Содержание
- 2. Organic Chemistry – the chemistry of the hydrocarbons and their derivatives; the chemistry of carbon compounds.
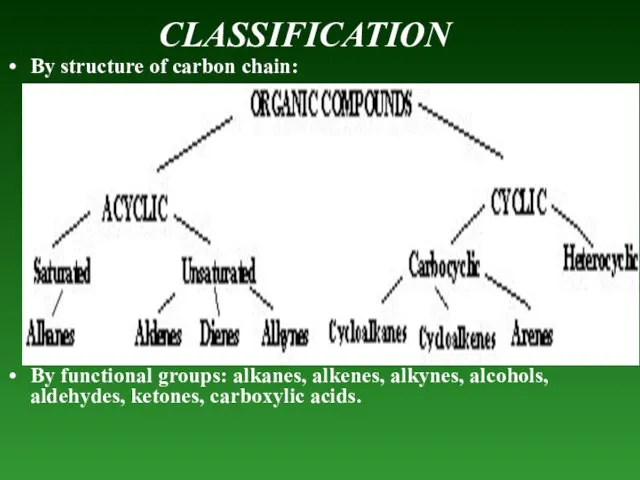
- 3. CLASSIFICATION By structure of carbon chain: By functional groups: alkanes, alkenes, alkynes, alcohols, aldehydes, ketones, carboxylic
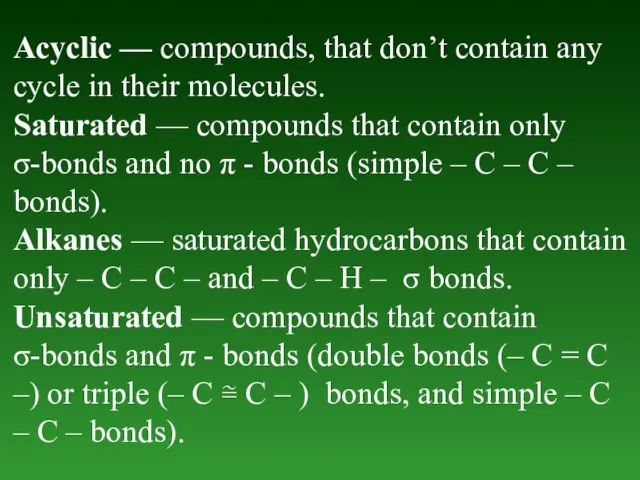
- 4. Acyclic — compounds, that don’t contain any cycle in their molecules. Saturated — compounds that contain
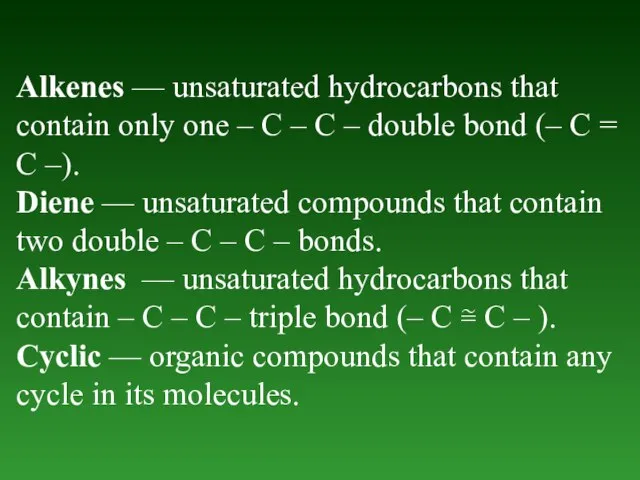
- 5. Alkenes — unsaturated hydrocarbons thаt contain only one – C – C – double bond (–
- 6. Carbocyclic - hydrocarbons containing а cycle that consists of only Carbon atoms. Cycloalkanes — saturated hydrocarbons
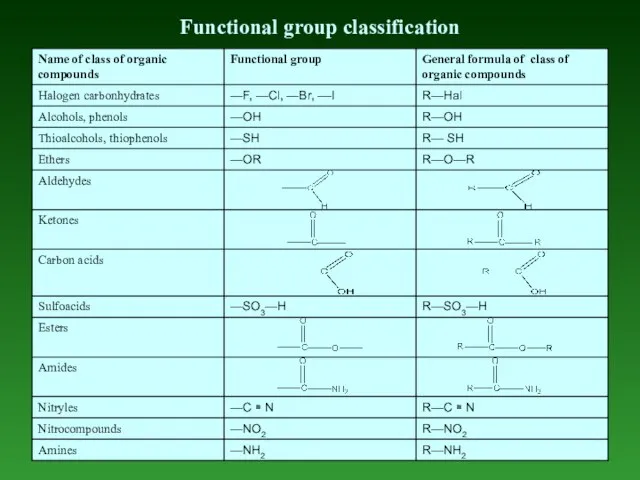
- 7. Functional group classification
- 8. Functional Group is any part of an organic compound, which is not а carbon-hydrogen or carbon-carbon
- 9. All organic compounds concerning to the same class form homological row – it is the row
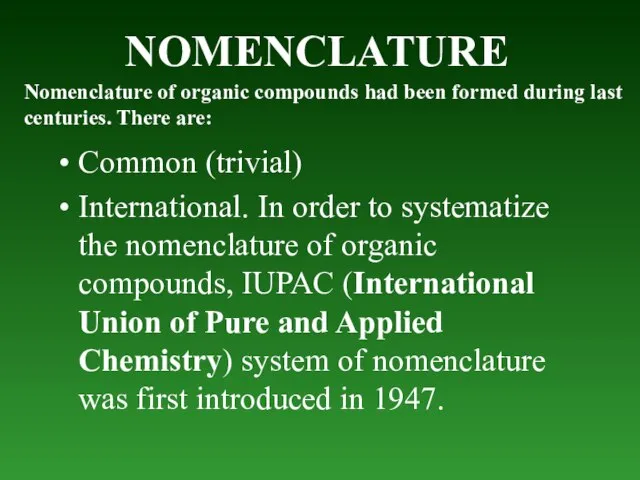
- 10. NOMENCLATURE Common (trivial) International. In order to systematize the nomenclature of organic compounds, IUPAC (International Union
- 11. Trivial nomenclature. At first organic compounds were named by chance, for example, because the natural sources
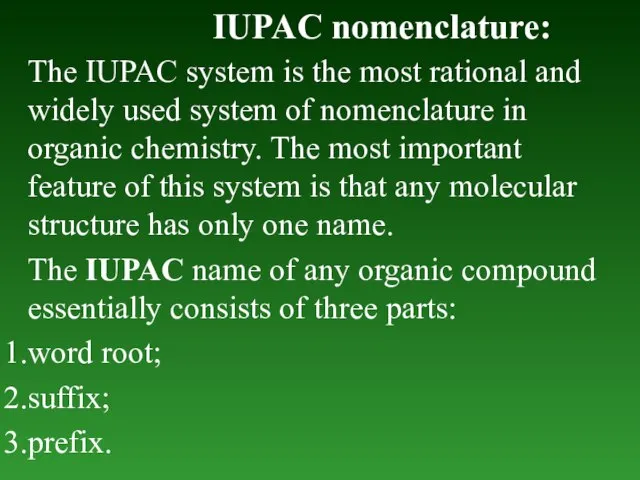
- 12. IUPAC nomenclature: The IUPAC system is the most rational and widely used system of nomenclature in
- 13. 1. Word root. It is the basic unit of the name. It denotes the number of
