Содержание
- 2. Content Introduction and Scope of Biochemistry Objectives of Biochemistry Outcomes of the Course Amino acids: characteristics
- 3. Introduction and Scope of Biochemistry Biochemistry is the branch of life science which deals with the
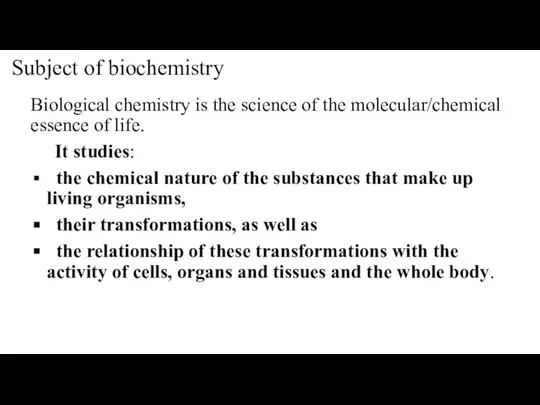
- 4. Subject of biochemistry Biological chemistry is the science of the molecular/chemical essence of life. It studies:
- 5. In other words, biochemistry studies the processes of development and functioning of organisms in the language
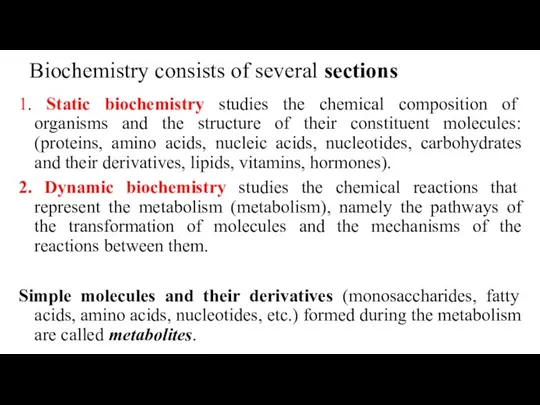
- 6. Biochemistry consists of several sections 1. Static biochemistry studies the chemical composition of organisms and the
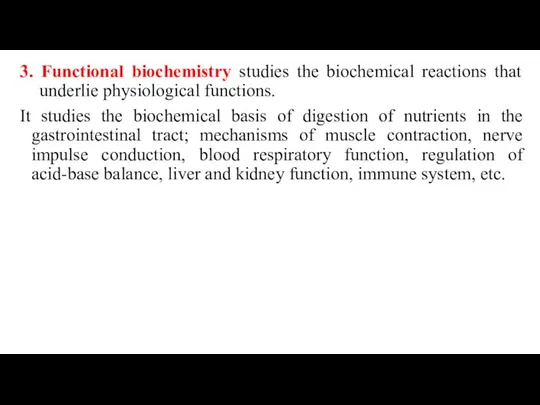
- 7. 3. Functional biochemistry studies the biochemical reactions that underlie physiological functions. It studies the biochemical basis
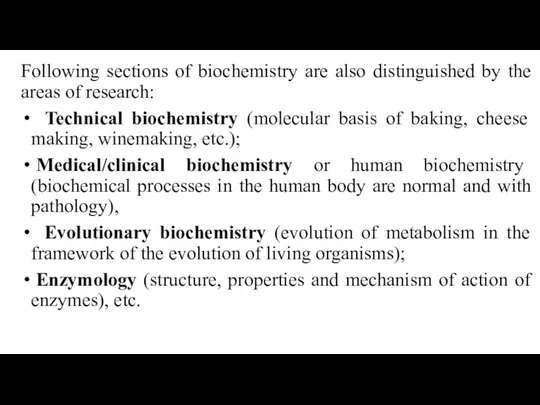
- 8. Following sections of biochemistry are also distinguished by the areas of research: Technical biochemistry (molecular basis
- 9. Outcomes of the “Biochemistry” Course As a result of studying the discipline, the student will be
