Содержание
- 2. A method of pulsed electronic beam (PEB) evaporation used for production: simple (ZnO, Al2O3, Fe2O3, CeO2)
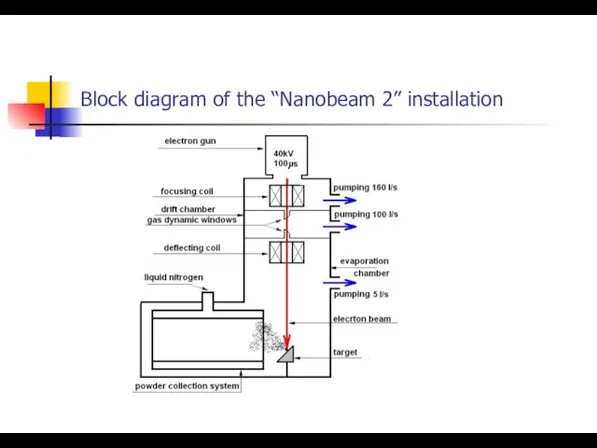
- 3. Block diagram of the “Nanobeam 2” installation
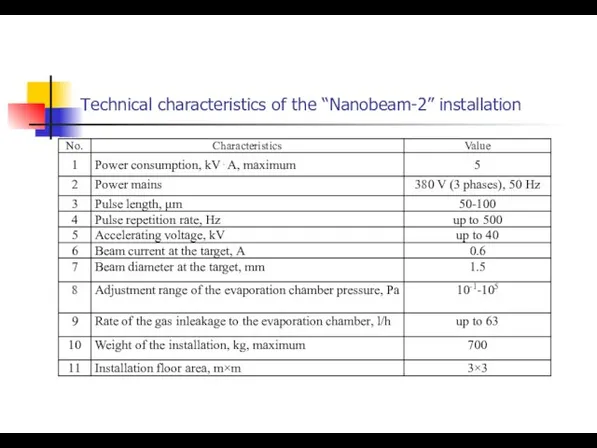
- 4. Technical characteristics of the “Nanobeam-2” installation
- 5. Photo of the “Nanobeam-2” installation
- 6. Beam scanning on a target is carried out continuously by a principle of development of a
- 7. Photo of the targets ZnO YSZ (ZrO2-10Y2O3) 1. fragments of the target 2. beam print
- 8. Photo of the “Nanobeam-2” powder collection system
