Содержание
- 2. ANNOUNCMENTS CW Test on CW 11, 12 (Special Relativity and Nuclear Physics) on Monday 27 Feb
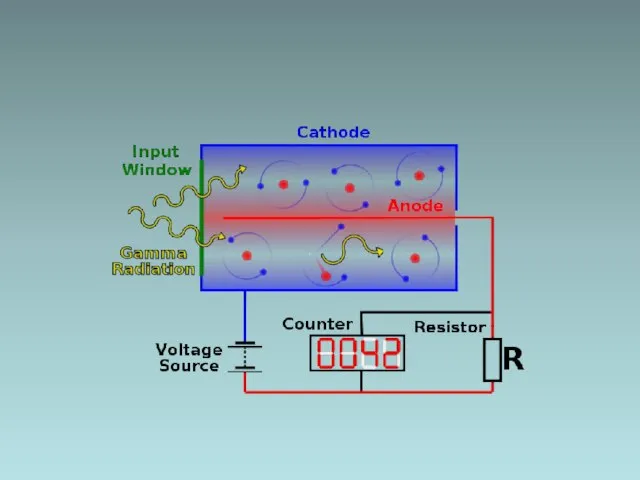
- 3. Detecting radiation and nuclear energy e.g., Geiger Muller tube Mica window Low pressure gas High voltage
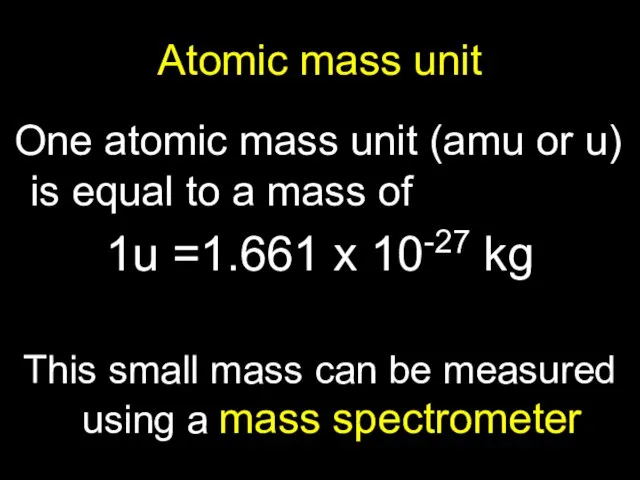
- 5. Atomic mass unit One atomic mass unit (amu or u) is equal to a mass of
- 6. Example 1: 1u =1.661 x 10-27 kg Find its equivalence in Joules of Energy, and MeV
- 7. Example 2: Using mass spectrometry, physicists have measured the masses of nuclei, protons and neutrons accurately.
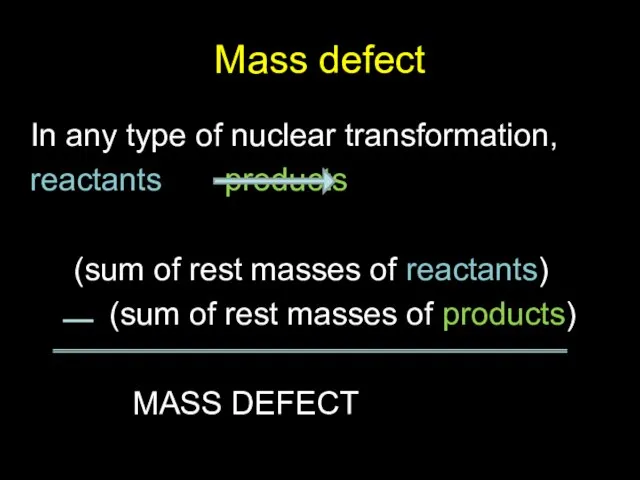
- 8. Mass defect In any type of nuclear transformation, reactants products (sum of rest masses of reactants)
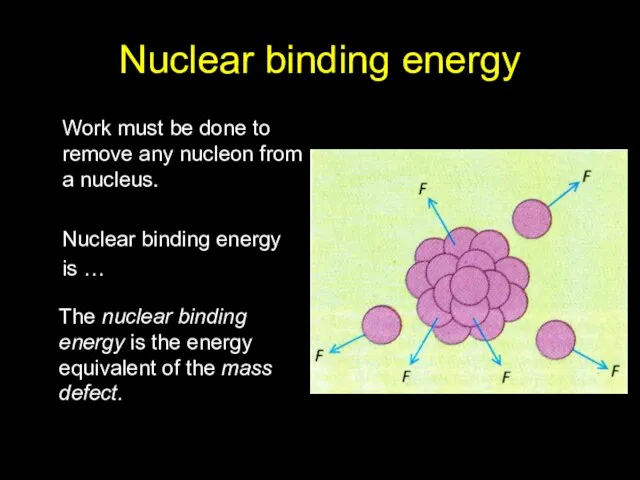
- 9. Nuclear binding energy Work must be done to remove any nucleon from a nucleus. Nuclear binding
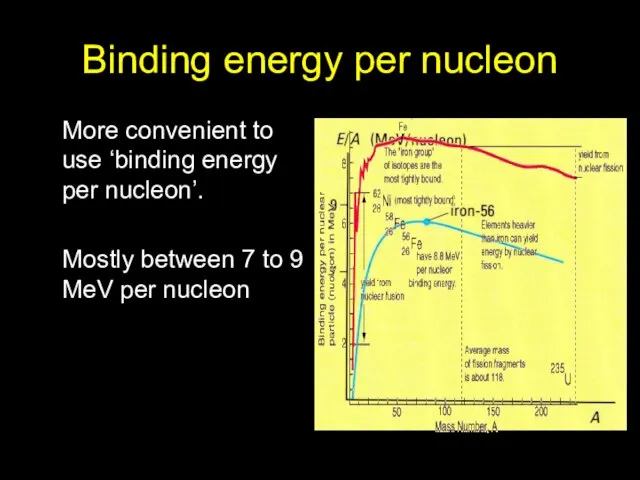
- 10. Binding energy per nucleon More convenient to use ‘binding energy per nucleon’. Mostly between 7 to
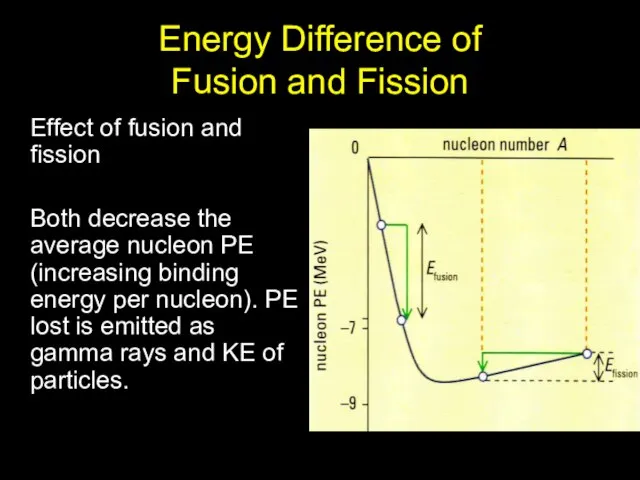
- 11. Energy Difference of Fusion and Fission Effect of fusion and fission Both decrease the average nucleon
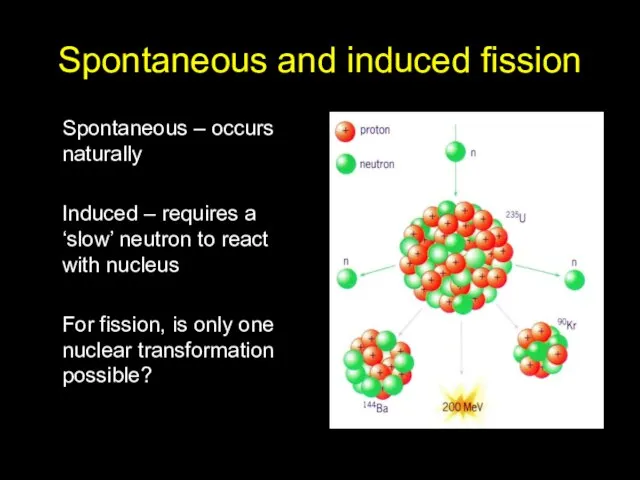