- 10. Proteins Almost all processes in living organisms are associated with the functioning of proteins and nucleic
- 11. Proteins Are Built from a set of 20 Amino Acids Amino acids are the building blocks
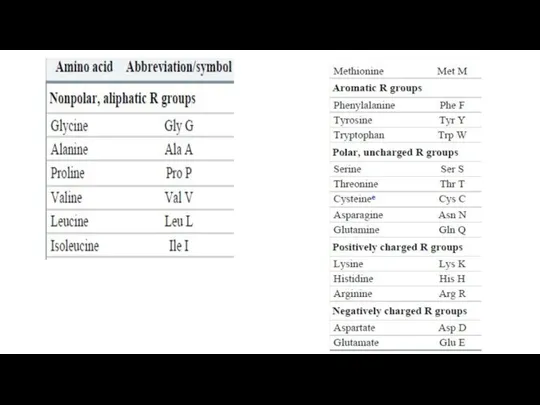
- 12. The common amino acids of proteins have been assigned three-letter abbreviations and one-letter symbols (Table 3-1),
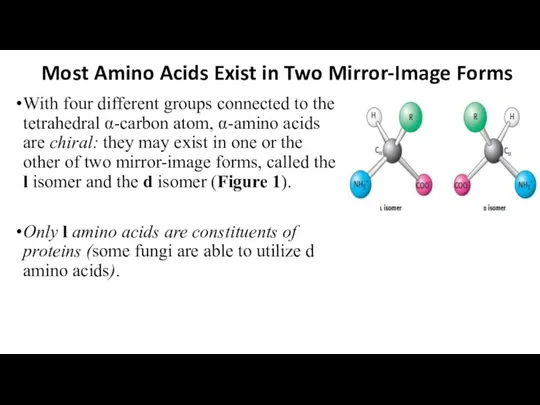
- 14. Most Amino Acids Exist in Two Mirror-Image Forms With four different groups connected to the tetrahedral
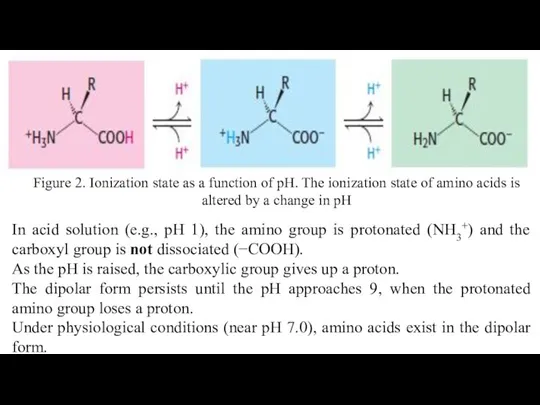
- 15. All Amino Acids Have at Least Two Charged Groups Free amino acids in solution at neutral
- 16. In acid solution (e.g., pH 1), the amino group is protonated (NH3+) and the carboxyl group
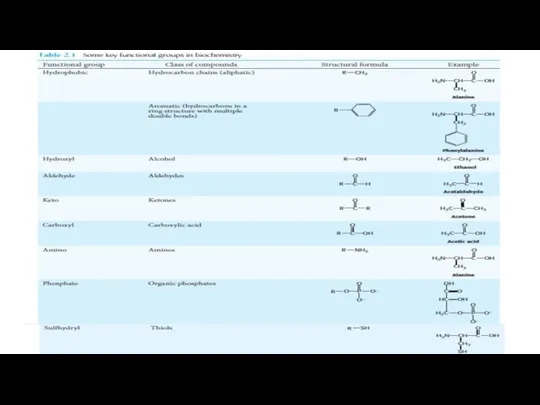
- 17. Amino Acids Contain a Wide Array of Functional Groups Twenty types of side chains varying in
- 19. Classification of amino acids Although there are many ways to classify amino acids, we will assort
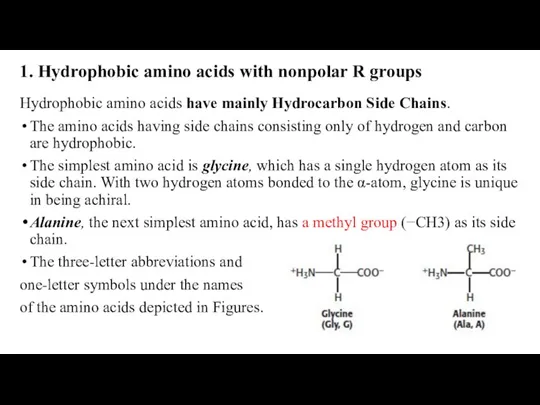
- 20. 1. Hydrophobic amino acids with nonpolar R groups Hydrophobic amino acids have mainly Hydrocarbon Side Chains.