- 9. For examination of the materials were used: The specific surface of the powders Ss was measured
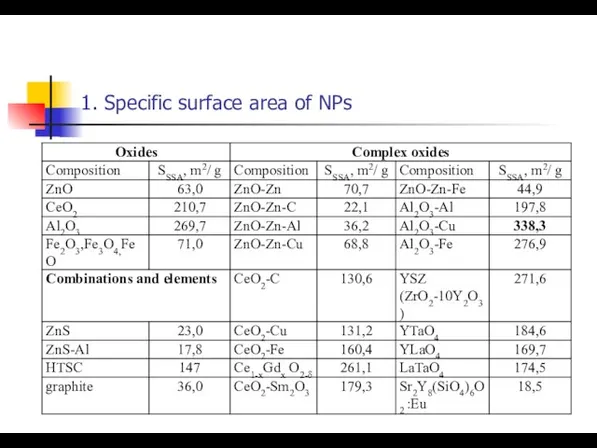
- 10. 1. Specific surface area of NPs
- 11. 2. Features in common of the morphology of the NPs were as follows: 1) coarse shapeless
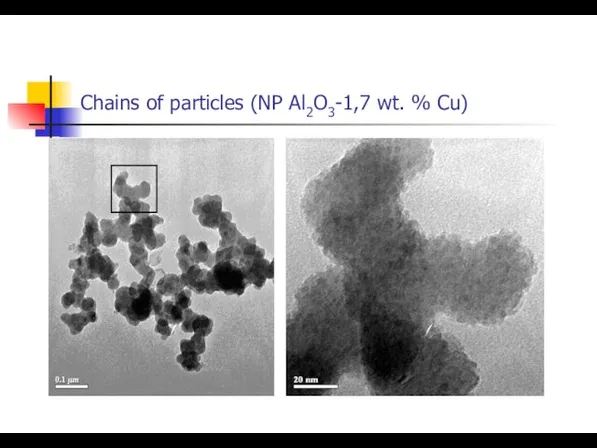
- 12. Chains of particles (NP Al2O3-1,7 wt. % Сu)
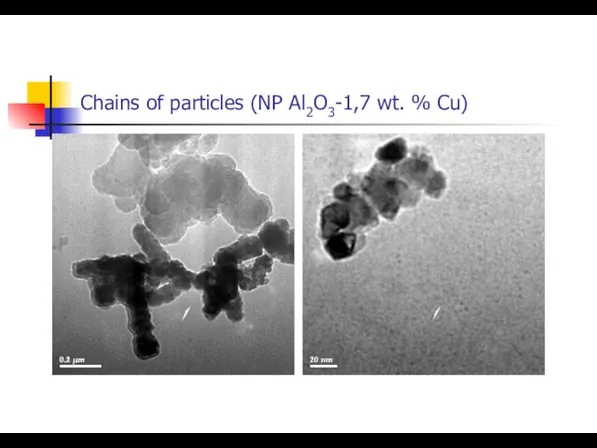
- 13. Chains of particles (NP Al2O3-1,7 wt. % Сu)
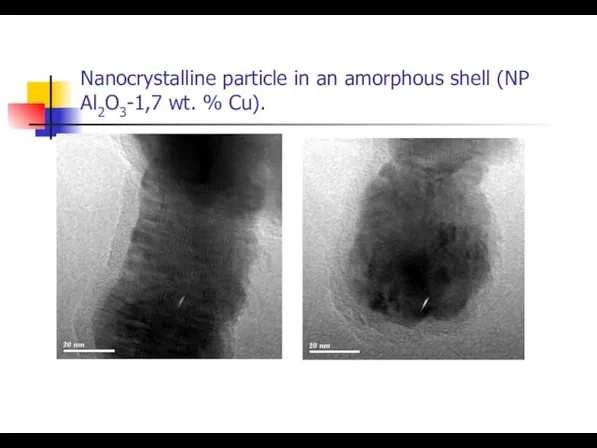
- 14. Nanocrystalline particle in an amorphous shell (NP Al2O3-1,7 wt. % Сu).
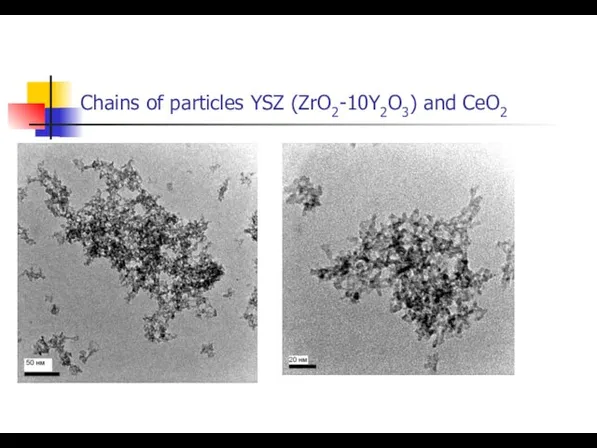
