Содержание
- 2. Nature of Electronic Transitions The total energy of a molecule is the sum of its electronic,
- 4. The saturated aliphatic hydrocarbons (alkanes) exhibit only σ σ* transitions but depending on the functional groups
- 5. Sigma and Pi orbitals
- 6. Electron transitions
- 8. Beer’s and Lambert’s Law The greater the number of molecules that absorb light of a given
- 12. Solvent Effects Highly pure, non-polar solvents such as saturated hydrocarbons do not interact with solute molecules
- 13. π Æ π* Transitions In case of π Æ π* transitions, the excited states are more
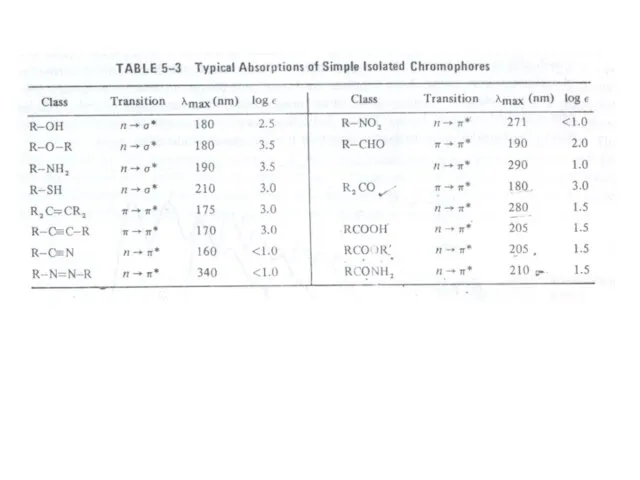
- 15. Chromophore: The energy of radiation being absorbed during excitation of electrons from ground state to excited
- 17. For example, alkanes contain only single bonds with only possible σ Æ σ* type electronic transitions.
- 18. Auxochrome: The substituents that themselves do not absorb ultraviolet radiations but their presence shifts the absorption
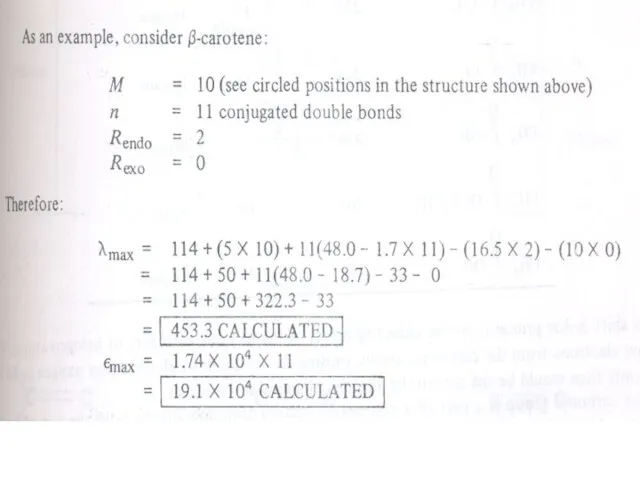
- 20. Conjugated Dienes, Trienes and Polyenes The presence of conjugate double bond decreases the energy difference between
- 21. The presence of alkyl substituents on double bond also produces bathochromic shift and hyperchromic effect. These