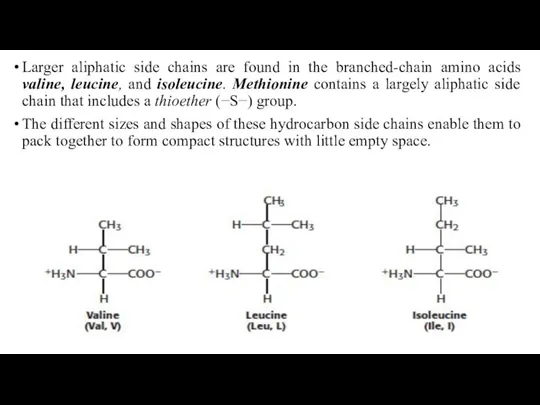
- 21. Larger aliphatic side chains are found in the branched-chain amino acids valine, leucine, and isoleucine. Methionine
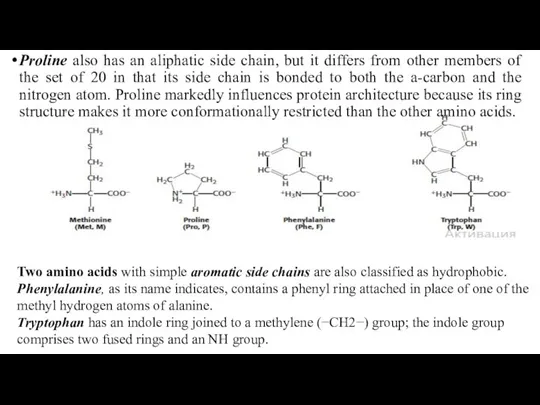
- 22. Proline also has an aliphatic side chain, but it differs from other members of the set
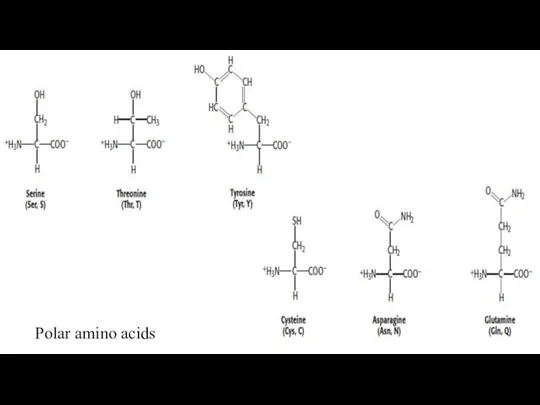
- 23. 2. Polar Amino acids The next group of amino acids that we will consider are those
- 24. Polar amino acids
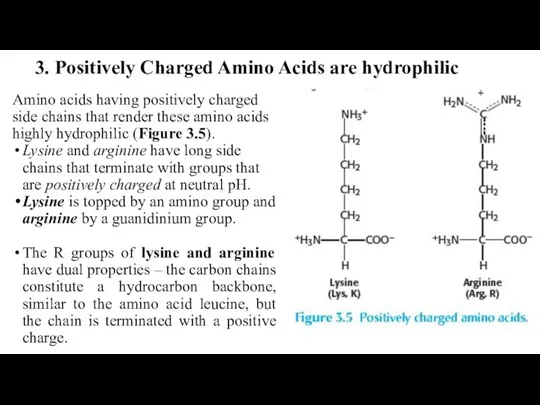
- 25. 3. Positively Charged Amino Acids are hydrophilic Amino acids having positively charged side chains that render
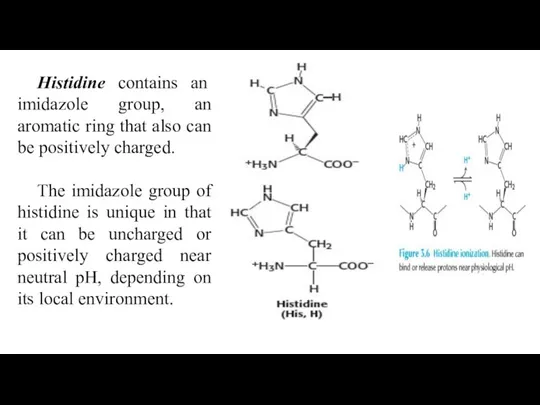
- 26. Histidine contains an imidazole group, an aromatic ring that also can be positively charged. The imidazole
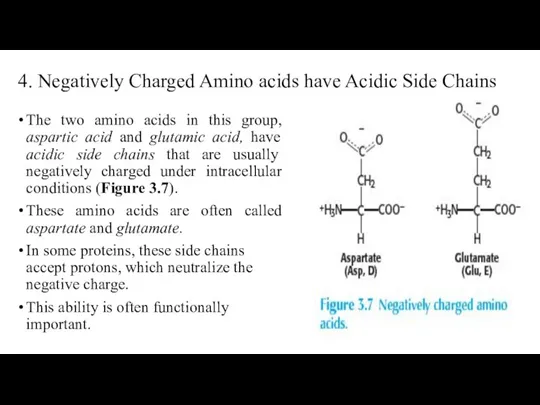
- 27. 4. Negatively Charged Amino acids have Acidic Side Chains The two amino acids in this group,
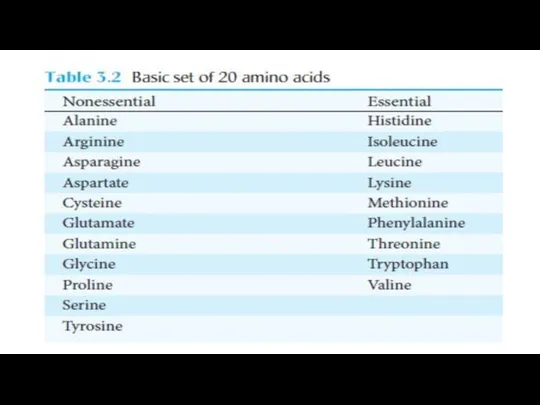
- 28. Essential Amino Acids must Be obtained from the Diet Most microorganisms can synthesize the entire basic
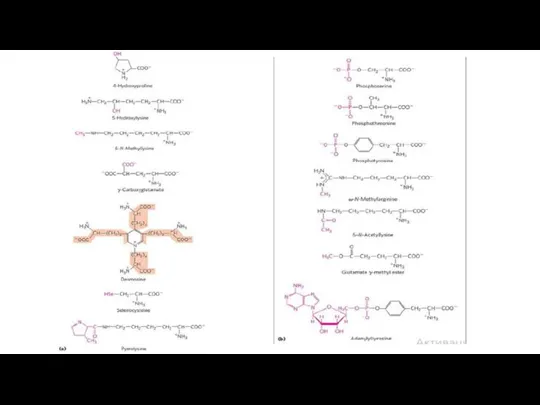
- 30. Uncommon Amino Acids also have important Functions In addition to the 20 common amino acids, proteins
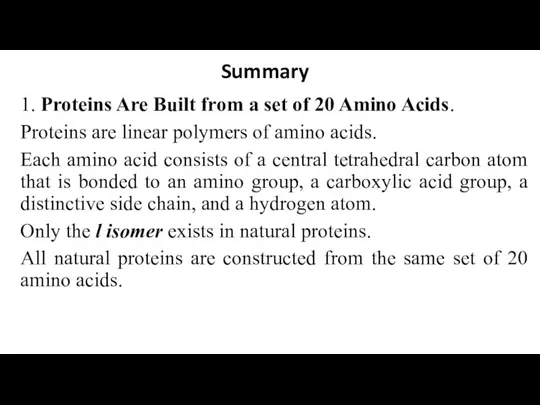
- 32. Summary 1. Proteins Are Built from a set of 20 Amino Acids. Proteins are linear polymers
- 33. 2. Amino Acids Contain a Wide Array of Functional Groups. The side chains of these 20
- 35. Скачать презентацию














 Закон Долло
Закон Долло Мышцы спины
Мышцы спины Fungi: recyclers, pathogens, parasites, and plant partners
Fungi: recyclers, pathogens, parasites, and plant partners Закономірності мінливості