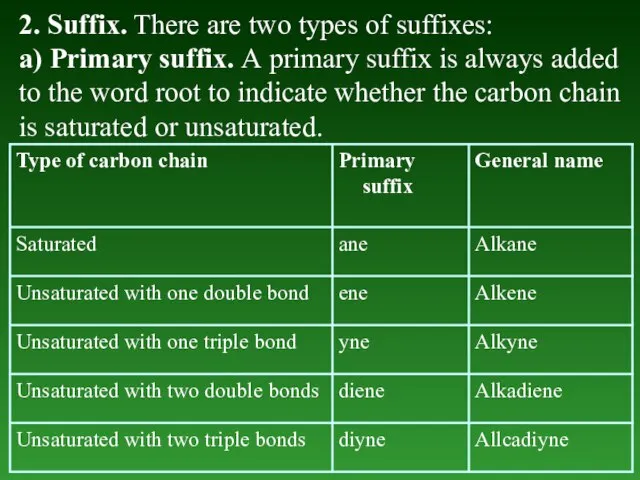
- 14. 2. Suffix. There are two types of suffixes: a) Primary suffix. А primary suffix is always
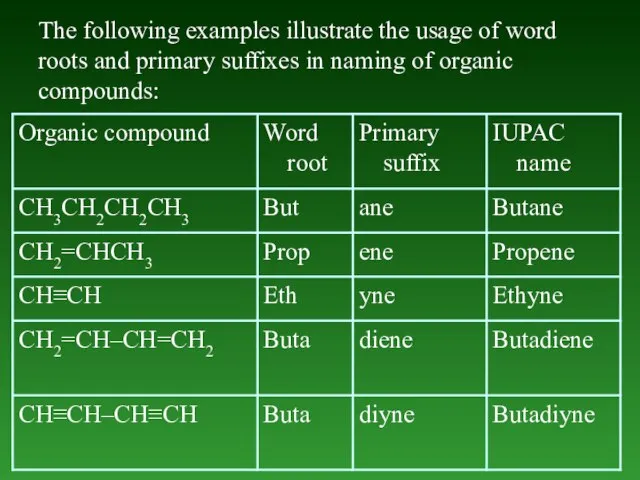
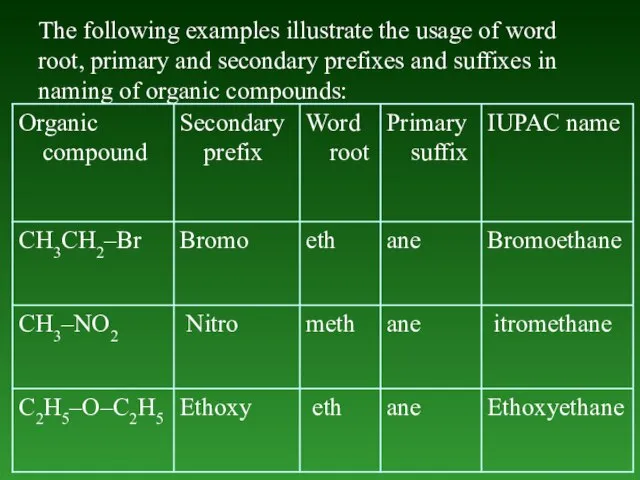
- 15. The following examples illustrate the usage of word roots and primary suffixes in naming of organic
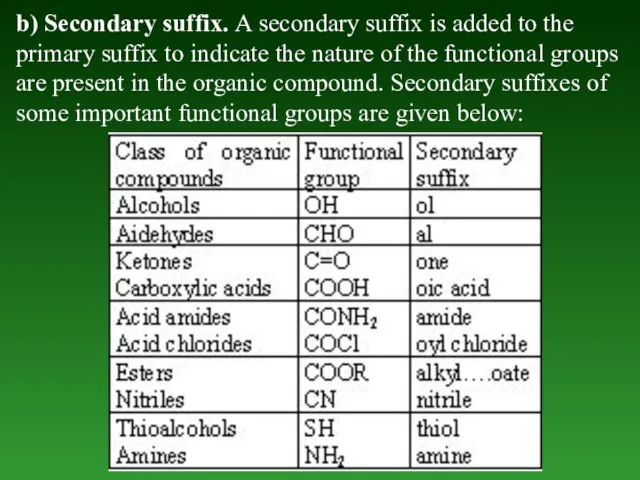
- 16. b) Secondary suffix. А secondary suffix is added to the primary suffix to indicate the nature
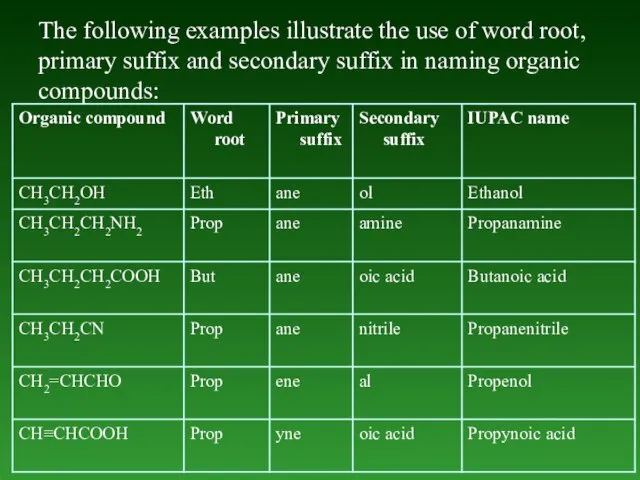
- 17. The following examples illustrate the use of word root, primary suffix and secondary suffix in naming
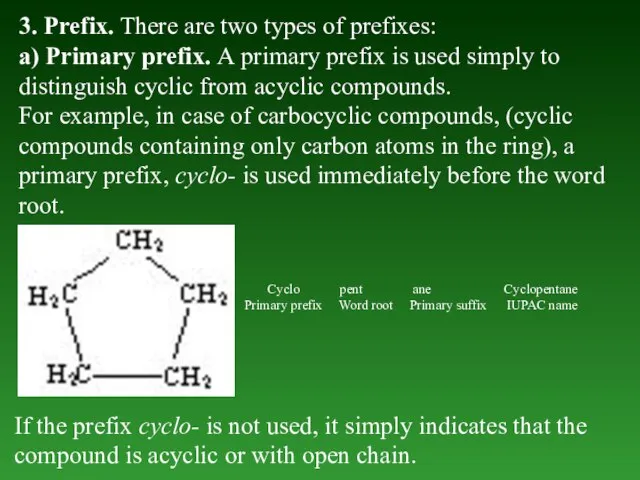
- 18. 3. Prefix. There are two types of prefixes: a) Primary prefix. А primary prefix is used
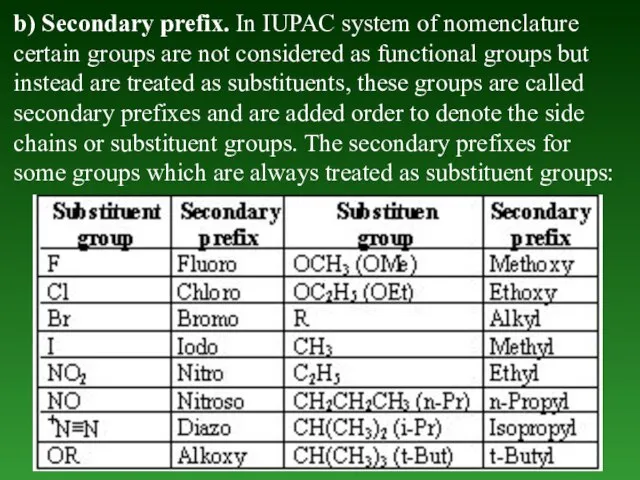
- 19. b) Secondary prefix. In IUPAC system of nomenclature certain groups are not considered as functional groups
- 20. Reactivity of functional group Sulphonic acids > carboxylic acids > anhydrides > esters > acid chlorides
- 21. The following examples illustrate the usage of word root, primary and secondary prefixes and suffixes in
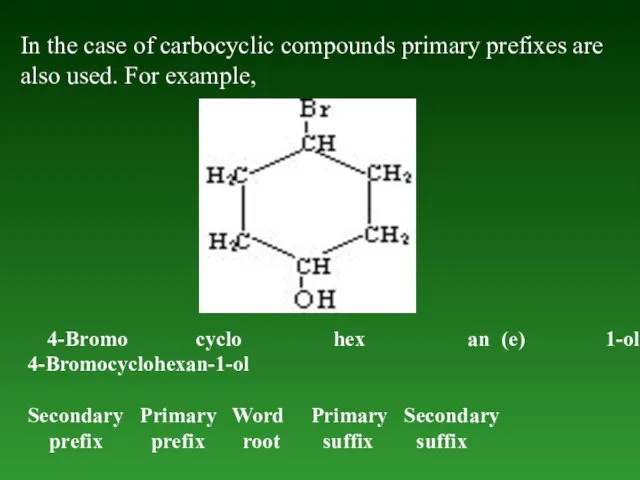
- 22. In the case of carbocyclic compounds primary prefixes are also used. For example, 4-Bromo cyclo hex
- 23. Complete IUPAC name of organic compound consists of the following parts: SECONDARY PREFIX + PRIMARY PREFIX
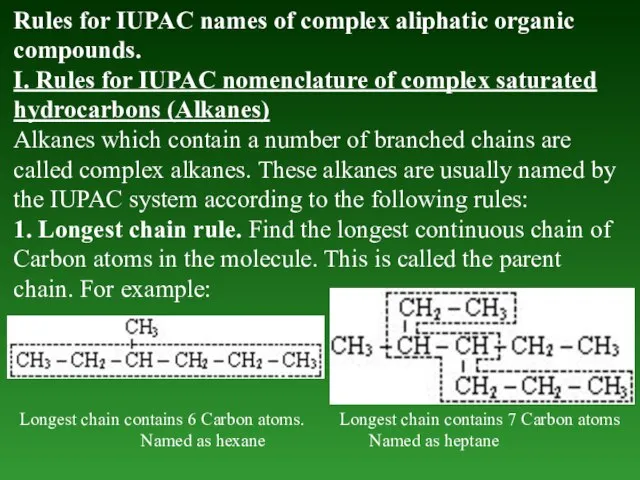
- 24. Rules for IUPAC names of complex aliphatic organic compounds. I. Rules for IUPAC nomenclature of complex