- 34. Carbonyl Compounds Carbonyl compounds have two principal UV radiations, the allowed π Æ π* transitions and
- 36. Aromatic Compounds The simplest aromatic compound is benzene. It shows two primary bands at 184 (ε
- 38. Скачать презентацию
Слайд 2 Nature of Electronic Transitions
The total energy of a molecule is the
Nature of Electronic Transitions
The total energy of a molecule is the
Слайд 4The saturated aliphatic hydrocarbons (alkanes) exhibit only
σ σ* transitions but depending on
The saturated aliphatic hydrocarbons (alkanes) exhibit only σ σ* transitions but depending on
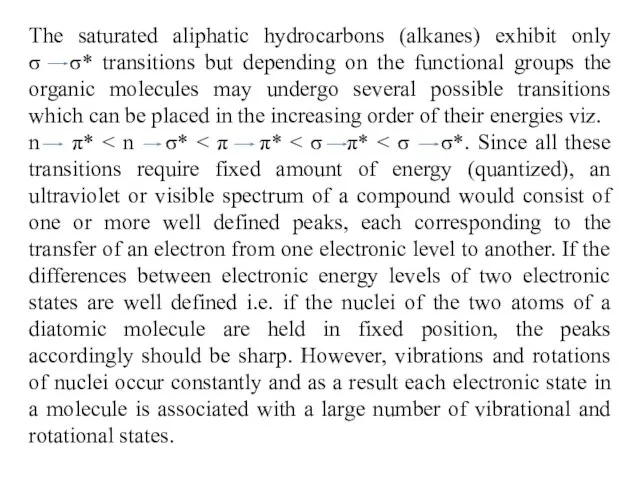
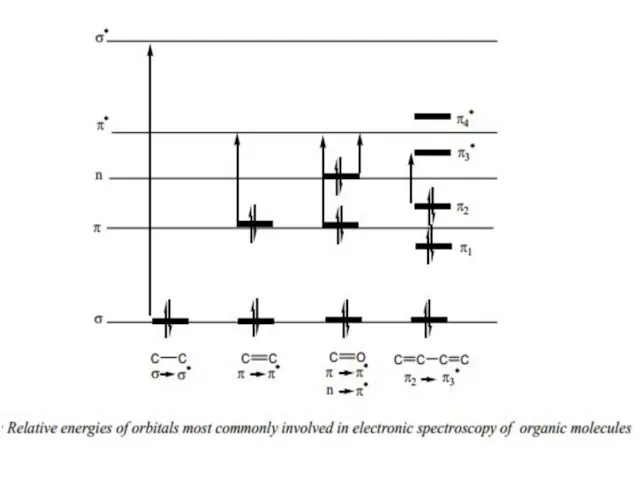
n π* < n σ* < π π* < σ π* < σ σ*. Since all these transitions require fixed amount of energy (quantized), an ultraviolet or visible spectrum of a compound would consist of one or more well defined peaks, each corresponding to the transfer of an electron from one electronic level to another. If the differences between electronic energy levels of two electronic states are well defined i.e. if the nuclei of the two atoms of a diatomic molecule are held in fixed position, the peaks accordingly should be