Содержание
- 2. Objectives of lecture –presentation: Study of different methods of nanosystems investigations
- 3. Plan of lecture –presentation: Lecture 5 1. Method of Electron Microscopy 2. Sonde Microscopy 3. Diffraction
- 4. The basic methods of nanoparticles’ sizes and some properties in gaseous phase determination: ionization by photons
- 5. Methods of study of particles on the surface: X-ray and scanning electron microscopy (information about sizes/forms
- 6. Methods of study of particles in volume: X-ray and scanning electron microscopy, electric conductivity, as well
- 7. 1. Method of Electron Microscopy Microscopy is the main method for determination nanoparticles’ sizes. They use
- 8. 1. Method of Electron Microscopy X-ray electron microscopy. Object in form of thin film is X-rayed
- 9. 1. Method of Electron Microscopy Scanning electron microscopy. This method is used mostly for study of
- 10. 1. Method of Electron Microscopy Several types of rays occur when electrons interact with the object:
- 11. 1. Method of Electron Microscopy The main value of this method – is that it is
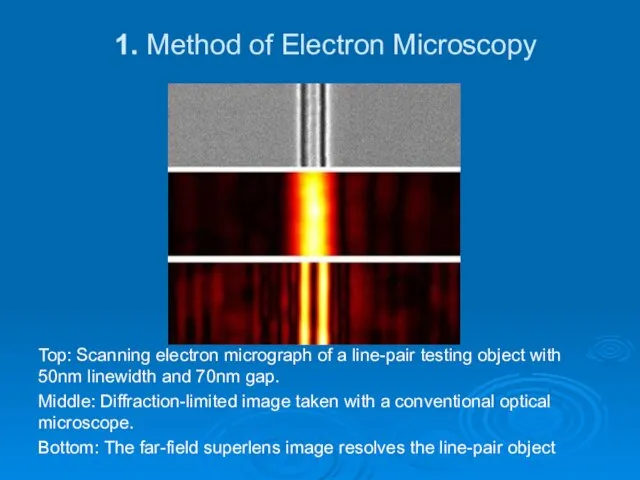
- 12. 1. Method of Electron Microscopy Top: Scanning electron micrograph of a line-pair testing object with 50nm
- 13. 2. Sonde Microscopy In 1981 Binnig and Rorer created scanning tunnel microscope (STM) and in 1986
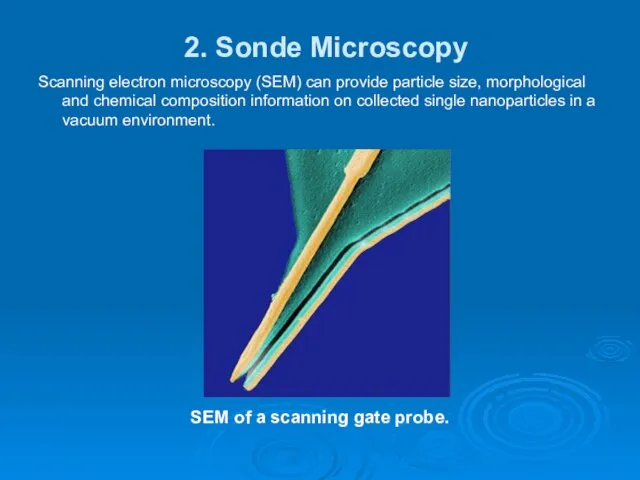
- 14. 2. Sonde Microscopy Scanning electron microscopy (SEM) can provide particle size, morphological and chemical composition information
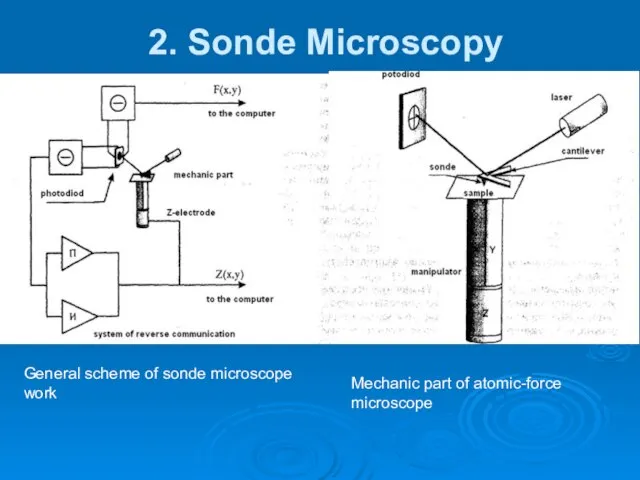
- 15. 2. Sonde Microscopy General scheme of sonde microscope work Mechanic part of atomic-force microscope
- 16. 3. Diffraction Methods . These methods include diffraction of X-rays and neutrons and are less general
- 17. 3. Diffraction Methods . Neutrons diffraction. Neutron is the particle which due to its properties is
- 18. Plan of lecture –presentation: Lecture 6 1. Mass-Spectrometry 2. Photoelectron Spectroscopy
- 19. 1.Mass-Spectrometry Mass spectrometry (MS) is an analytical technique that produces spectra (singular spectrum) of the masses
- 20. 1.Mass-Spectrometry Mass spectrometry works by ionizing chemical compounds to generate charged molecules or molecule fragments and
- 21. Steps of mass-spectrometry: 1. Ionisation - Gaseous atoms of a particular element are bombarded with electrons
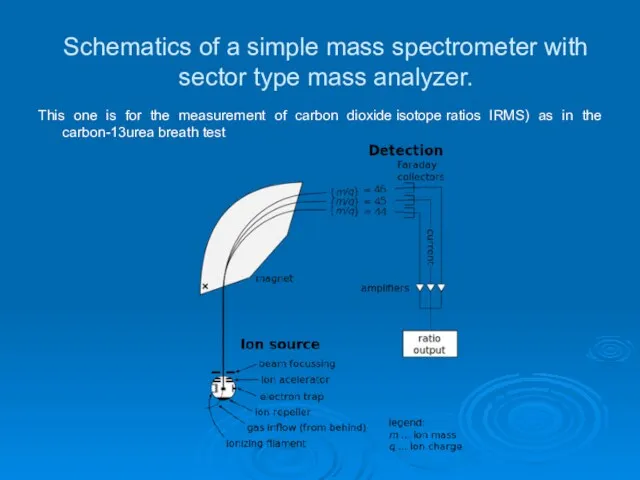
- 22. Schematics of a simple mass spectrometer with sector type mass analyzer. This one is for the
- 23. 2. Photoelectron Spectroscopy This method is based on the measurement of spectrum energies of electrons which
- 24. 2. Photoelectron Spectroscopy They determine energies of interactions of electrons and their energy levels in studied