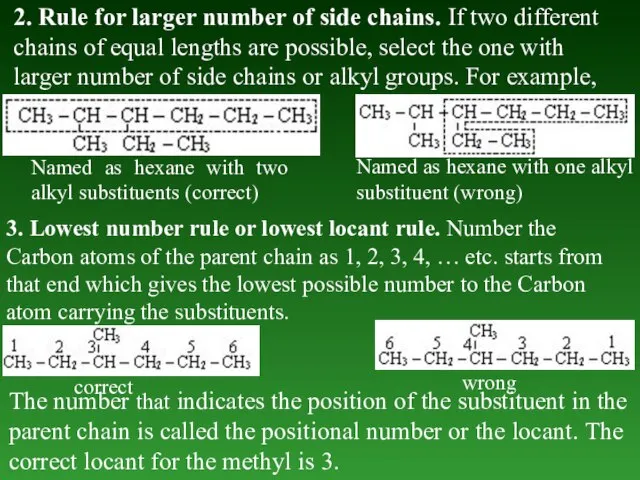
- 25. 2. Rule fоr larger number of side chains. If two different chains of equal lengths are
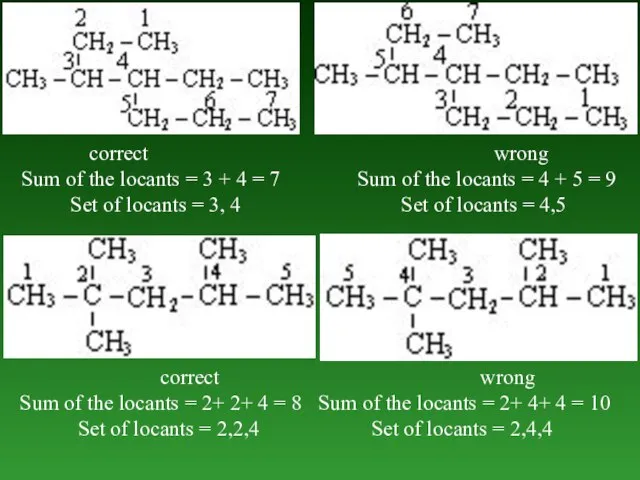
- 26. 4. Lowest sum rule and lowest set of locants rule. When two or more substituents are
- 27. correct wrong Sum of the locants = 3 + 4 = 7 Sum of the locants
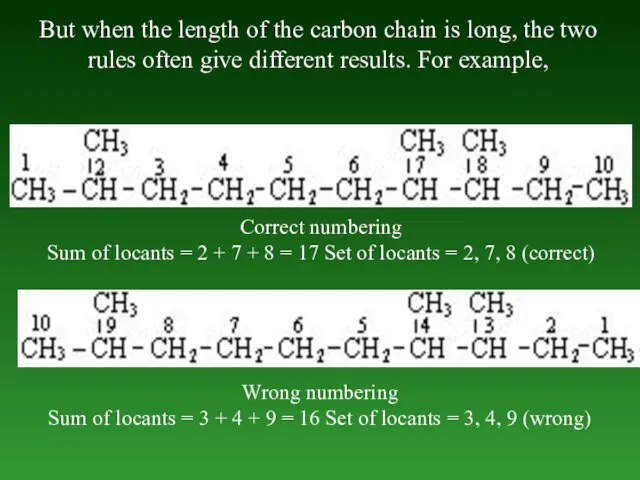
- 28. But when the length of the carbon chain is long, the two rules often give different
- 29. 5. Name of the complex alkane. We use prefix to indicate the position of substituent оn
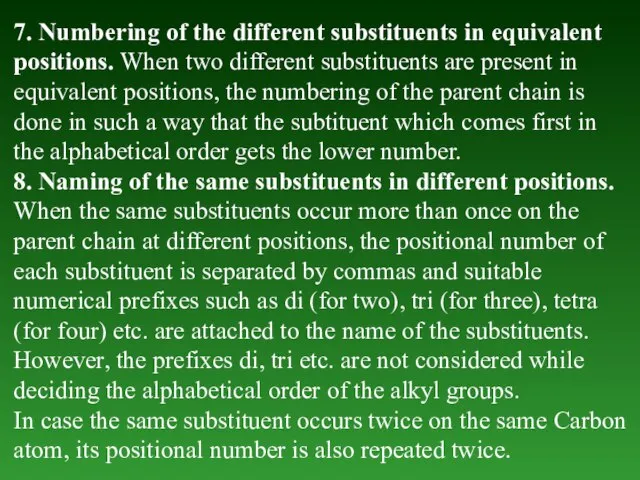
- 30. 7. Numbering of the different substituents in equivalent positions. When two different substituents are present in
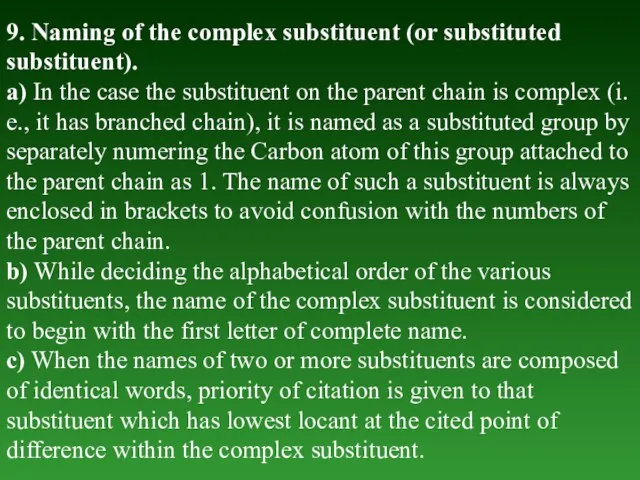
- 31. 9. Naming of the complex substituent (or substituted substituent). а) In the case the substituent on
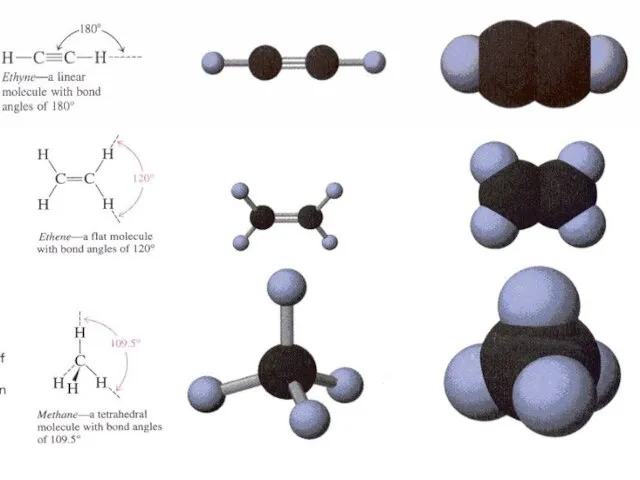
- 32. II. Rules for IUPAC nomenclature of unsaturated hydrocarbons (Alkenes and Alkynes). When naming compounds containing multiple
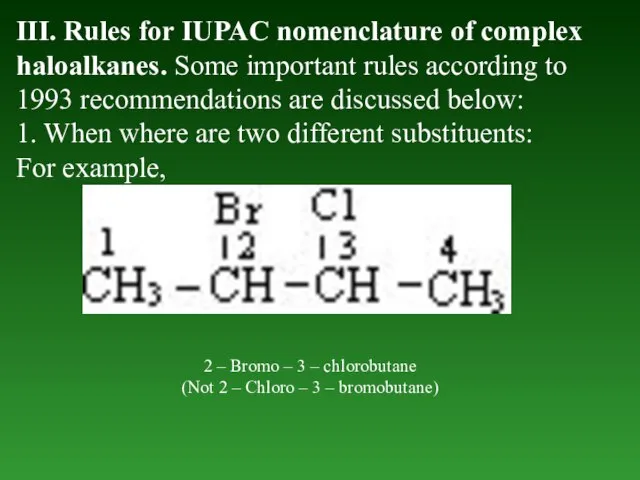
- 33. III. Rules for IUPAC nomenclature of complex haloalkanes. Some important rules according to 1993 recommendations are
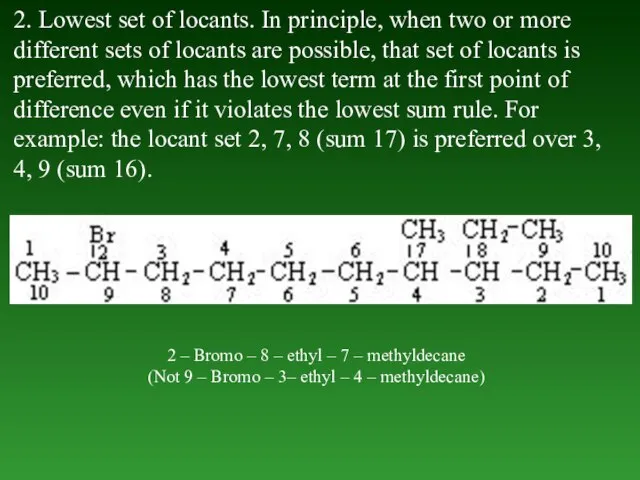
- 34. 2. Lowest set of locants. In principle, when two or more different sets of locants are
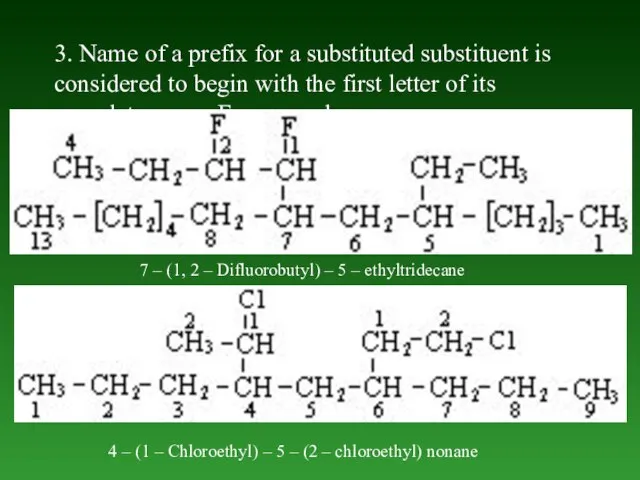
- 35. 3. Name of а prefix for a substituted substituent is considered to begin with the first