sharp. However, vibrations and rotations of nuclei occur constantly and as a result each electronic state in a molecule is associated with a large number of vibrational and rotational states.
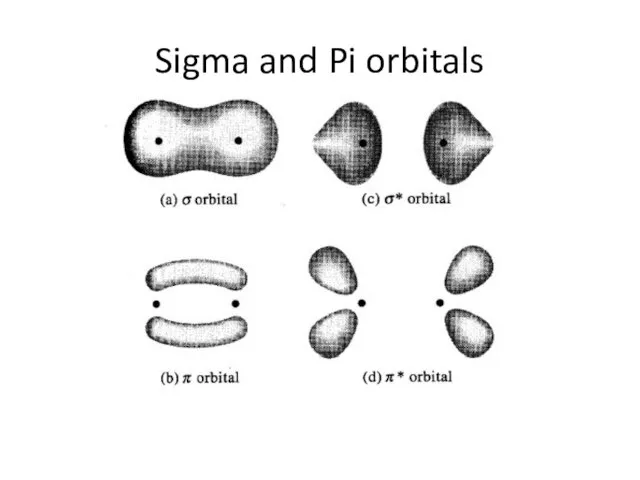
Слайд 5Sigma and Pi orbitals
Sigma and Pi orbitals
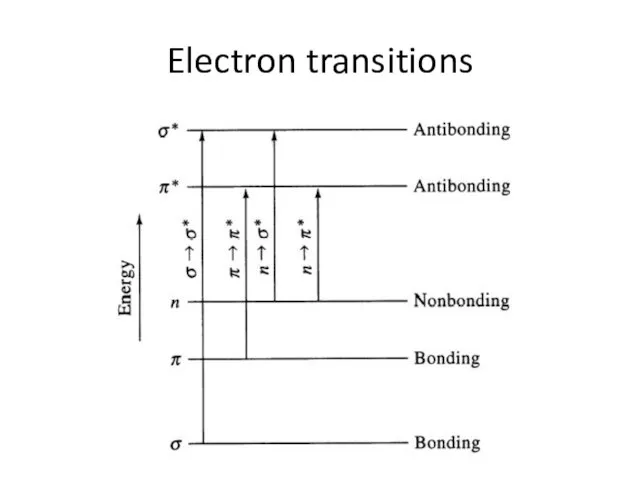
Слайд 6Electron transitions
Electron transitions
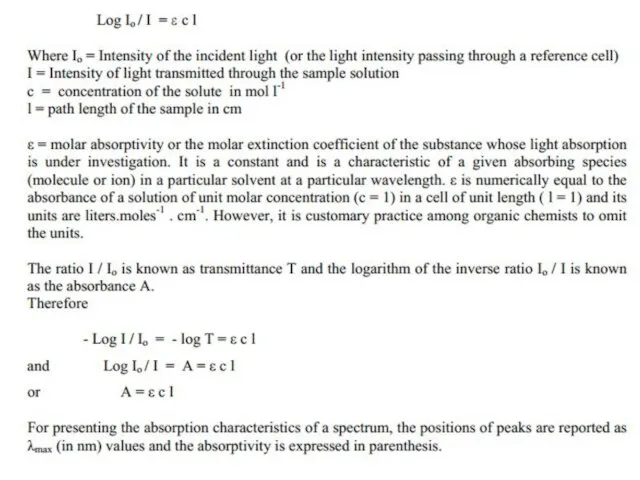
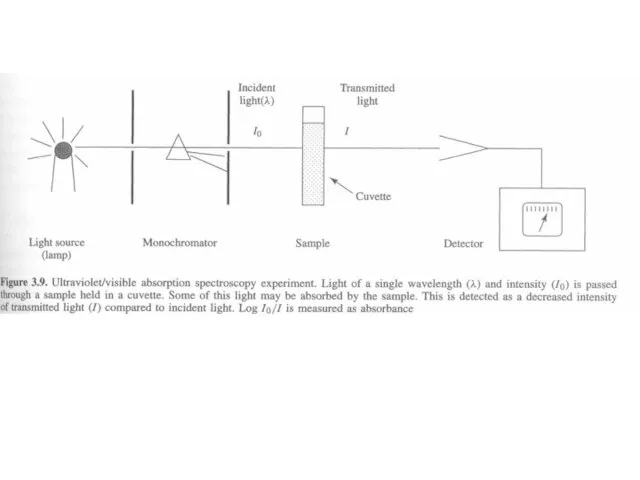
Слайд 8 Beer’s and Lambert’s Law
The greater the number of molecules that absorb
Beer’s and Lambert’s Law
The greater the number of molecules that absorb
When the radiation passes through a solution, the amount of light absorbed or transmitted is an exponential function of the molecular concentration of the solute and also a function of length of the path of radiation through the sample. Therefore,
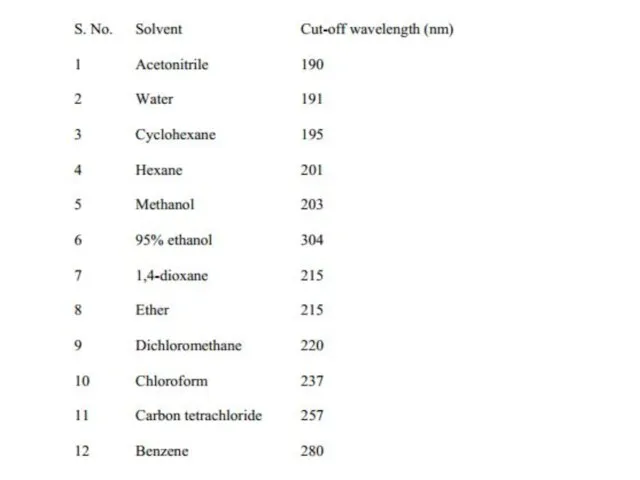
Слайд 12Solvent Effects
Highly pure, non-polar solvents such as saturated hydrocarbons do not
Solvent Effects
Highly pure, non-polar solvents such as saturated hydrocarbons do not
ground state or in excited state and the spectrum of a compound in these solvents may significantly vary from the one recorded in a hydrocarbon solvent.