- 12. Spontaneous and induced fission Spontaneous – occurs naturally Induced – requires a ‘slow’ neutron to react
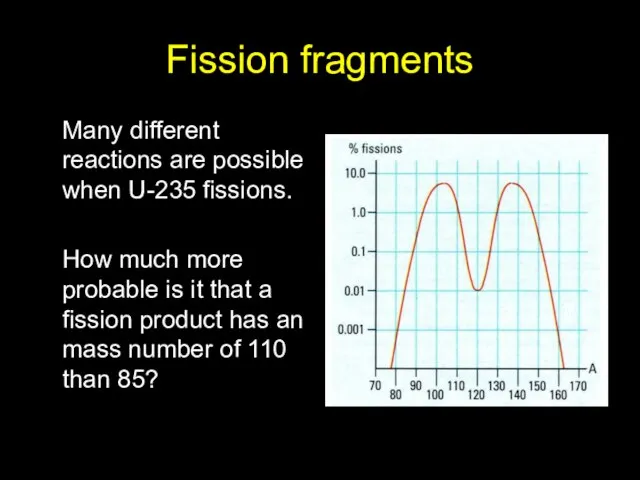
- 13. Fission fragments Many different reactions are possible when U-235 fissions. How much more probable is it
- 14. Example 3 a) A possible fission reaction is shown above. Given that the masses of U,
- 15. Some facts about reactors As of Feb, 2012 worldwide, 31 countries have 435 active reactors producing
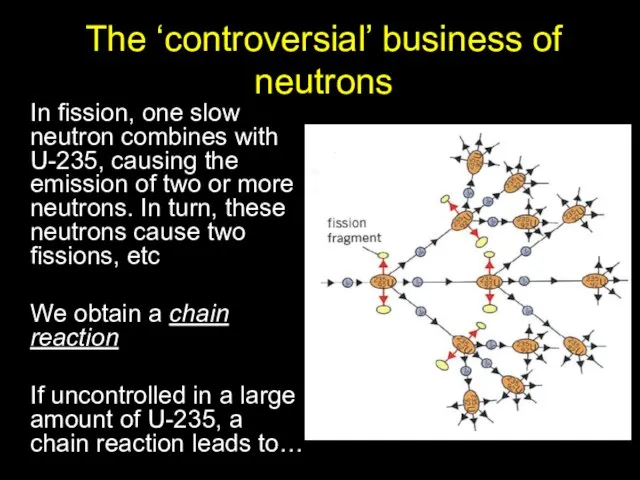
- 16. The ‘controversial’ business of neutrons In fission, one slow neutron combines with U-235, causing the emission
- 17. Why mushrooms?
- 18. Atomic bombs (using fission)
- 20. What do you know about the history of nuclear tests within Kazakh territory? Semipalatinsk Test Site
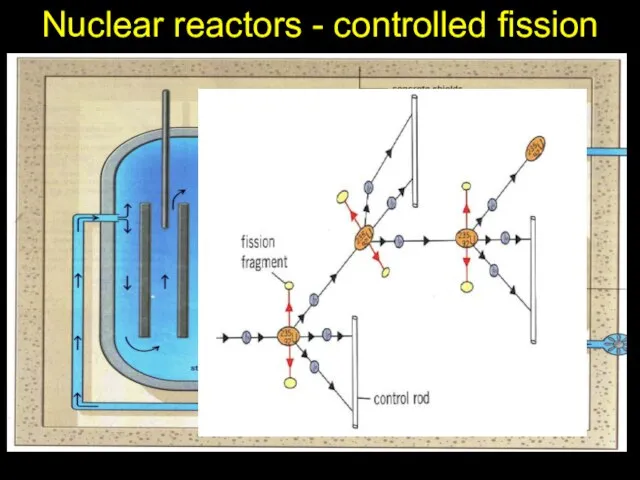
- 21. Nuclear reactors - controlled fission
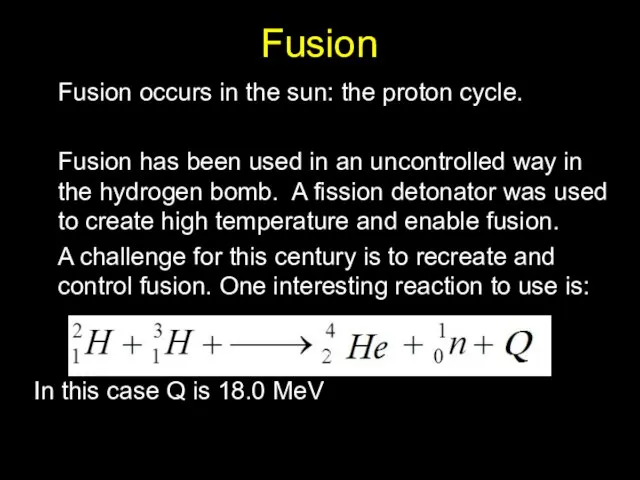
- 22. Fusion Fusion occurs in the sun: the proton cycle. Fusion has been used in an uncontrolled
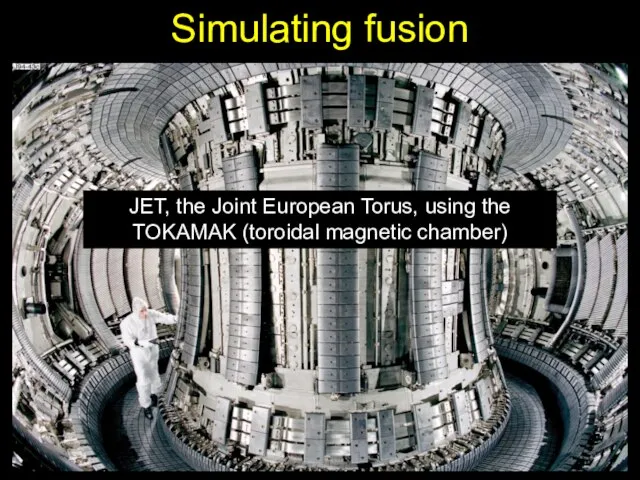
- 23. Simulating fusion JET, the Joint European Torus, using the TOKAMAK (toroidal magnetic chamber)
- 24. READING Adams and Allday: 8.26 to 8.32, inclusive. At the end of this lecture you should
- 26. Скачать презентацию











 Инструментальные средства для работы в системе Moodle