- 36. IV. Rules for IUPAC nomenclature of compounds containing one functional group, multiple bonds and substituents. While
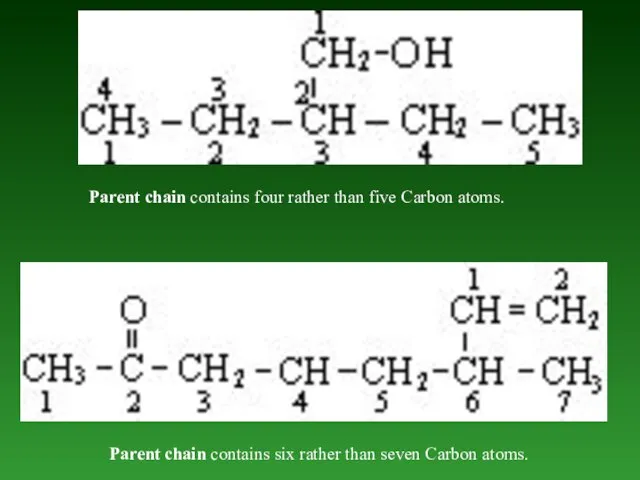
- 37. Parent chain contains four rather than five Carbon atoms. Parent chain contains six rather than seven
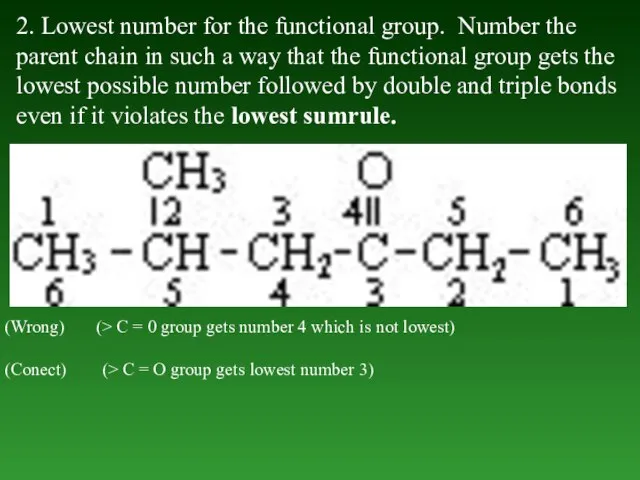
- 38. 2. Lowest number for the functional group. Number the parent chain in such а way that
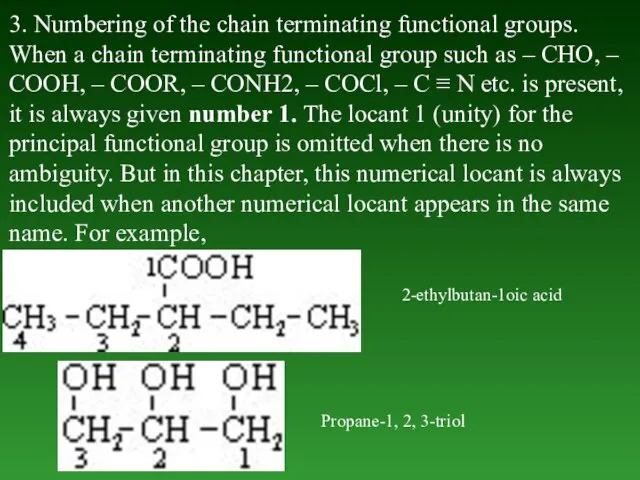
- 39. 3. Numbering of the chain terminating functional groups. When а chain terminating functional group such as
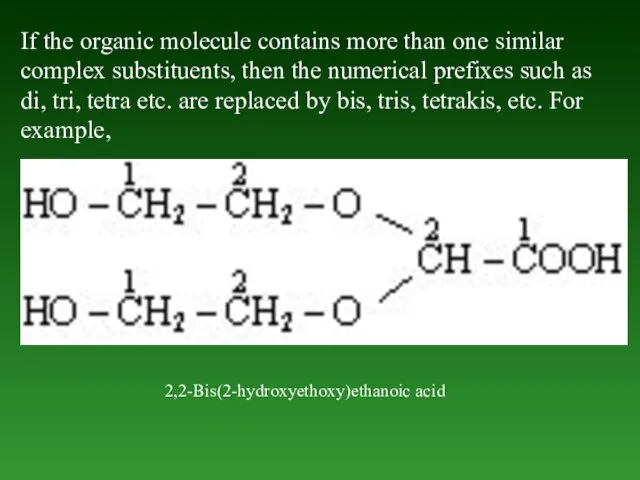
- 40. If the organic molecule contains more than one similar complex substituents, then the numerical prefixes such
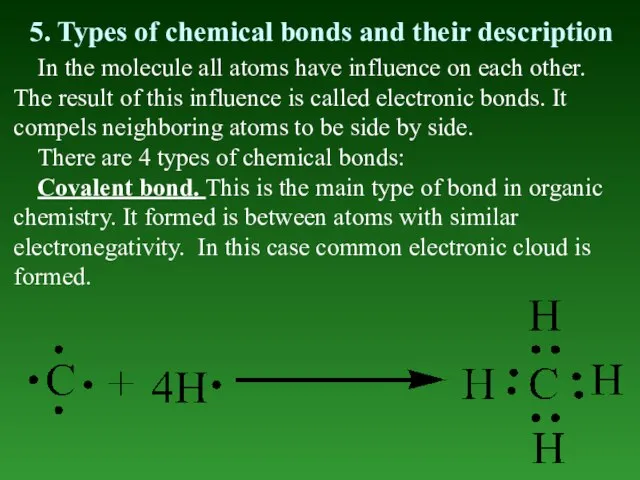
- 41. 5. Types of chemical bonds and their description In the molecule all atoms have influence on
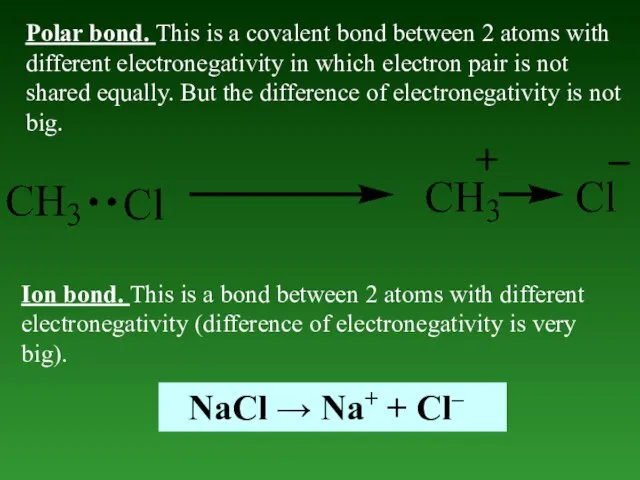
- 42. Polar bond. This is a covalent bond between 2 atoms with different electronegativity in which electron
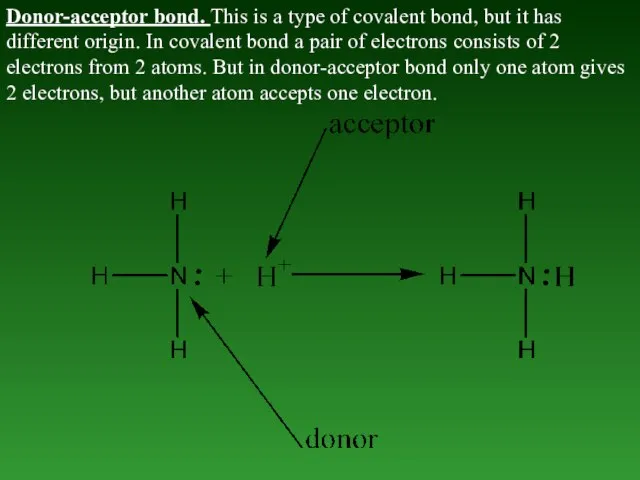
- 43. Donor-acceptor bond. This is a type of covalent bond, but it has different origin. In covalent
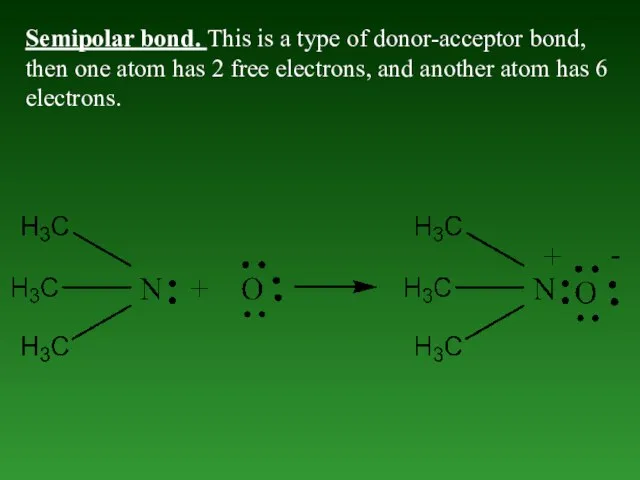
- 44. Semipolar bond. This is a type of donor-acceptor bond, then one atom has 2 free electrons,
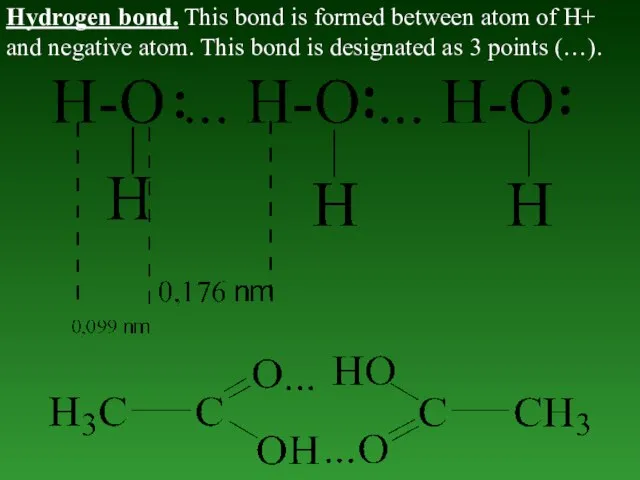
- 45. Hydrogen bond. This bond is formed between atom of H+ and negative atom. This bond is
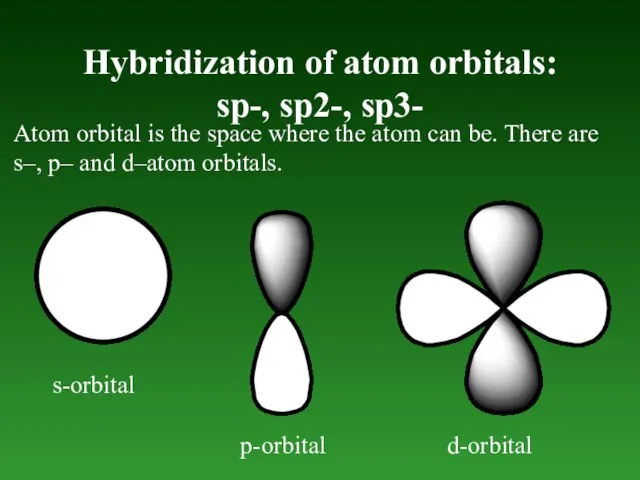