- 15. Chains of particles YSZ (ZrO2-10Y2O3) and CeO2
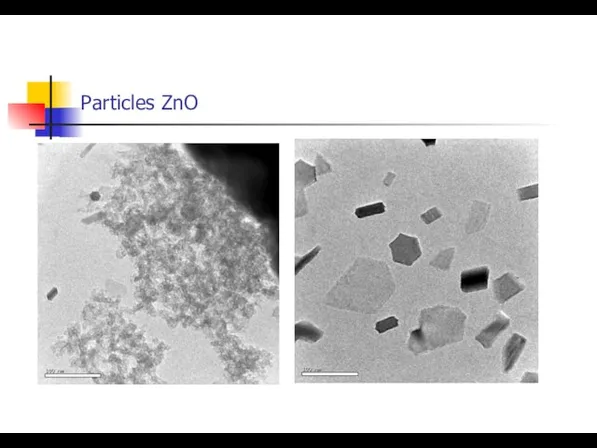
- 16. Particles ZnO
- 17. 3. There is formation NP different on the phase structure from an evaporated material An XRD
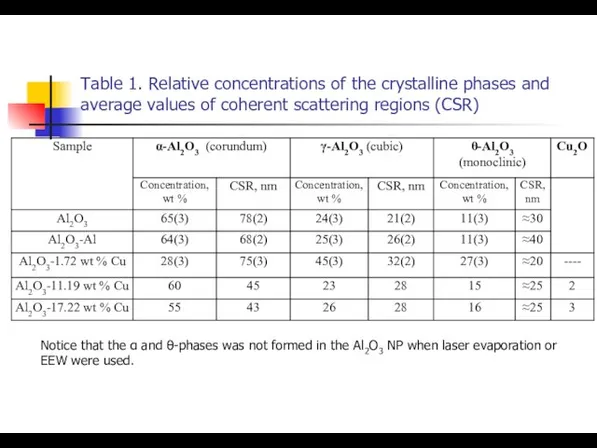
- 18. Table 1. Relative concentrations of the crystalline phases and average values of coherent scattering regions (CSR)
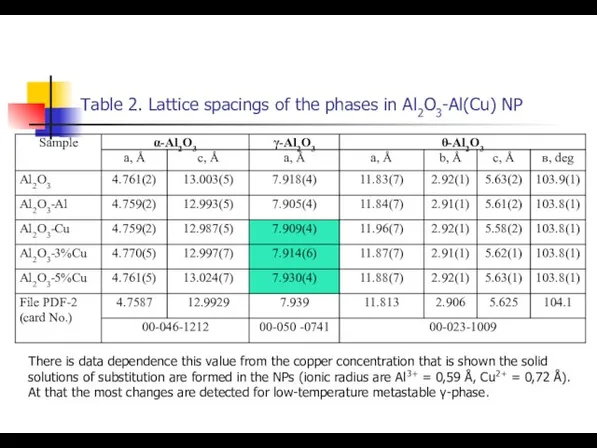
- 19. Table 2. Lattice spacings of the phases in Al2O3-Al(Cu) NP There is data dependence this value
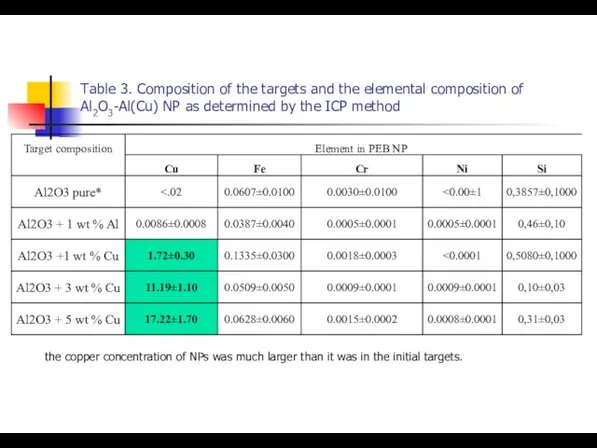
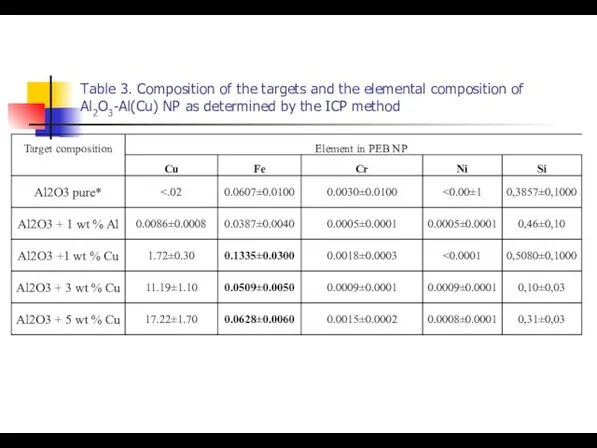