Инструментальные средства для работы в системе Moodle Семья в историческом интерьере
Семья в историческом интерьере Устойчивость транспортных потоков Евразии
Устойчивость транспортных потоков Евразии Составление уравнения по условию задачи6 класс
Составление уравнения по условию задачи6 класс КОНСТРУЦИОННЫЕ СТАЛИ
КОНСТРУЦИОННЫЕ СТАЛИ Классификация коньяка
Классификация коньяка  Как готовить системных программистов
Как готовить системных программистов Физминутка Крошка Енот
Физминутка Крошка Енот Вектора
Вектора Tag-questions (разделительные вопросы)
Tag-questions (разделительные вопросы)  Макет комнаты подростка
Макет комнаты подростка Школа
Школа День эффективности HR08 Апреля, 2011. Москва.
День эффективности HR08 Апреля, 2011. Москва. Писатель-романтик А.С. Грин Когда дни начинают пылиться и краски блекнуть, я беру Грина. Когда дни начинают пылиться и краски блекну
Писатель-романтик А.С. Грин Когда дни начинают пылиться и краски блекнуть, я беру Грина. Когда дни начинают пылиться и краски блекну Информатика и ИКТ
Информатика и ИКТ Орфография. Морфемика. Словообразование
Орфография. Морфемика. Словообразование Работа и мощность постоянного тока. Закон Джоуля-Ленца.
Работа и мощность постоянного тока. Закон Джоуля-Ленца. О Компании Роснефть и ОЦО – Общем Центре Обслуживания