- 46. Hybridization of atom orbitals: sp-, sp2-, sp3- Atom orbital is the space where the atom can
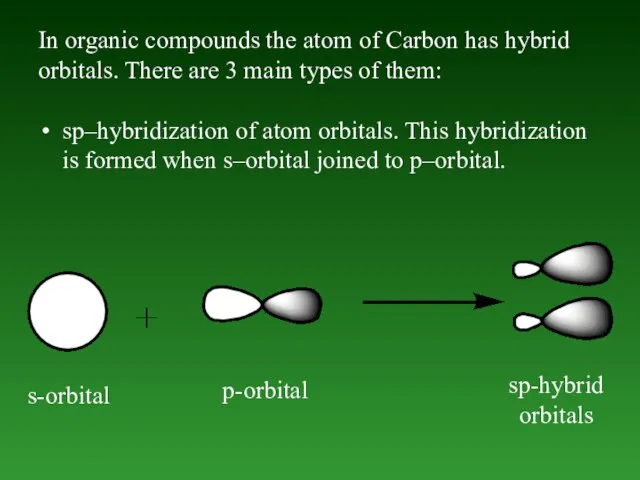
- 47. In organic compounds the atom of Carbon has hybrid orbitals. There are 3 main types of
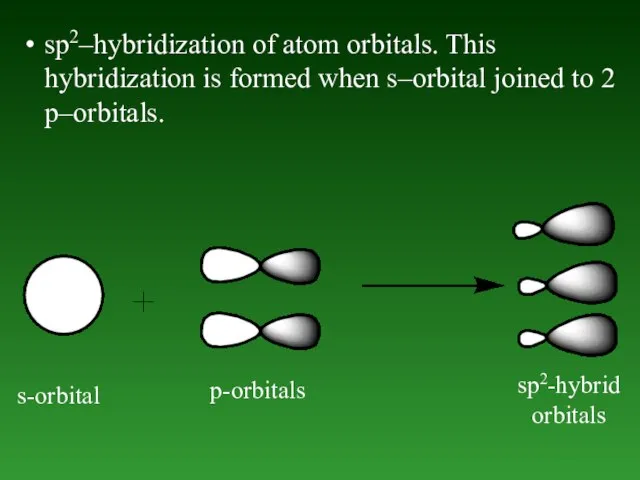
- 48. sp2–hybridization of atom orbitals. This hybridization is formed when s–orbital joined to 2 p–orbitals. s-orbital p-orbitals
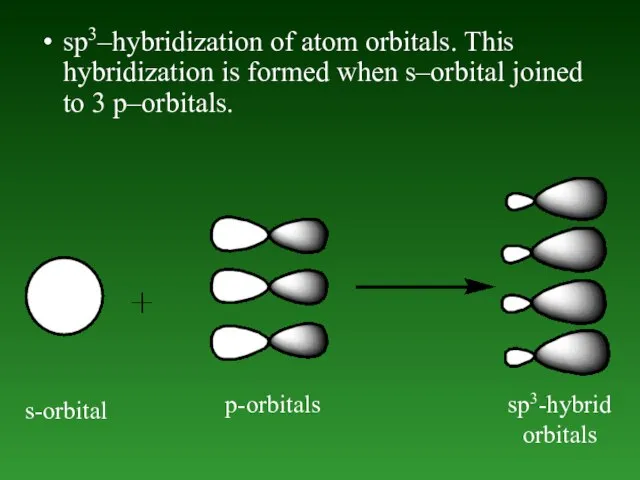
- 49. sp3–hybridization of atom orbitals. This hybridization is formed when s–orbital joined to 3 p–orbitals. s-orbital p-orbitals
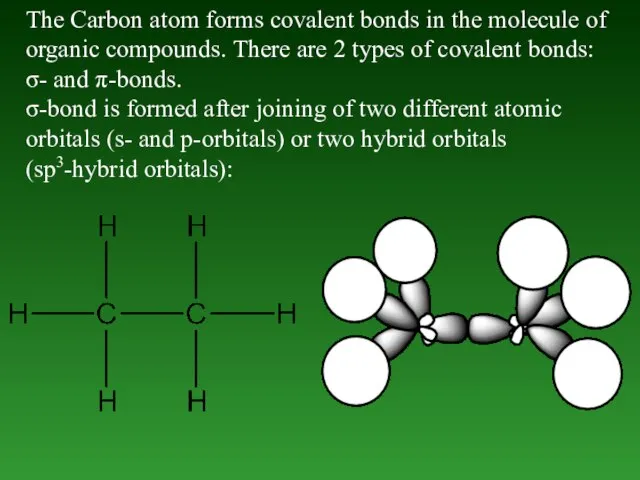
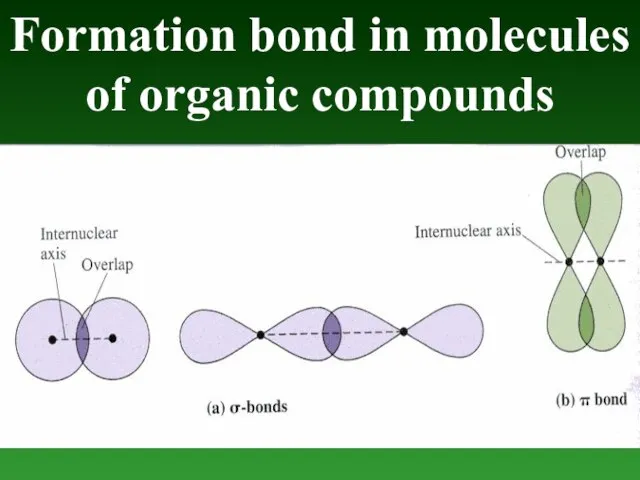
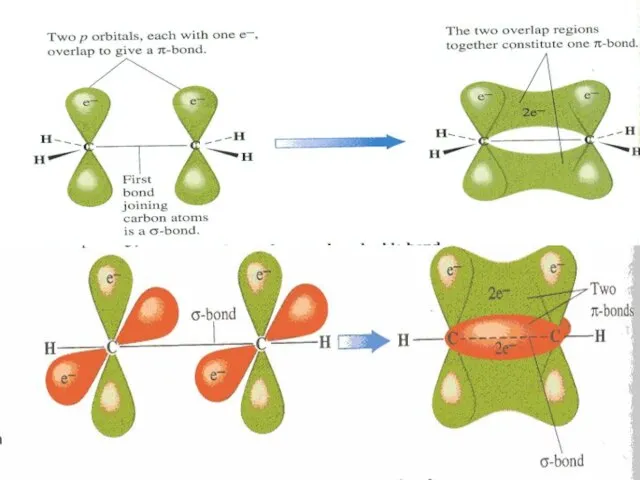
- 50. The Carbon atom forms covalent bonds in the molecule of organic compounds. There are 2 types
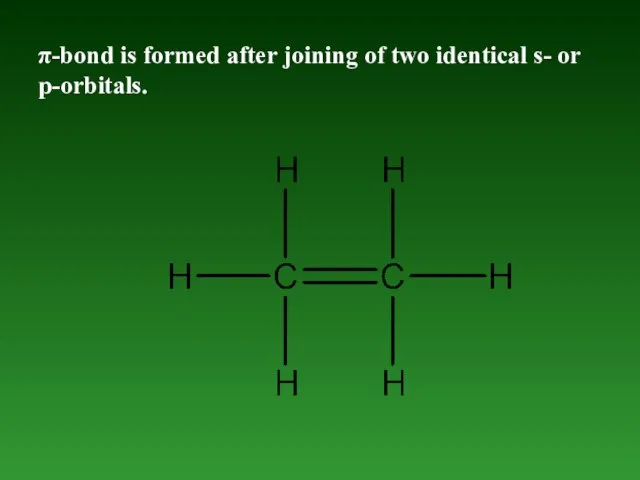
- 51. π-bond is formed after joining of two identical s- or p-orbitals.
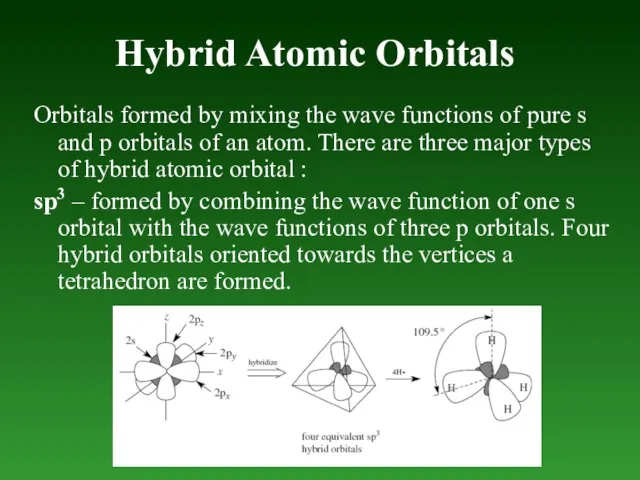
- 52. Hybrid Atomic Orbitals Orbitals formed by mixing the wave functions of pure s and p orbitals
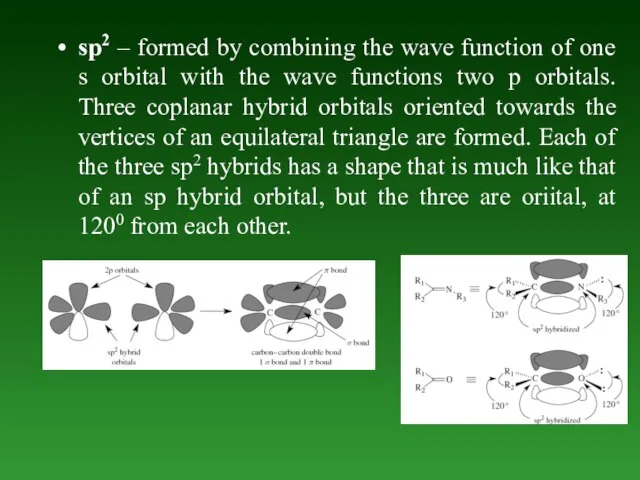
- 53. sp2 – formed by combining the wave function of one s orbital with the wave functions
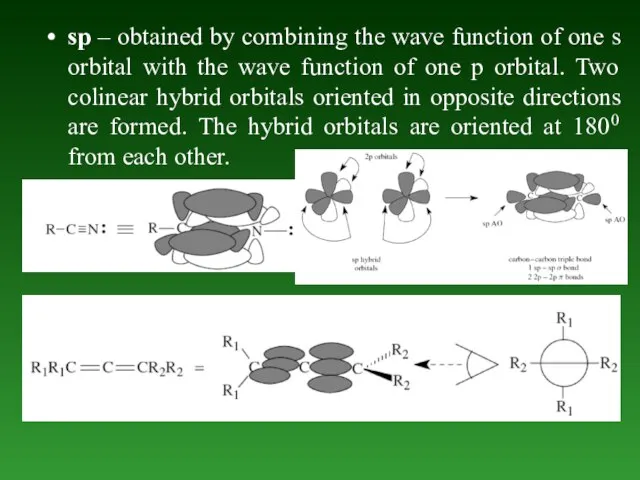
- 54. sp – obtained by combining the wave function of one s orbital with the wave function
- 56. Formation bond in molecules of organic compounds
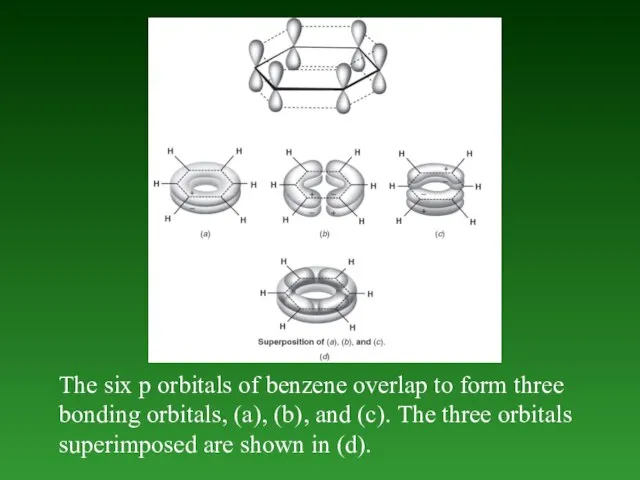
- 58. The six p orbitals of benzene overlap to form three bonding orbitals, (a), (b), and (c).