Слайд 13 π Æ π* Transitions
In case of π Æ π* transitions,
π Æ π* Transitions
In case of π Æ π* transitions,

8(ii) n Æ π* Transitions
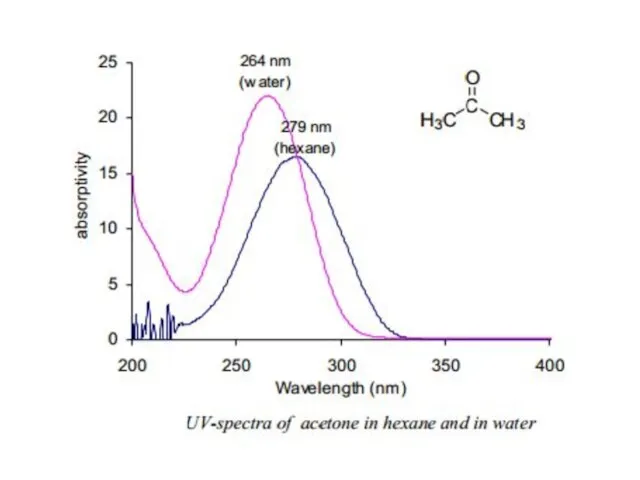
In case of n Æ π* transitions, the polar solvents form hydrogen bonds with the ground state of polar molecules more readily than with their excited states. Therefore, in polar solvents the energies of electronic transitions are increased. For example, the figure 5 shows that theabsorption maximum of acetone in hexane appears at 279 nm which in water is shifted to 264 nm, with a blue shift of 15 nm.
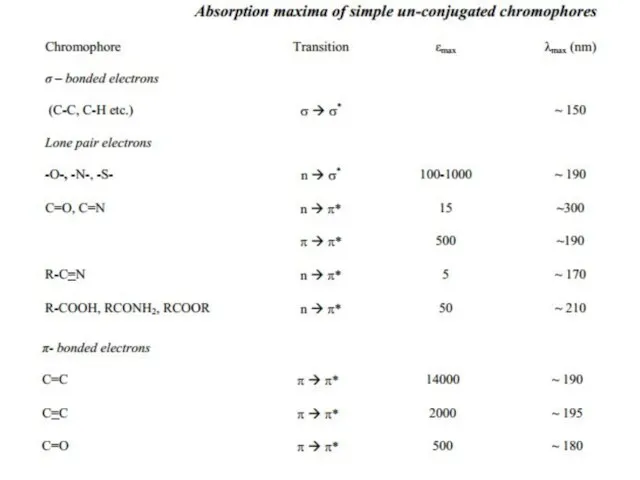
Слайд 15Chromophore: The energy of radiation being absorbed during excitation of electrons from
Chromophore: The energy of radiation being absorbed during excitation of electrons from

transitions of little use. Some of these transitions have been listed in table:
Слайд 17For example, alkanes contain only single bonds with only possible σ Æ
For example, alkanes contain only single bonds with only possible σ Æ
Слайд 18Auxochrome: The substituents that themselves do not absorb ultraviolet radiations but their
Auxochrome: The substituents that themselves do not absorb ultraviolet radiations but their

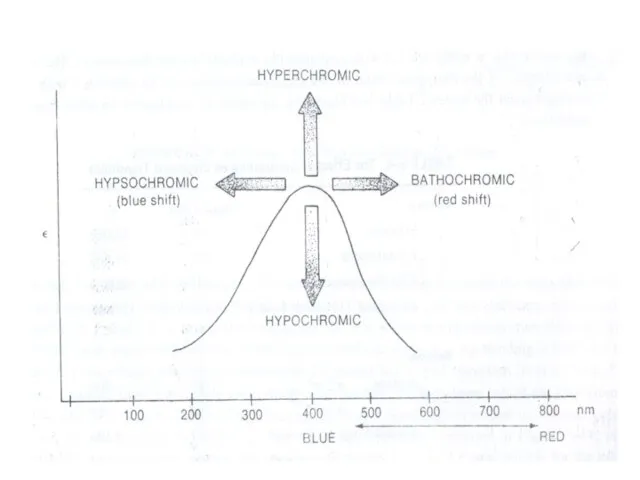
(iii) Bathochromic Shift or Red shift: A shift of an absorption maximum towards longer wavelength or lower energy.