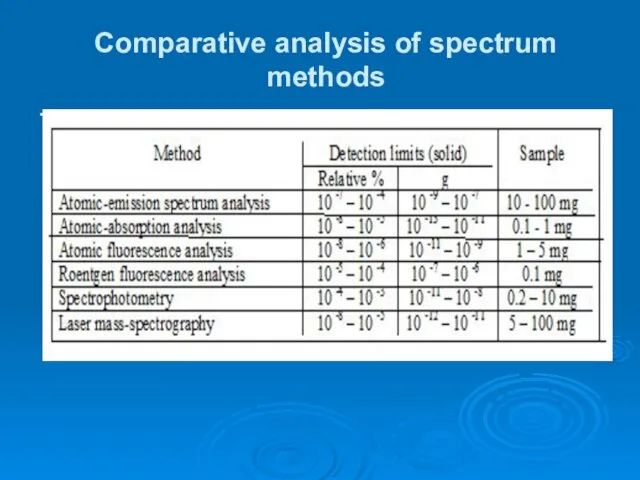
- 25. Comparative analysis of spectrum methods .
- 26. Check Yourself 1. What is the most significant for study of chemical interactions? 2. What are
- 27. Check Yourself 13. What restricts the resolution of conventional lenses? 14. Describe the method suggested by
- 28. Check Yourself 22. Which are the main requirements for crystals to be studied by roentgenography method?
- 30. Скачать презентацию




















 Процессы жизнедеятельности одноклеточных животных
Процессы жизнедеятельности одноклеточных животных Живой организм и его свойства
Живой организм и его свойства Финансирование научных исследований
Финансирование научных исследований Операционные системы и сети ЭВМ Operating Systems and Networking Лекция 26
Операционные системы и сети ЭВМ Operating Systems and Networking Лекция 26 АЙСБЕРГ
АЙСБЕРГ Морфология культуры
Морфология культуры Онлайн-курс СПбПУ. Введение в инженерную деятельность. Трек 2
Онлайн-курс СПбПУ. Введение в инженерную деятельность. Трек 2 Органическая химия. Предельные углеводороды
Органическая химия. Предельные углеводороды Безопасность жизни
Безопасность жизни Май 2012
Май 2012 Реализация производственной системы Сбербанка в Западно-Уральском банке ОАО Сбербанка России: задачи, инструменты, достижения
Реализация производственной системы Сбербанка в Западно-Уральском банке ОАО Сбербанка России: задачи, инструменты, достижения кодировка
кодировка Сырные палочки
Сырные палочки Первая встреча Костромского Клуба Тестировщиков (ClubQA)Что должен знать тестировщик
Первая встреча Костромского Клуба Тестировщиков (ClubQA)Что должен знать тестировщик Сущность и содержание миссии. Формулирование миссии
Сущность и содержание миссии. Формулирование миссии 3_urok_9-e_kl_informatika
3_urok_9-e_kl_informatika Dlya_postera
Dlya_postera Схема расположения участка в структуре города
Схема расположения участка в структуре города От газеты Телесемь в Краснодаре
От газеты Телесемь в Краснодаре Презентация на тему: Красная Шапочка
Презентация на тему: Красная Шапочка Решение нестандартных уравнений
Решение нестандартных уравнений СЧЕТ ОТ 0 ДО 10
СЧЕТ ОТ 0 ДО 10 Модель психологической службы ГБОУ СОШ № 426
Модель психологической службы ГБОУ СОШ № 426 Где живут люди? ИЗО 4 класс
Где живут люди? ИЗО 4 класс С ДНЕМ УЧИТЕЛЯ! В большую жизнь Вы нам открыли двери,Вы нас не только азбуке учили.Учитель! Мы Вас любим, мы Вам верим!Мы доброты ур
С ДНЕМ УЧИТЕЛЯ! В большую жизнь Вы нам открыли двери,Вы нас не только азбуке учили.Учитель! Мы Вас любим, мы Вам верим!Мы доброты ур Простые механизмы. Рычаг.
Простые механизмы. Рычаг. MTC Skills
MTC Skills Исторические лица земли Тверской, их жизнь и деяния, составляющие гордость региона
Исторические лица земли Тверской, их жизнь и деяния, составляющие гордость региона