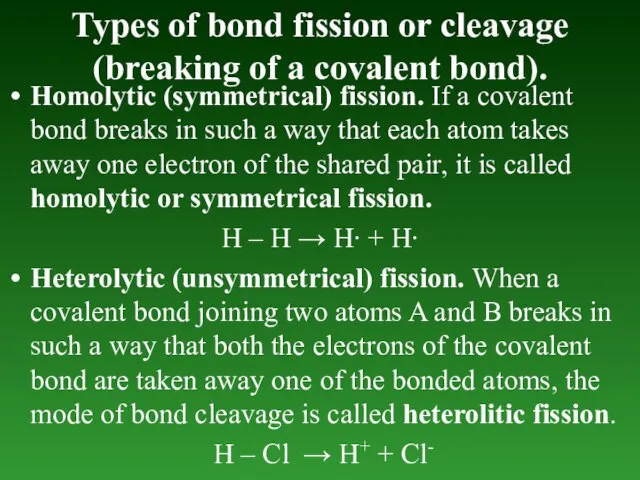
- 59. Types of bond fission or cleavage (breaking of а covalent bond). Homolytic (symmetrical) fission. If а
- 60. Electrophilic are electron loving chemical species. Their attraction for electrons is due to the presence of
- 61. Nucleophiles are nucleus loving chemical species. Since the nucleus of any atom is positively charged, therefore,
- 62. Types of organic reactions. All the organic reactions can be broadly classified into the following four
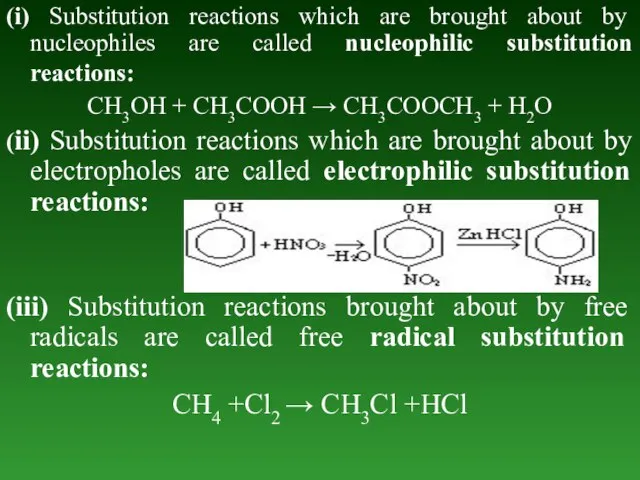
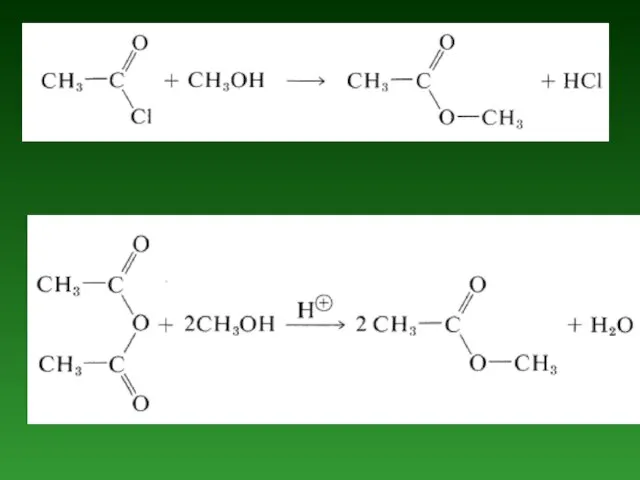
- 63. (i) Substitution reactions which are brought about by nucleophiles are called nucleophilic substitution reactions: CH3OH +
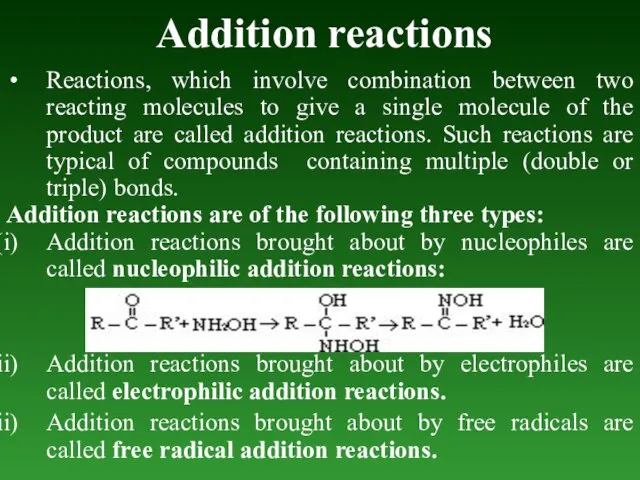
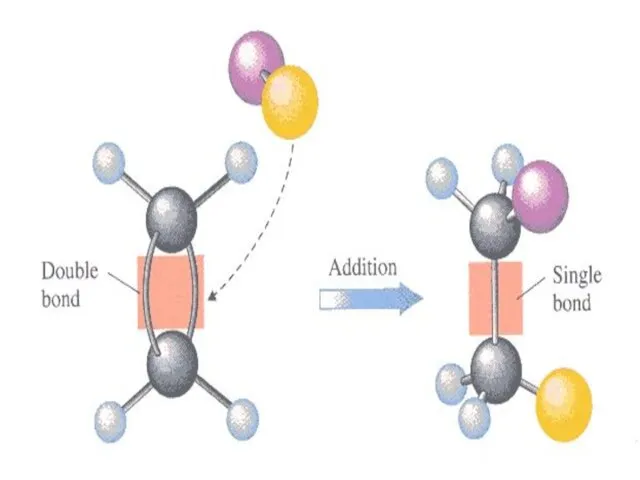
- 64. Addition reactions Reactions, which involve combination between two reacting molecules to give a single molecule of
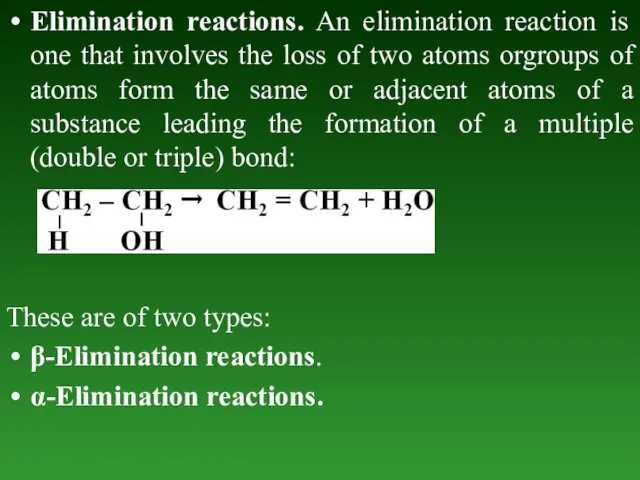
- 66. Elimination reactions. An elimination reaction is one that involves the loss of two atoms orgroups of
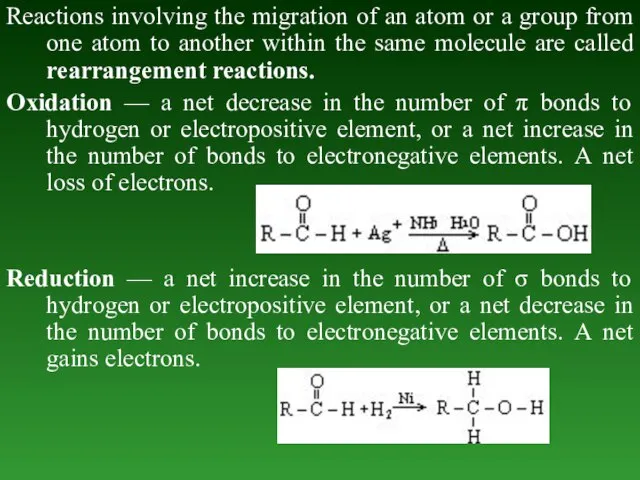
- 67. Reactions involving the migration of an atom or a group from one atom to another within
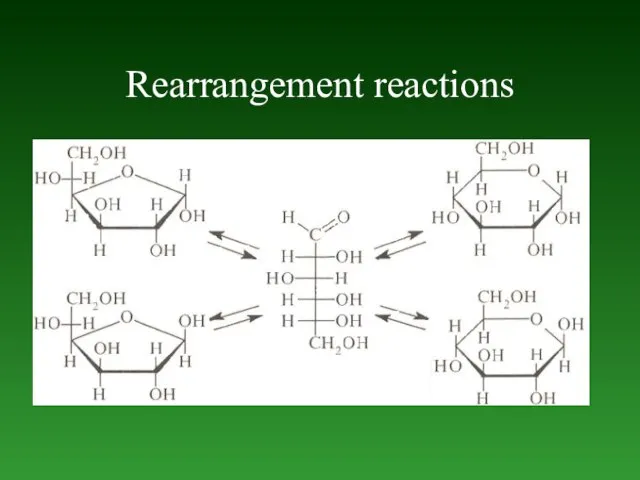
- 68. Rearrangement reactions