Закономірності мінливості Твой организм. Основные части тела человека
Твой организм. Основные части тела человека Обитатели моря
Обитатели моря Чому людина не може жити без рослин
Чому людина не може жити без рослин Биохимия соединительной ткани и кости
Биохимия соединительной ткани и кости Возможная родословная человека. Антропогенез. Эволюция человека. Часть 1
Возможная родословная человека. Антропогенез. Эволюция человека. Часть 1 15. Тип Кольчатые черви. легкий (1)
15. Тип Кольчатые черви. легкий (1) Физические качества
Физические качества Степь да степь кругом
Степь да степь кругом Эволюция животного мира . Филогенез
Эволюция животного мира . Филогенез Большой Арктический государственній природный заповедник
Большой Арктический государственній природный заповедник Продукты выделения организмов
Продукты выделения организмов Значение насекомых
Значение насекомых Метаболизм белков
Метаболизм белков Хвостатые земноводные
Хвостатые земноводные Збудження і гальмування. Передача імпульсів у нервовій системі. Синапс
Збудження і гальмування. Передача імпульсів у нервовій системі. Синапс Модификационная изменчивость
Модификационная изменчивость Лук растим – быть здоровыми хотим
Лук растим – быть здоровыми хотим Папоротники, мхи и водоросли
Папоротники, мхи и водоросли § 47. Первые и современные люди 9 класс биология
§ 47. Первые и современные люди 9 класс биология Певчие птицы. Кроссворд
Певчие птицы. Кроссворд Тип хордовые
Тип хордовые Сравнение способов выращивания фиалок вегетативно
Сравнение способов выращивания фиалок вегетативно Презентация на тему Иод и его роль в живом организме
Презентация на тему Иод и его роль в живом организме  1666861337135100
1666861337135100