О Компании Роснефть и ОЦО – Общем Центре Обслуживания Гравийно-песчаные заводы
Гравийно-песчаные заводы Click to edit Master title style Click to edit Master subtitle style
Click to edit Master title style Click to edit Master subtitle style  10 принципов хорошего управления проектами в компаниях сферы услугВита Кравчук, управляющий партнер компании Business.People Киев, 1 ок
10 принципов хорошего управления проектами в компаниях сферы услугВита Кравчук, управляющий партнер компании Business.People Киев, 1 ок МЕТОДЫ ХРАНЕНИЯ ИЕРАРХИЧЕСКИХ СТРУКТУР В РЕЛЯЦИОННЫХ БАЗАХ ДАННЫХ
МЕТОДЫ ХРАНЕНИЯ ИЕРАРХИЧЕСКИХ СТРУКТУР В РЕЛЯЦИОННЫХ БАЗАХ ДАННЫХ Достопримечательности Омска
Достопримечательности Омска Инвестиционная политика предприятия в современных условиях
Инвестиционная политика предприятия в современных условиях Презентация_шаблон
Презентация_шаблон Презентация на тему Изобразительное искусство России второй половины XIX века
Презентация на тему Изобразительное искусство России второй половины XIX века 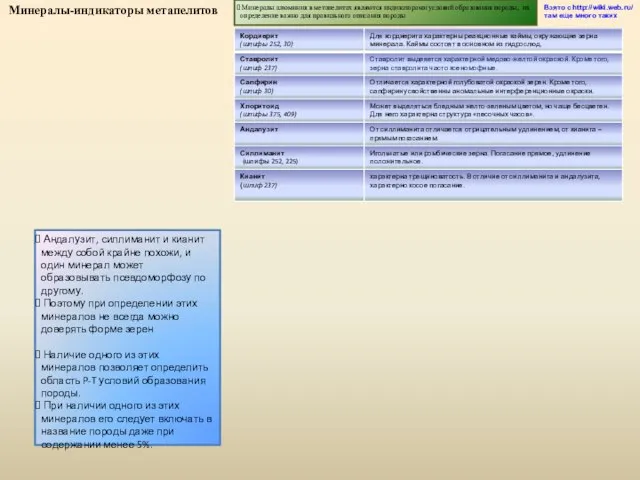 Минералы-индикаторы метапелитов
Минералы-индикаторы метапелитов Методика планирования воспитательной работы в классе
Методика планирования воспитательной работы в классе