- 20. Table 3. Composition of the targets and the elemental composition of Al2O3-Al(Cu) NP as determined by
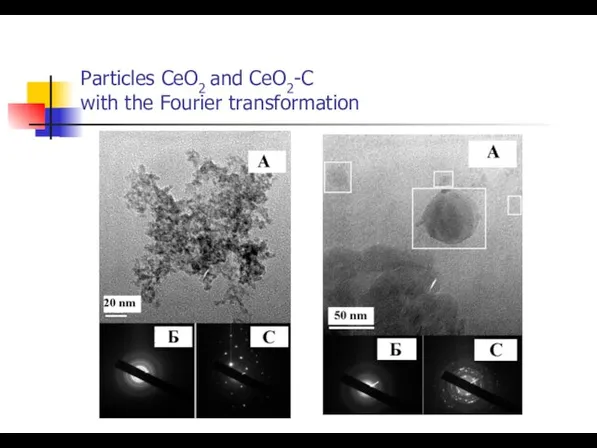
- 21. Particles CeO2 and CeO2-C with the Fourier transformation
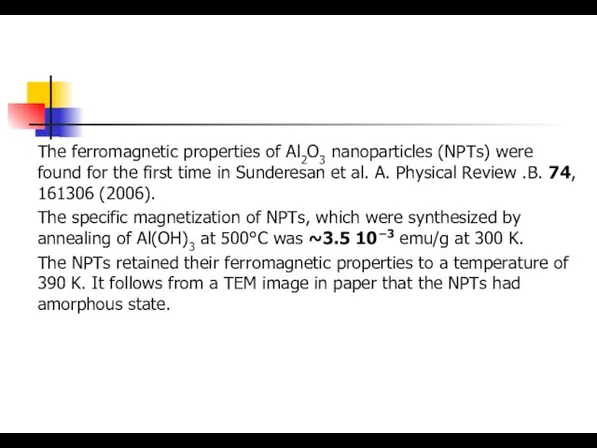
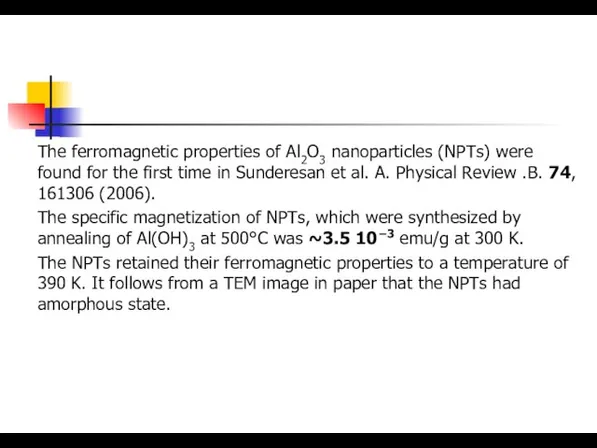
- 22. The ferromagnetic properties of Al2O3 nanoparticles (NPTs) were found for the first time in Sunderesan et
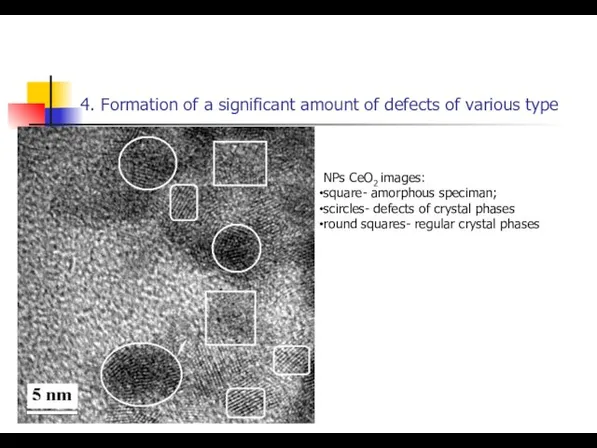
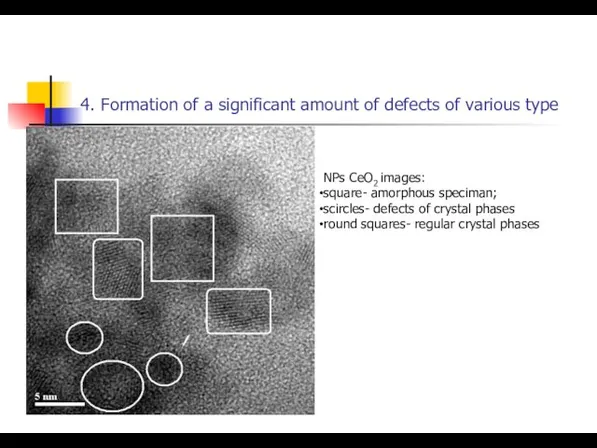
- 23. 4. Formation of a significant amount of defects of various type NPs СeO2 images: square- amorphous
- 24. 4. Formation of a significant amount of defects of various type NPs СeO2 images: square- amorphous
