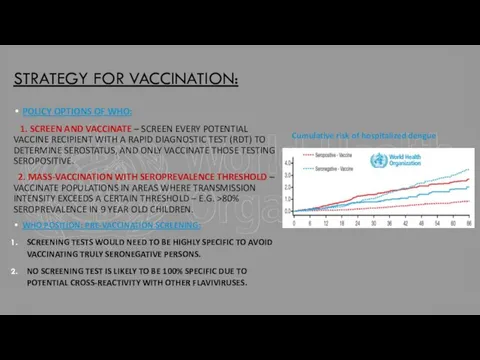
EVERY POTENTIAL VACCINE RECIPIENT WITH A RAPID DIAGNOSTIC TEST (RDT) TO DETERMINE SEROSTATUS, AND ONLY VACCINATE THOSE TESTING SEROPOSITIVE.
2. MASS-VACCINATION WITH SEROPREVALENCE THRESHOLD – VACCINATE POPULATIONS IN AREAS WHERE TRANSMISSION INTENSITY EXCEEDS A CERTAIN THRESHOLD – E.G. >80% SEROPREVALENCE IN 9 YEAR OLD CHILDREN.
WHO POSITION: PRE-VACCINATION SCREENING:
SCREENING TESTS WOULD NEED TO BE HIGHLY SPECIFIC TO AVOID VACCINATING TRULY SERONEGATIVE PERSONS.
NO SCREENING TEST IS LIKELY TO BE 100% SPECIFIC DUE TO POTENTIAL CROSS-REACTIVITY WITH OTHER FLAVIVIRUSES.
Cumulative risk of hospitalized dengue


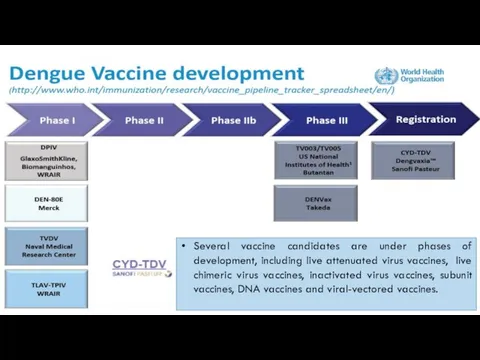





 Эндодонтиялық ем жүргізу кезінде қолданылатын коффердам
Эндодонтиялық ем жүргізу кезінде қолданылатын коффердам Уровни алгоритмов лучевого исследования
Уровни алгоритмов лучевого исследования Острый живот
Острый живот Алгоритм диагностики и лечения синдрома раздраженного кишечника
Алгоритм диагностики и лечения синдрома раздраженного кишечника Системы для определения уровня глюкозы в крови
Системы для определения уровня глюкозы в крови Аномалии таза
Аномалии таза Травмы конечностей
Травмы конечностей Еркін нарық оңтайлылық. Аяқталмаған нарығы. Еркін нарық денсаулық жағдайларына жұмыс істеуі
Еркін нарық оңтайлылық. Аяқталмаған нарығы. Еркін нарық денсаулық жағдайларына жұмыс істеуі Строение коркового и мозгового вещества почки
Строение коркового и мозгового вещества почки Уменьшение преднагрузки и посленагрузки на левый желудочек (лечение периферическими вазодилататорами)
Уменьшение преднагрузки и посленагрузки на левый желудочек (лечение периферическими вазодилататорами) Вторичный сифилис
Вторичный сифилис Паразитизм. Пути эволюции паразитов. Заболевания
Паразитизм. Пути эволюции паразитов. Заболевания Тромбоэмболия легочной артерии
Тромбоэмболия легочной артерии Нейротропные средства
Нейротропные средства Денсаулықпен байланысты өмір сапасы
Денсаулықпен байланысты өмір сапасы Лечебная физическая культура
Лечебная физическая культура Сестринский процесс при деформирующем остеоартрозе
Сестринский процесс при деформирующем остеоартрозе 7
7 Тыныс жетіспеушілігі
Тыныс жетіспеушілігі Коньюктивит
Коньюктивит Терапевтическая функция философии
Терапевтическая функция философии Алкоголизмның этнокультурасы және гендрлік ерекшеліктері
Алкоголизмның этнокультурасы және гендрлік ерекшеліктері Ортодонтическое лечение пациентов с дисфункцией зубочелюстной системы
Ортодонтическое лечение пациентов с дисфункцией зубочелюстной системы Физиология нормального сна
Физиология нормального сна Кардиогенный шок
Кардиогенный шок Синдро́м дли́тельного сдавле́ния
Синдро́м дли́тельного сдавле́ния Съемное протезирование зубов
Съемное протезирование зубов Структуры желчевыводящих путей
Структуры желчевыводящих путей