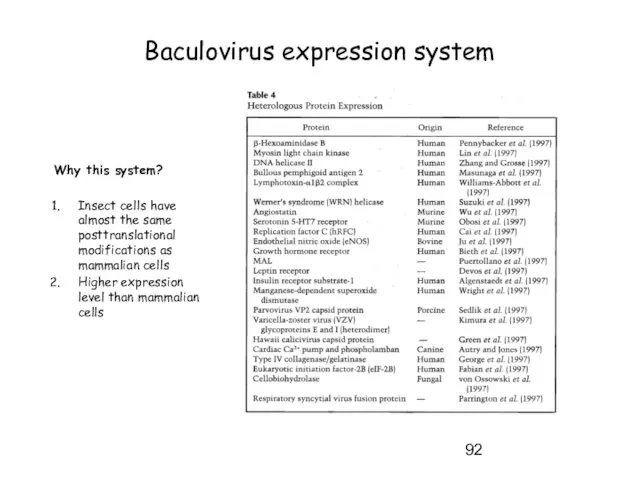
- 92. Why this system? Insect cells have almost the same posttranslational modifications as mammalian cells Higher expression
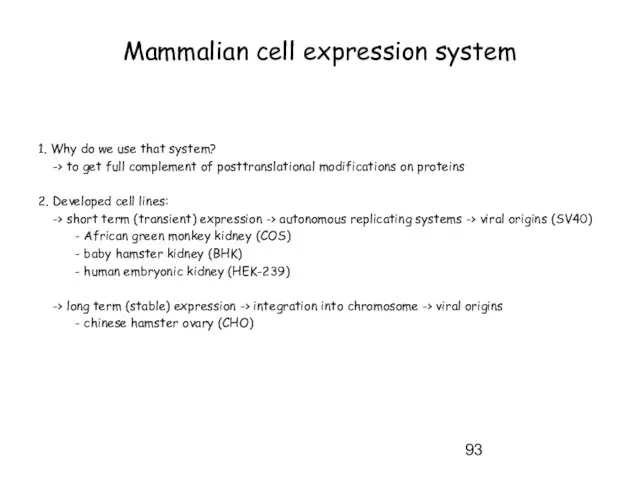
- 93. Mammalian cell expression system 1. Why do we use that system? -> to get full complement
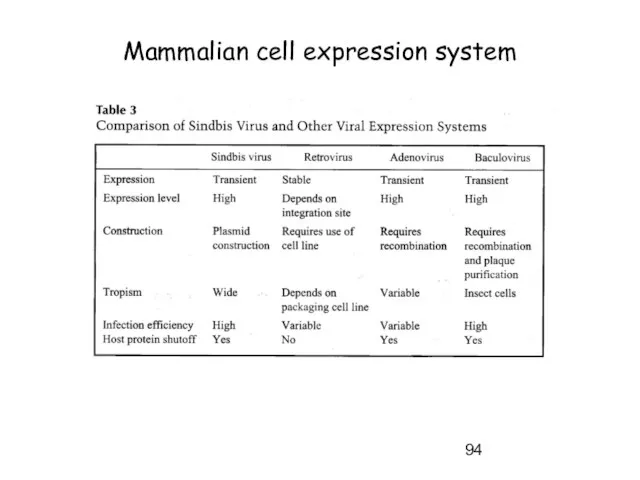
- 94. Mammalian cell expression system
- 95. Gene expression in mammalian cell lines A convenient alternative for setting up mammalian cell facilities –
- 97. Скачать презентацию





 Маркетинг территории в России: возможности и технологии
Маркетинг территории в России: возможности и технологии Презентация на тему Физиология почки
Презентация на тему Физиология почки  Практика распределенной разработки в Open-Source проектах.
Практика распределенной разработки в Open-Source проектах. Таможенные операции и таможенные процедуры
Таможенные операции и таможенные процедуры Найти расстояние от берега до корабля.