(iv) Hypsochromic Shift or Blue Shift: A shift of an absorption maximum towards shorter wavelength or higher energy.
(v) Hypochromic Effect: An effect that results in decreased absorption intensity.
vi) Hyperchromic Effect: An effect that results in increased absorption intensity.
Слайд 20Conjugated Dienes, Trienes and Polyenes
The presence of conjugate double bond decreases
Conjugated Dienes, Trienes and Polyenes
The presence of conjugate double bond decreases
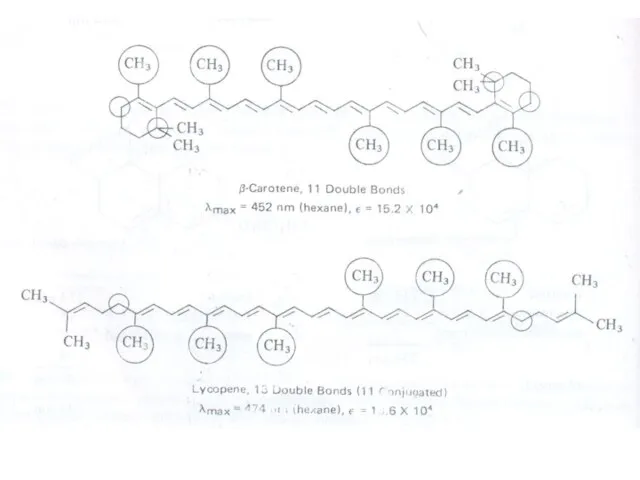
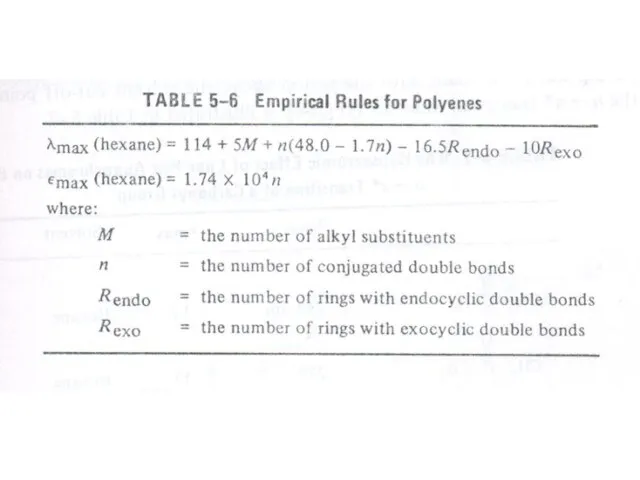
Therefore, the increase in size of the conjugated system gradually shifts the absorption maximum (λmax) to longer wavelength and also increases the absorption. For example, ethylene absorbs at 10175 nm (ε = 1000) and the conjugation in butadiene gives a strong absorption at longer wavelength at 230 nm and with higher intensity (ε = >1000).