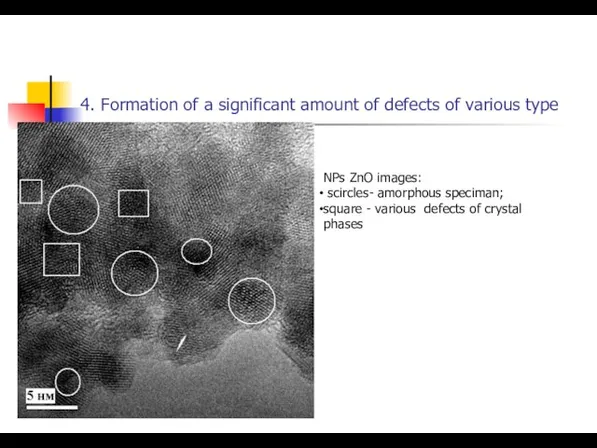
- 25. 4. Formation of a significant amount of defects of various type NPs ZnO images: scircles- amorphous
- 26. The ferromagnetic properties of Al2O3 nanoparticles (NPTs) were found for the first time in Sunderesan et
- 27. The ferromagnetic properties of Al2O3 nanoparticles (NPTs) were found for the first time in Sunderesan et
- 28. Al2O3 and Al2O3-Al NPs were extremely inhomogeneous in the magnetic respect as could be seen from
- 29. Generally, magnetization of the Al2O3-Cu powders was higher than that of Al2O3 NPs [Sunderesan et al.