Найти расстояние от берега до корабля. Взаимодействие пути и подвижного состава
Взаимодействие пути и подвижного состава Process Analysis
Process Analysis Уральский экономический район
Уральский экономический район ТЕХНИЧЕСКОЕ ЗАДАНИЕ
ТЕХНИЧЕСКОЕ ЗАДАНИЕ Праздник Пасхи
Праздник Пасхи Город творческой интеллигенции
Город творческой интеллигенции Доклад Партнерские связи Лешуконского, Мезенского и Пинежского районов как способ развития территории
Доклад Партнерские связи Лешуконского, Мезенского и Пинежского районов как способ развития территории Географические названия на буквы Н и К
Географические названия на буквы Н и К Труд и семья с точки зрения закона
Труд и семья с точки зрения закона Проблемы, возникающие при регулировании тарифов методом доходности инвестированного капитала
Проблемы, возникающие при регулировании тарифов методом доходности инвестированного капитала ЭЛЕКТРОСНАБЖЕНИЕ_Ч1(заочн)
ЭЛЕКТРОСНАБЖЕНИЕ_Ч1(заочн) Малинова О.Ю. Конструирование «общепринятого»: использование прошлого для легитимации политического курса (на примере анализа е
Малинова О.Ю. Конструирование «общепринятого»: использование прошлого для легитимации политического курса (на примере анализа е Семейный эколого-туристический клуб
Семейный эколого-туристический клуб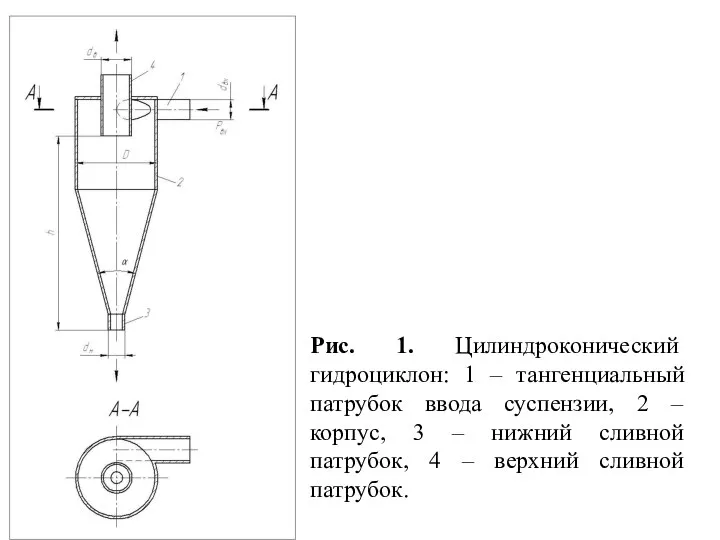 Гидромеханические установки. Цилиндроконический гидроциклон. (Лекция 5)
Гидромеханические установки. Цилиндроконический гидроциклон. (Лекция 5) Виды печатных машинок
Виды печатных машинок Этикет 2 класс
Этикет 2 класс Недаром помнит вся Россия про день Бородина! 6 класс
Недаром помнит вся Россия про день Бородина! 6 класс ШЕЙНГАУЗ АЛЕКСАНДР СОЛОМОНОВИЧ заведующий отделом института экономических исследований ДВО РАН, д.с-х.н., профессор
ШЕЙНГАУЗ АЛЕКСАНДР СОЛОМОНОВИЧ заведующий отделом института экономических исследований ДВО РАН, д.с-х.н., профессор Возможности Emerson по Продукции и Сервису $ 22,6 Миллиарда - доходы более чем 60 подразделений
Возможности Emerson по Продукции и Сервису $ 22,6 Миллиарда - доходы более чем 60 подразделений Презентация на тему Насекомые вредители поля и огорода
Презентация на тему Насекомые вредители поля и огорода  Русь в XIII – XV веках Монгольское нашествие на Русь
Русь в XIII – XV веках Монгольское нашествие на Русь Мой выбор - терапия
Мой выбор - терапия