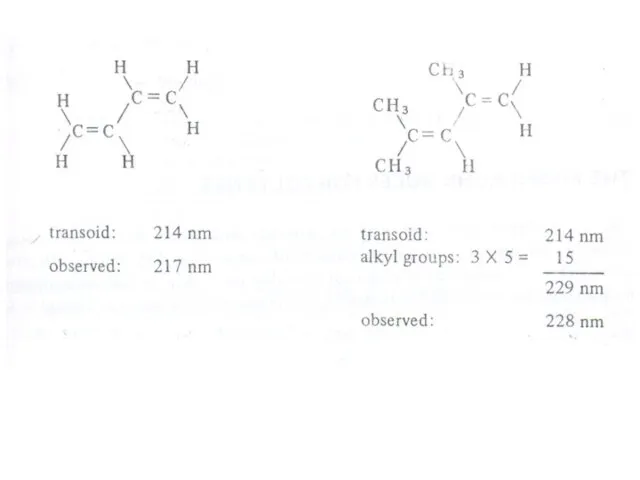
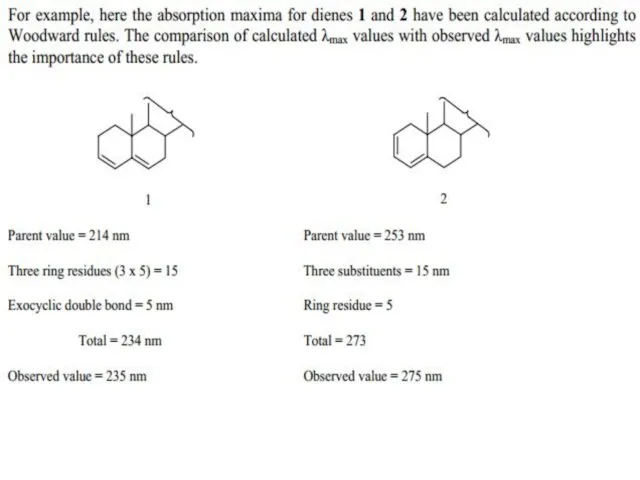
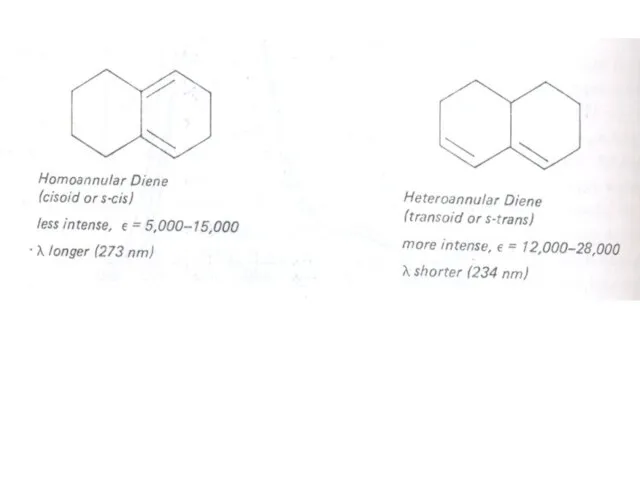
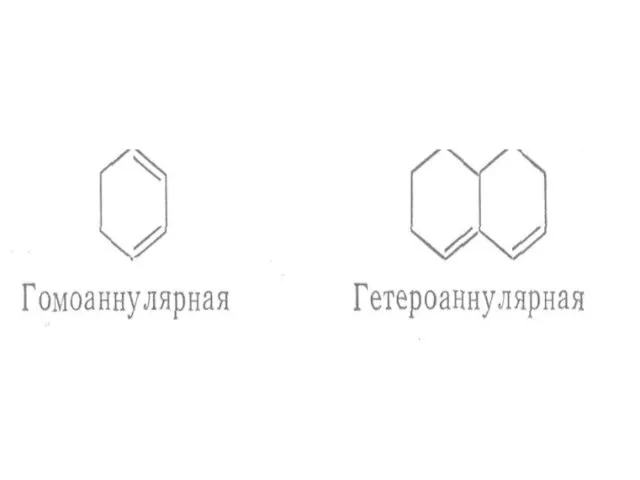
Слайд 21 The presence of alkyl substituents on double bond also produces bathochromic shift
The presence of alkyl substituents on double bond also produces bathochromic shift
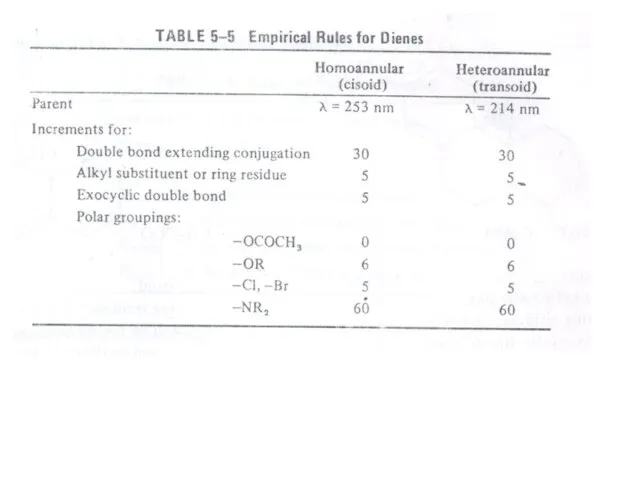
Слайд 34 Carbonyl Compounds
Carbonyl compounds have two principal UV radiations, the allowed
Carbonyl Compounds
Carbonyl compounds have two principal UV radiations, the allowed
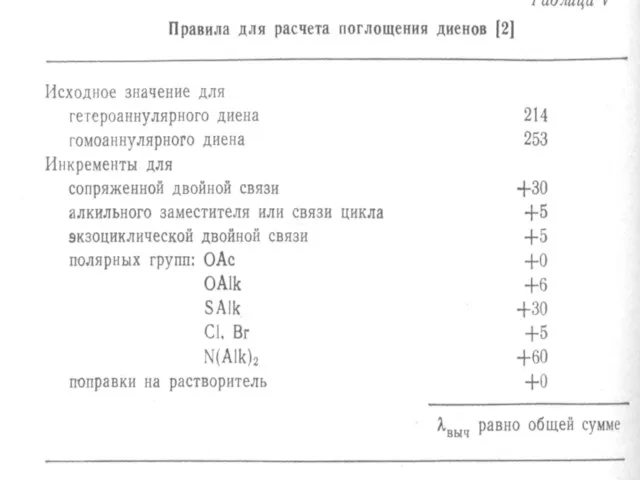
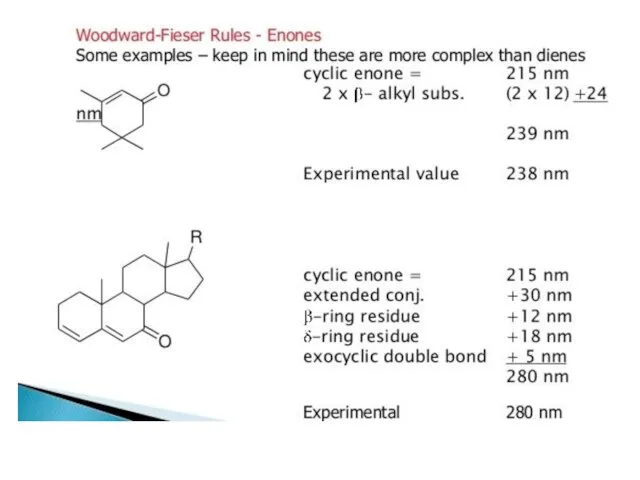
atoms. The heteroatom withdraws electrons from carbonyl carbon and makes carbonyl oxygen lone pair of electrons more stabilized due to its involvement in increasing C=O bond order. As a result, the n Æ π* transition of these compounds is shifted to 200-215 nm range relative to 270 nm in aldehydes and ketones. Conjugation of the carbonyl group with double bond shifts both n Æ π* and π Æ π* transitions to longer wavelengths. The effect on π Æ π* band is more pronounced. Woodward formulated rules to predict the position of an absorption maximum inan unknown enone. These rules have been summarized in table 6.