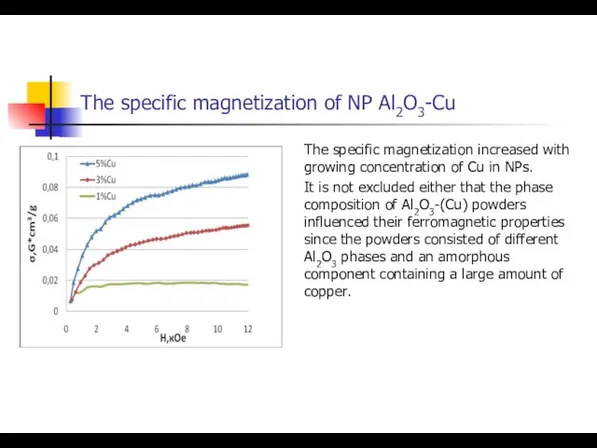
- 30. The specific magnetization of NP Al2O3-Cu The specific magnetization increased with growing concentration of Cu in
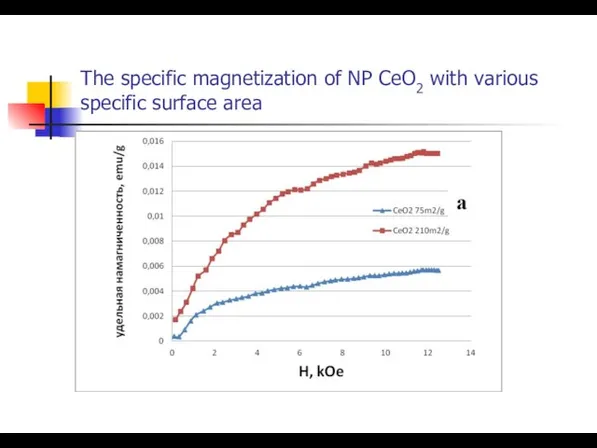
- 31. The specific magnetization of NP CeO2 with various specific surface area
- 32. The specific magnetization of NP Al2O3-Cu The specific magnetization increased with growing concentration of Cu in
- 33. Nonreproducibility of magnetization of pure Al2O3 and Al2O3-Al NPs containing comparable concentrations of iron impurities in
- 34. Table 3. Composition of the targets and the elemental composition of Al2O3-Al(Cu) NP as determined by
- 35. 5. Defective structure NP was reflected in their luminescent properties The spectra of Al2O3 and Al2O3-Al
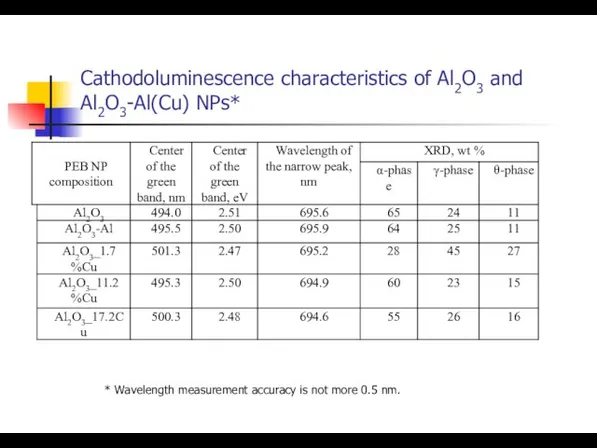
- 36. Cathodoluminescence characteristics of Al2O3 and Al2O3-Al(Cu) NPs* * Wavelength measurement accuracy is not more 0.5 nm.
