shots Given 3 weeks (21 days) apart
When Fully Vaccinated: 2 weeks after your second shot
Additional Dose: Recommended for moderately to severely immunocompromised people, given 4 weeks after second shot
Booster Dose: Recommended for some people who are at higher risk for COVID-19 exposure or severe illness , given 6 or more months after second shot
Efficiency: 95%
Common side effects: Chills, headache, pain, tiredness, and/or redness and swelling at the injection site, all of which generally resolve within a day or two of rest, hydration, and medications like acetaminophen. On rare occasions, the vaccine has appeared to trigger anaphylaxis.
As far as the Delta variant, two studies reported by Public Health England that have not yet been peer reviewed showed that full vaccination after two doses is 88% effective against symptomatic disease and 96% effective against hospitalization.

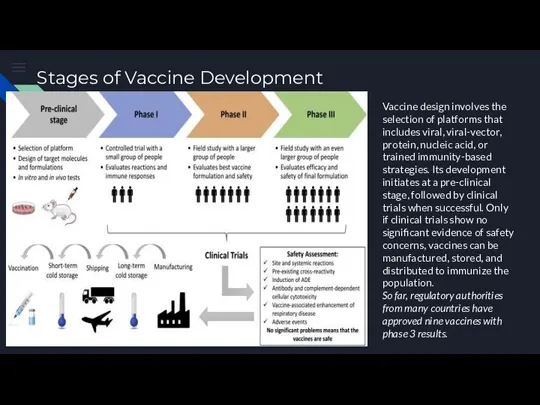









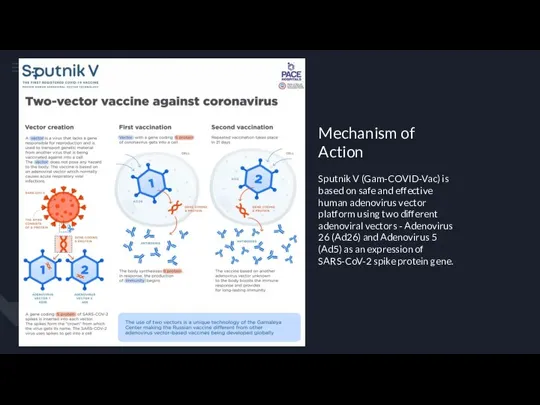
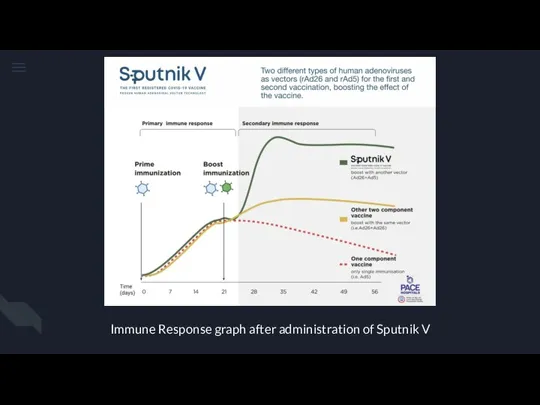
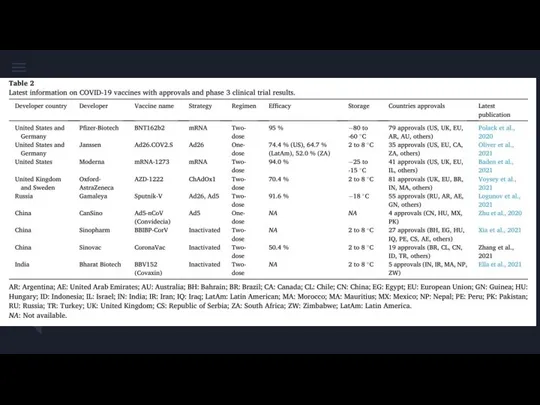

 Робототизация сестринского дела
Робототизация сестринского дела Covid-19 что после …
Covid-19 что после … Дифференциальная диагностика бронхолегочной патологии
Дифференциальная диагностика бронхолегочной патологии Краткое пособие по ЭКГ
Краткое пособие по ЭКГ Дерматоздардағы көздің зақымдалуы
Дерматоздардағы көздің зақымдалуы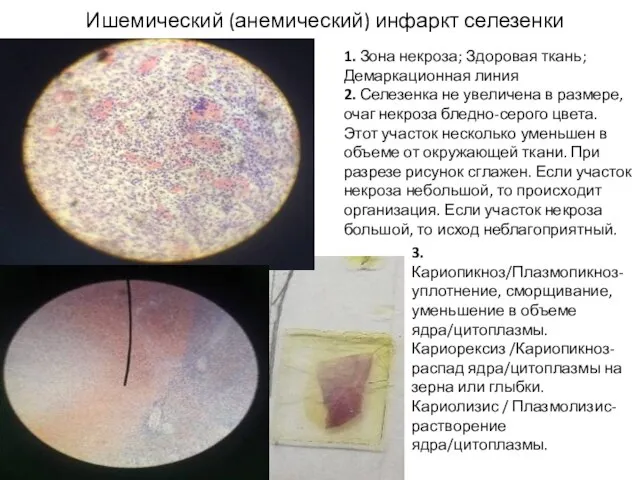 Ишемический (анемический) инфаркт селезенки
Ишемический (анемический) инфаркт селезенки Лечебная физкультура при лечении дефектов осанки
Лечебная физкультура при лечении дефектов осанки Средства индивидуальной защиты кожи
Средства индивидуальной защиты кожи Диабет. Еда, которая лечит болезнь
Диабет. Еда, которая лечит болезнь Аборт. Прерывание беременности
Аборт. Прерывание беременности Спортивная медицина
Спортивная медицина Базовые модели профилактики аддиктивных расстройств
Базовые модели профилактики аддиктивных расстройств Жировая эмболия
Жировая эмболия ПОДАГРА
ПОДАГРА Ампутации и экзартикуляции конечностей
Ампутации и экзартикуляции конечностей Covid-19 для интенсивиста. Эпидемиологическая безопасность при ИВЛ
Covid-19 для интенсивиста. Эпидемиологическая безопасность при ИВЛ Бычий цепень
Бычий цепень Правильное питание - залог здоровья
Правильное питание - залог здоровья Лучевая диагностика опорнодвигательного аппарата
Лучевая диагностика опорнодвигательного аппарата Апробация в школах г. Надыма программы ЛадьЯ для профилактики аддикций и девиантного поведения среди учащихся 7-8 классов
Апробация в школах г. Надыма программы ЛадьЯ для профилактики аддикций и девиантного поведения среди учащихся 7-8 классов Беременность и острый аппендицит
Беременность и острый аппендицит Бронхолегочная дисплазия новорожденных
Бронхолегочная дисплазия новорожденных Сестринский уход. Документация к сестринскому уходу
Сестринский уход. Документация к сестринскому уходу Пищевые токсикоинфекции
Пищевые токсикоинфекции Эпителиалды құйымшақты жол
Эпителиалды құйымшақты жол Вызовы и угрозы санитарно-эпидемиологическому здоровью населения
Вызовы и угрозы санитарно-эпидемиологическому здоровью населения Генная инженерия
Генная инженерия Врожденная ветряная оспа
Врожденная ветряная оспа