Слайд 36 Aromatic Compounds
The simplest aromatic compound is benzene. It shows two
Aromatic Compounds
The simplest aromatic compound is benzene. It shows two
electron-donating and electron-withdrawing groups.


 Тревожные дети
Тревожные дети Специальные захваты грузоподъёмных машин
Специальные захваты грузоподъёмных машин ООО Название компании. Шаблон презентации
ООО Название компании. Шаблон презентации Окружность, круг
Окружность, круг Влияние каллиграфии на мозговую активность. 3 часть
Влияние каллиграфии на мозговую активность. 3 часть Законодательствов области обращенияс опасными отходами
Законодательствов области обращенияс опасными отходами Mon métier
Mon métier КУЛЬТУРА ЗАПАДНОЕВРОПЕЙСКОГОСРЕДНЕВЕКОВЬЯ
КУЛЬТУРА ЗАПАДНОЕВРОПЕЙСКОГОСРЕДНЕВЕКОВЬЯ Спортивный коллектив
Спортивный коллектив Приемы сжатия текста
Приемы сжатия текста презент орелй
презент орелй Організація дистанційного навчання, за допомогою платформи MOODLE
Організація дистанційного навчання, за допомогою платформи MOODLE Метод полуреакций или электронно-ионного баланса
Метод полуреакций или электронно-ионного баланса Дистанционное обучение
Дистанционное обучение Перспективы развития платежной системы Банка России в свете Федерального закона «О национальной платежной системе»
Перспективы развития платежной системы Банка России в свете Федерального закона «О национальной платежной системе» Used Car Market in India – A Quikr Review
Used Car Market in India – A Quikr Review Биография и творчество А. А. Вознесенского
Биография и творчество А. А. Вознесенского ФЕДЕРАЛЬНОЕ АГЕНТСТВО ПО ОБРАЗОВАНИЮ Государственное образовательное учреждение высшего профессионального образования «МОСКОВ
ФЕДЕРАЛЬНОЕ АГЕНТСТВО ПО ОБРАЗОВАНИЮ Государственное образовательное учреждение высшего профессионального образования «МОСКОВ Смерть и оживление
Смерть и оживление Аренда земельных участков
Аренда земельных участков Презентация на тему Таможня в 21-ом веке
Презентация на тему Таможня в 21-ом веке Динамика уровня экономического и социального развития Санкт-Петербурга
Динамика уровня экономического и социального развития Санкт-Петербурга 2
2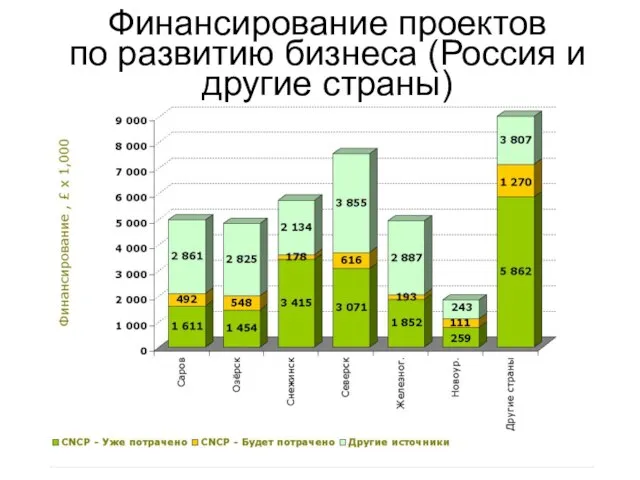 Финансирование проектов по развитию бизнеса (Россия и другие страны)
Финансирование проектов по развитию бизнеса (Россия и другие страны) ??????? ????????????????
??????? ???????????????? Рациональные уравнения
Рациональные уравнения Russian Fashion Retail Forum
Russian Fashion Retail Forum Применение жиров
Применение жиров