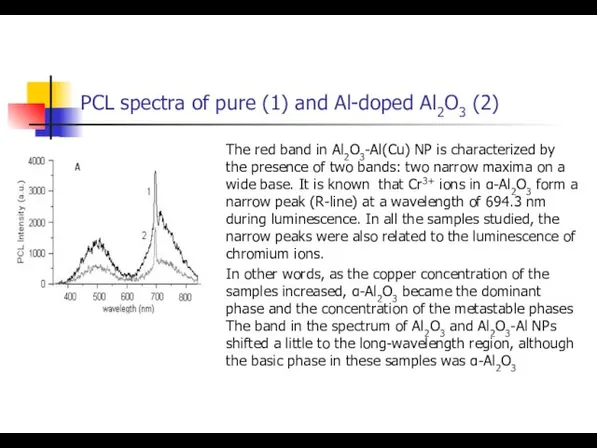
- 37. PCL spectra of pure (1) and Al-doped Al2O3 (2) The red band in Al2O3-Al(Cu) NP is
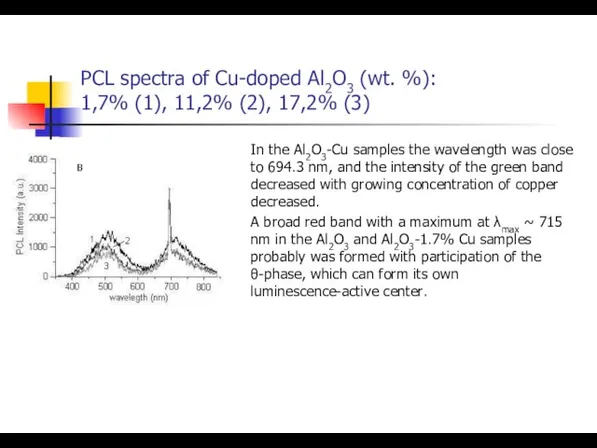
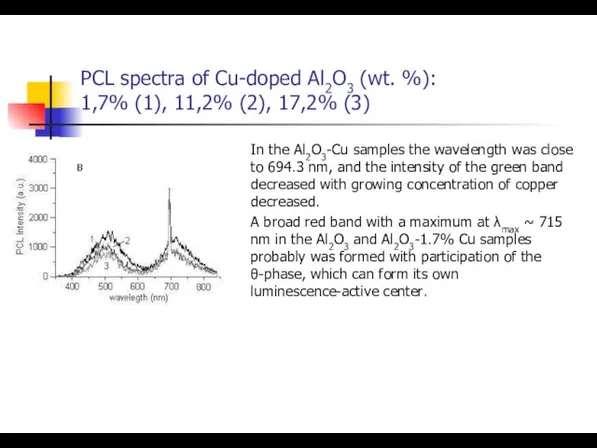
- 38. PCL spectra of Cu-doped Al2O3 (wt. %): 1,7% (1), 11,2% (2), 17,2% (3) In the Al2O3-Cu
- 39. PCL spectra of Cu-doped Al2O3 (wt. %): 1,7% (1), 11,2% (2), 17,2% (3) In the Al2O3-Cu
- 40. OSL and TL of Nanostructured Aluminum Oxide Thin Layers To define the absorbed doses of short
- 41. OSL and TL of Nanostructured Aluminum Oxide Thin Layers The TL glow curve shows an unusually
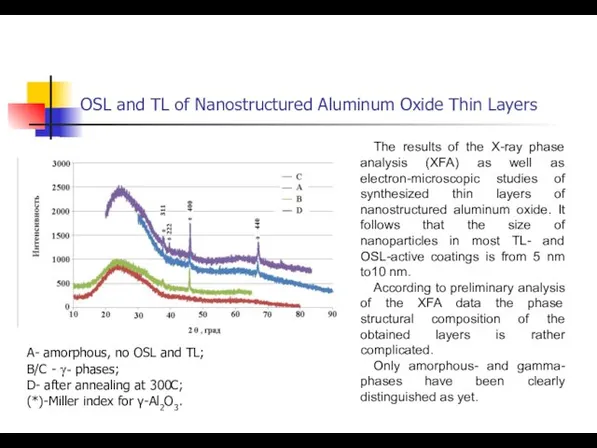
- 42. OSL and TL of Nanostructured Aluminum Oxide Thin Layers The results of the X-ray phase analysis
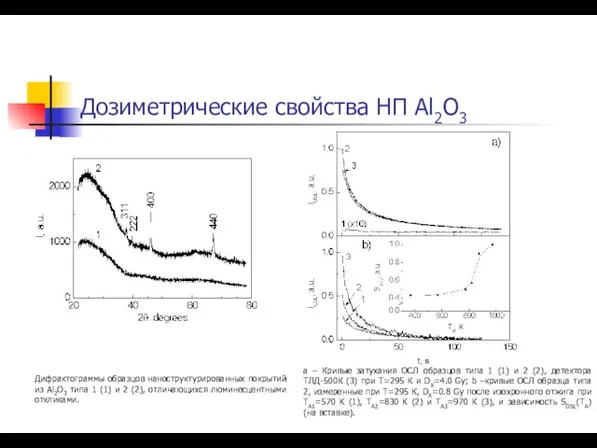
- 43. Дозиметрические свойства НП Al2O3 Дифрактограммы образцов наноструктурированных покрытий из Al2O3 типа 1 (1) и 2 (2),
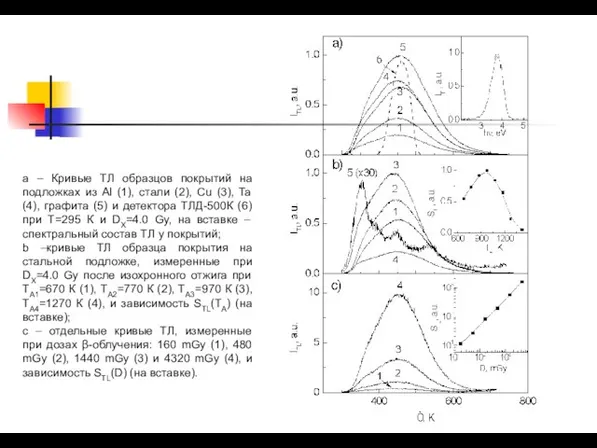
- 44. a – Кривые ТЛ образцов покрытий на подложках из Al (1), стали (2), Сu (3), Ta
- 45. Conclusions The conducted researches have shown that by means of PEB evaporation it is possible to
- 47. Скачать презентацию











 HIPAK Kinetic 2400 AF / 2600 AF
HIPAK Kinetic 2400 AF / 2600 AF Прыжок в длину с места
Прыжок в длину с места МКОУ «Приреченская основная общеобразовательная школа Верхнемамонского муниципального района Воронежской области»ЛАГЕРЬ С
МКОУ «Приреченская основная общеобразовательная школа Верхнемамонского муниципального района Воронежской области»ЛАГЕРЬ С  Презентация без названия
Презентация без названия Disaster