- 69. Alcohols Alcohols are a family of compounds containing a hydroxyl ( OH) group bonded to an
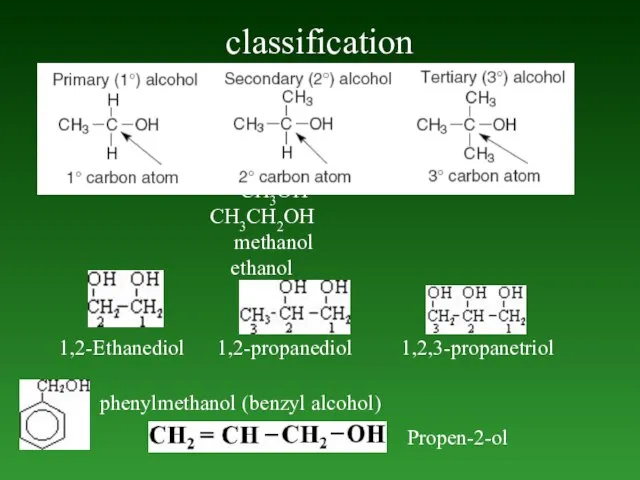
- 70. classification 1,2-Ethanediol 1,2-propanediol 1,2,3-propanetriol СН3ОН СН3СН2ОН methanol ethanol phenylmethanol (benzyl alcohol) Propen-2-ol
- 71. Preparation of Alcohols
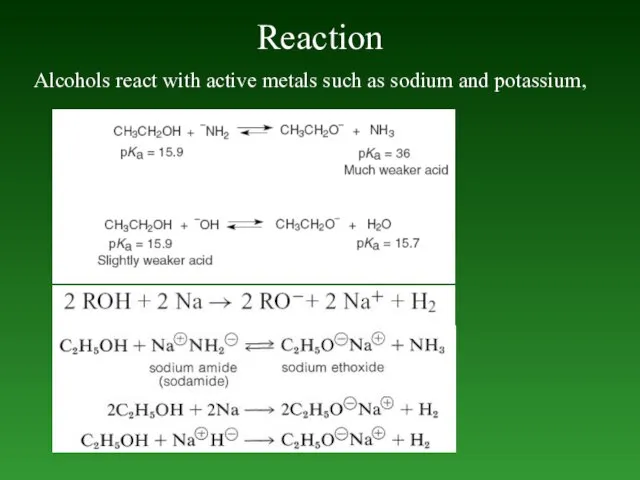
- 72. Reaction Alcohols react with active metals such as sodium and potassium,
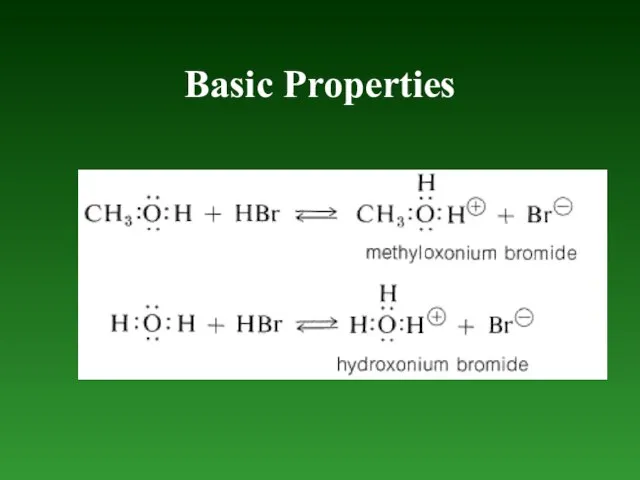
- 73. Basic Properties
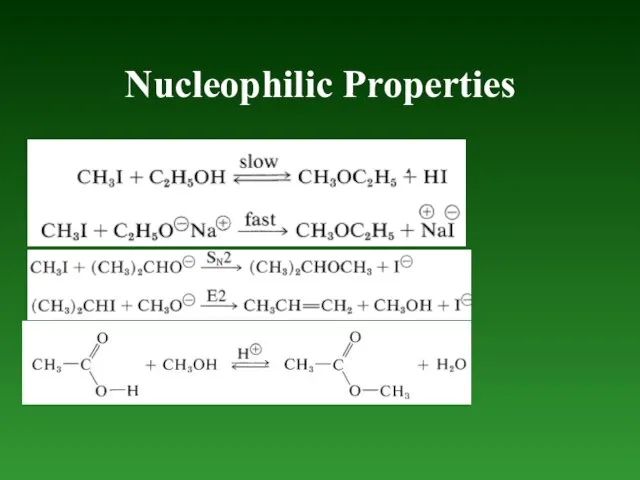
- 74. Nucleophilic Properties
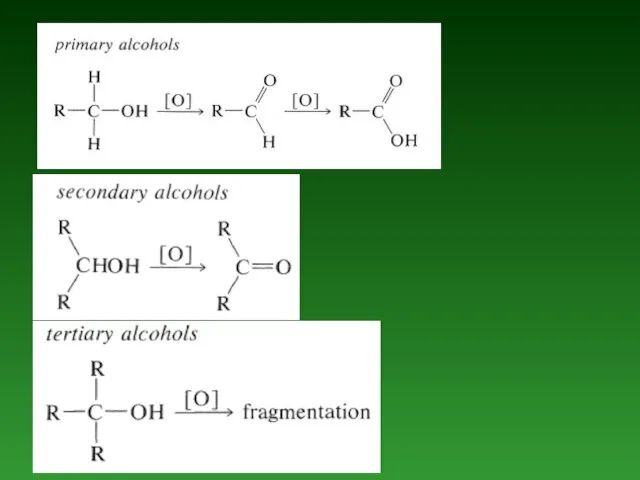
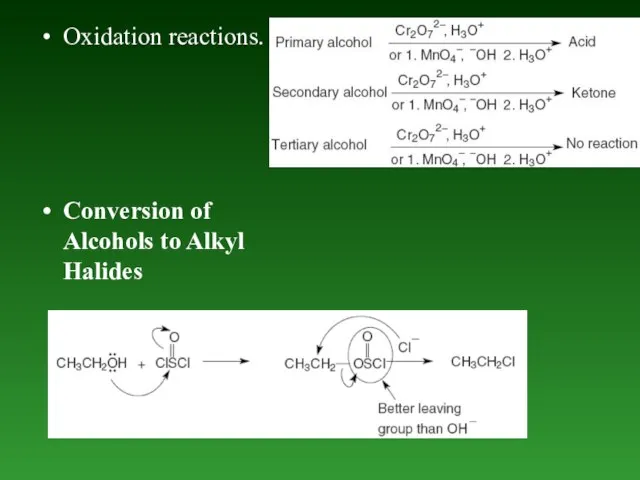
- 77. Oxidation reactions. Conversion of Alcohols to Alkyl Halides
- 78. Dehydration Reactions CH3CH2OH ==== CH2= CH2 + H2O
- 79. Carbonyl campounds compounds which contain a carbonyl group - a carbon-oxygen double bond. Adehyde - а
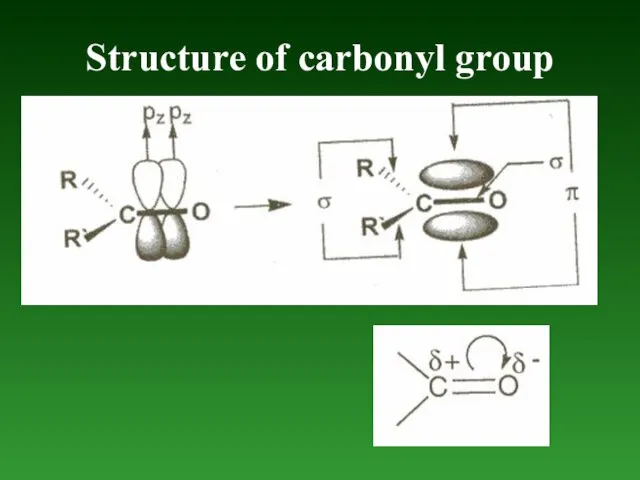
- 80. Structure of carbonyl group
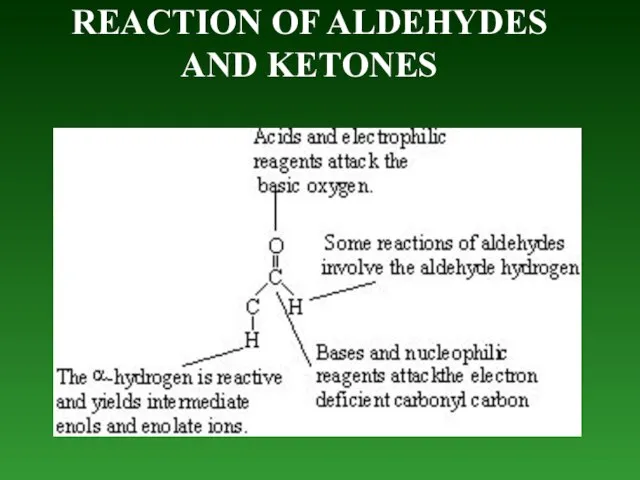
- 81. REACTION OF ALDEHYDES AND KETONES
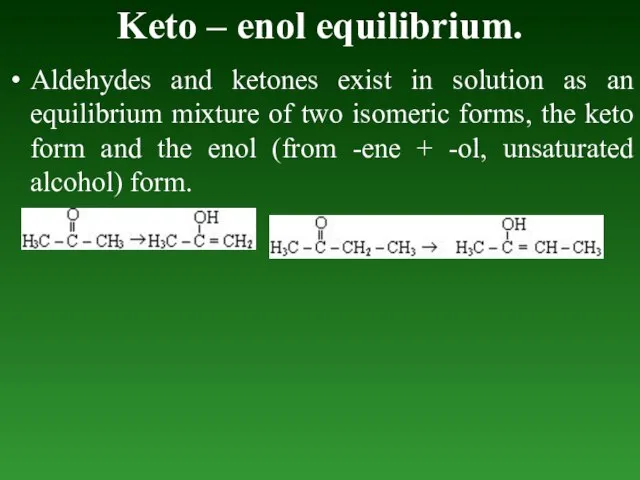
- 82. Keto – enol equilibrium. Aldehydes and ketones exist in solution as an equilibrium mixture of two
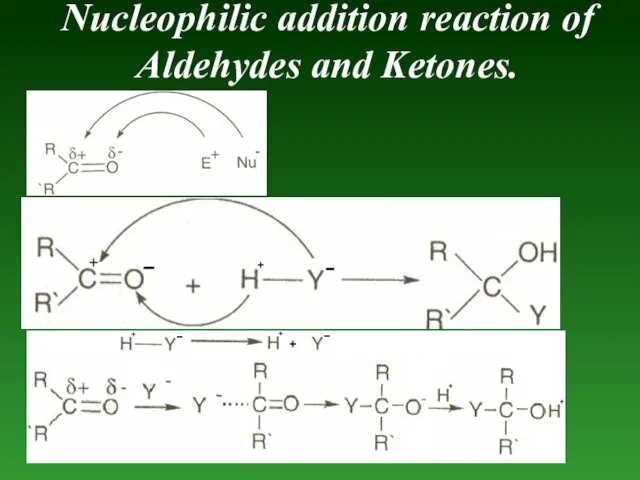
- 83. Nucleophilic addition reaction of Aldehydes and Ketones.
