Содержание
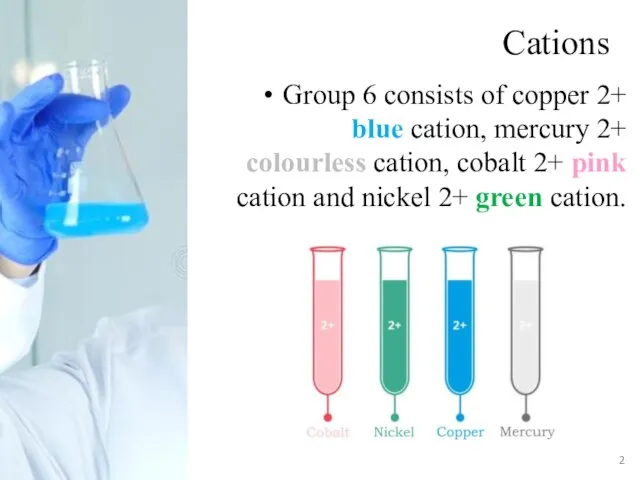
- 2. Cations Group 6 consists of copper 2+ blue cation, mercury 2+ colourless cation, cobalt 2+ pink
- 3. Common reagent The common reagent of this group is aqueous ammonia solution NH4OH. When ammonia solution
- 4. When excess ammonia solution is added, these precipitates will dissolve into colorful solutions.
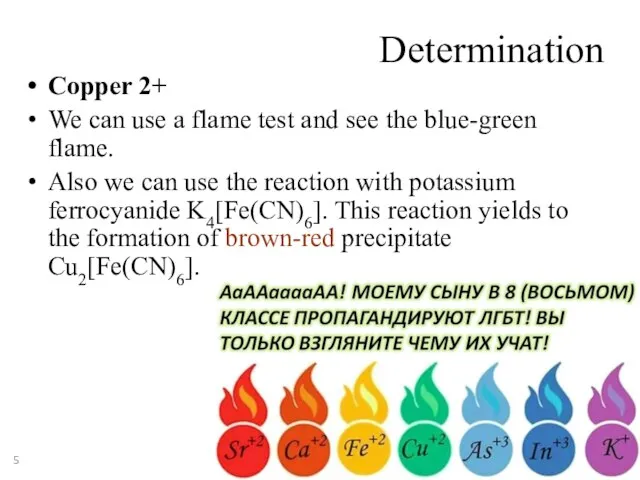
- 5. Determination Copper 2+ We can use a flame test and see the blue-green flame. Also we
- 6. Mercury 2+ We can use the reaction with potassium iodide KI. The brown-red precipitate of mercury
- 7. Cobalt 2+ We can use the reaction ammonium thiocyanate NH4SCN. The solution turns to blue. For
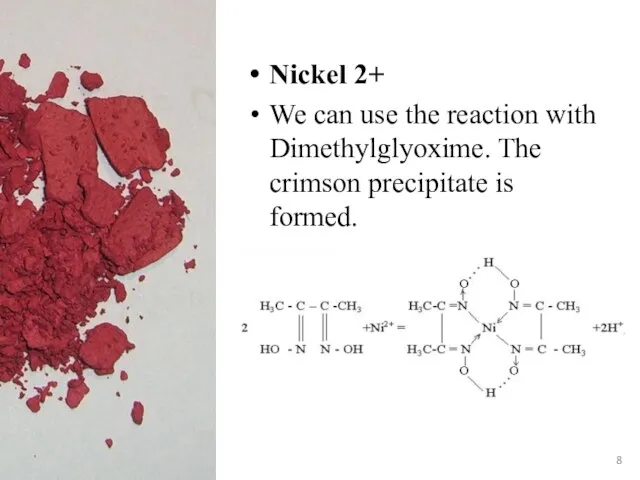
- 8. Nickel 2+ We can use the reaction with Dimethylglyoxime. The crimson precipitate is formed.
- 9. Application Compounds of Group 6 cations found a use in medicine and pharmacy. For example, mercury
- 11. Скачать презентацию





 HCl - кислота
HCl - кислота Декоративная краска с эффектом окисленной меди
Декоративная краска с эффектом окисленной меди Окислительно-восстановительные реакции. Метод электронного баланса
Окислительно-восстановительные реакции. Метод электронного баланса Основы химической термодинамики
Основы химической термодинамики Биосинтез пиримидиновых нуклеотидов
Биосинтез пиримидиновых нуклеотидов Металлическая химическая связь
Металлическая химическая связь Органическая химия. Введение
Органическая химия. Введение Кислоты, основания и соли как электролиты
Кислоты, основания и соли как электролиты Молярная масса вещества
Молярная масса вещества Основания как электролиты
Основания как электролиты Номенклатура органических соединений. Упражнения
Номенклатура органических соединений. Упражнения Углеводороды: соединения, состоящие из углерода и водорода
Углеводороды: соединения, состоящие из углерода и водорода Презентация на тему Генетическая связь между классами неорганических веществ
Презентация на тему Генетическая связь между классами неорганических веществ  Сплавы металлов
Сплавы металлов Углеводороды
Углеводороды Pomalé a rýchle reakcie v bežnom živote
Pomalé a rýchle reakcie v bežnom živote Тренажёр: знаки химических элементов
Тренажёр: знаки химических элементов Серная кислота
Серная кислота ОВР
ОВР Оксиды. Физические свойства
Оксиды. Физические свойства Svins. Iegūšana
Svins. Iegūšana Основания
Основания Иерархия классов методов моделирования. Атомистические и микроскопические методы
Иерархия классов методов моделирования. Атомистические и микроскопические методы Важнейшие породообразующие минералы
Важнейшие породообразующие минералы Влияние давления на скорость химических реакций с участием газов
Влияние давления на скорость химических реакций с участием газов Вероятность протекания химических реакций
Вероятность протекания химических реакций Метод проектов: организация деятельности по химии
Метод проектов: организация деятельности по химии Металлы. Физические свойства металлов
Металлы. Физические свойства металлов