Disaster Экзаменационный стресс
Экзаменационный стресс 1
1 Святой праведный старец Феодор Томский
Святой праведный старец Феодор Томский Современное потребление
Современное потребление  Изменение экономической конъюнктуры и эволюция маркетинговых концепций на примере ВТБ24
Изменение экономической конъюнктуры и эволюция маркетинговых концепций на примере ВТБ24 Характер философского знания и задачи философии
Характер философского знания и задачи философии Агентство Соединенных Штатов по Международному Развитию Винрок Интернэшнл
Агентство Соединенных Штатов по Международному Развитию Винрок Интернэшнл Источники права в Грузии
Источники права в Грузии Презентация кабинета химии
Презентация кабинета химии 5 ПСХЭ символы
5 ПСХЭ символы Ослабление влияния помех на аудиоаппаратуру сетевыми фильтрами
Ослабление влияния помех на аудиоаппаратуру сетевыми фильтрами Презентация на тему Отряд Вши
Презентация на тему Отряд Вши  Круглый стол «Социальная активность молодежи в реальном и виртуальном социальном пространстве».
Круглый стол «Социальная активность молодежи в реальном и виртуальном социальном пространстве». Эффект лотоса
Эффект лотоса Гибридизация
Гибридизация Паукообразные 2 класс
Паукообразные 2 класс Нагрузочное тестирование Описание услуг
Нагрузочное тестирование Описание услуг Проектная деятельность
Проектная деятельность Презентация на тему Буллинг
Презентация на тему Буллинг Happy Holidays
Happy Holidays Нейропсихология продаж
Нейропсихология продаж Презентация на тему Как жили на Руси
Презентация на тему Как жили на Руси Вирусы и антивирусы
Вирусы и антивирусы