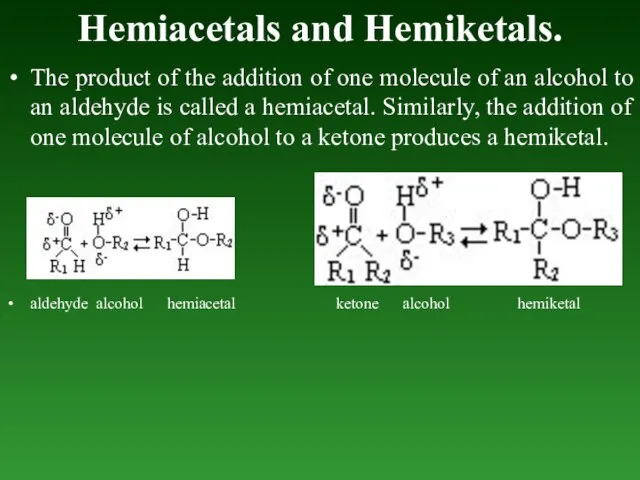
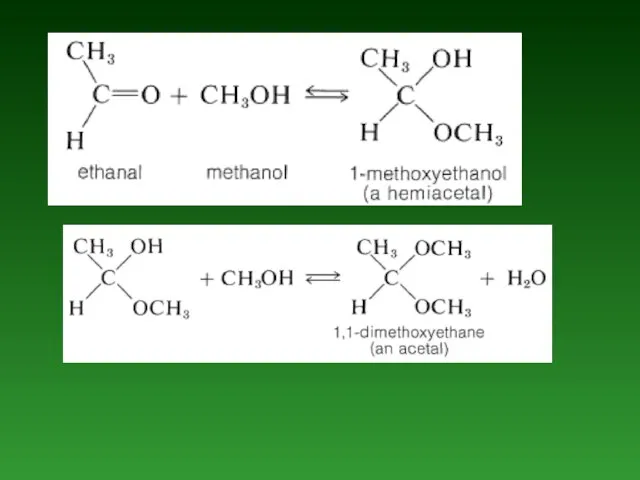
- 84. Hemiacetals and Hemiketals. The product of the addition of one molecule of an alcohol to an
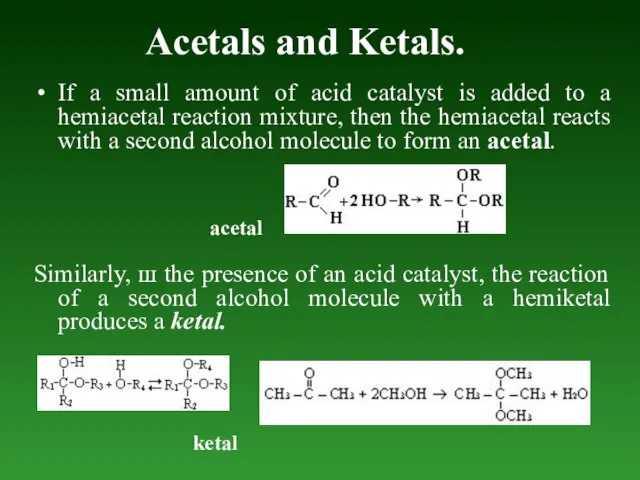
- 85. Acetals and Ketals. If а small amount of acid catalyst is added to а hemiacetal reaction
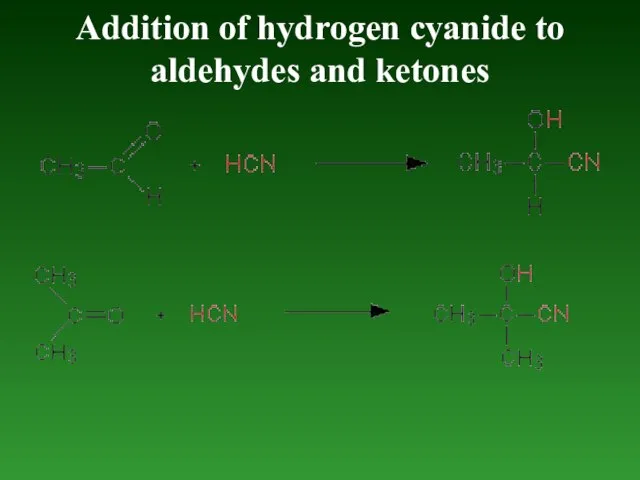
- 87. Addition of hydrogen cyanide to aldehydes and ketones
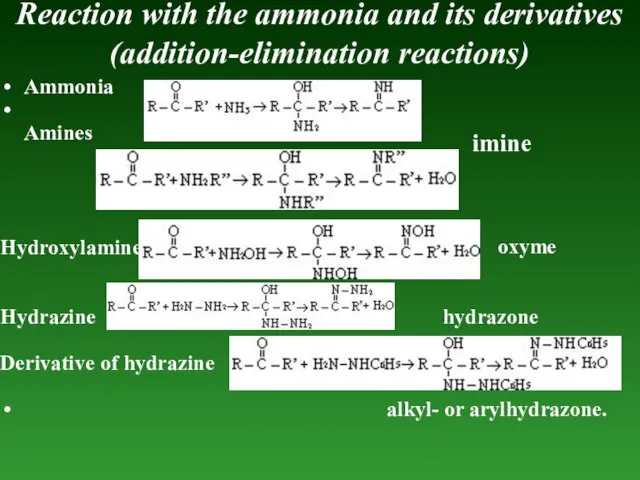
- 88. Reaction with the ammonia and its derivatives (addition-elimination reactions) Ammonia Amines Hydroxylamine Hydrazine hydrazone Derivative of
- 89. Oxidation of Aldehydes and Ketones. a) Using Tollens' reagent (the silver mirror test) Tollens' reagent contains
- 90. The reduction of an aldehyde and a ketone In general terms, reduction of an aldehyde leads
- 92. Скачать презентацию





















 “Роль внешних консультантов в управлении рисками организации”
“Роль внешних консультантов в управлении рисками организации” Програми стажування в сільському господарстві США
Програми стажування в сільському господарстві США Презентация на тему Мы печем тортики из песка
Презентация на тему Мы печем тортики из песка  Решение уравнений, содержащих знак абсолютной величины
Решение уравнений, содержащих знак абсолютной величины Управление собственным капиталом корпорации
Управление собственным капиталом корпорации Rugby School Private boarding school for boys
Rugby School Private boarding school for boys Лев Семенович Выготский
Лев Семенович Выготский Работа с родителями Учитель начальных классов высшей категории,классный руководитель
Работа с родителями Учитель начальных классов высшей категории,классный руководитель  Человек читающий – человек успешный
Человек читающий – человек успешный Люби живое. Б. С. Житков. «Про обезьянку»
Люби живое. Б. С. Житков. «Про обезьянку» Обитель Пречистой
Обитель Пречистой Время суток (фотографии)
Время суток (фотографии) Новый год 2018
Новый год 2018 airunit
airunit Актуальность преподавания изобразительного искусства в школе.
Актуальность преподавания изобразительного искусства в школе. Влияние курения и алкоголя на организм человека
Влияние курения и алкоголя на организм человека ФИАТА основные направления деятельности и перспективы развития Бондарева Е., Белоглазова Ю., Безнощук Б.
ФИАТА основные направления деятельности и перспективы развития Бондарева Е., Белоглазова Ю., Безнощук Б. Тема 1 БОУП
Тема 1 БОУП Ия Ишемицкая. Не сезон. Как поднять продажи в период спада
Ия Ишемицкая. Не сезон. Как поднять продажи в период спада Сроки проведения вступительных испытаний в гимназию в 2012 году
Сроки проведения вступительных испытаний в гимназию в 2012 году Комбинаторика
Комбинаторика Храм Неба
Храм Неба 12 колен Израиля. Часть 1
12 колен Израиля. Часть 1 Великая Отечественная Война глазами современности
Великая Отечественная Война глазами современности 20141118_igrovye_tekhnologii_na_urokakh_geografii_i_biologii
20141118_igrovye_tekhnologii_na_urokakh_geografii_i_biologii Effective communication skills for managers
Effective communication skills for managers Права матери и ребенка и механизм их защиты
Права матери и ребенка и механизм их защиты Организационно-технологическое обеспечение ГИА-9 в новой форме в рамках формирующейся РСОКО Кемеровской области
Организационно-технологическое обеспечение ГИА-9 в новой форме в рамках формирующейся РСОКО Кемеровской области