Содержание
- 2. Disclaimer This document was prepared by Hemoglobin Oxygen Therapeutics LLC (“HbO2 Therapeutics” or the “Company”) to
- 3. Mission Statement To develop and commercialize the first and best in class technology platform for oxygen-carrying
- 4. Company at a Glance Delaware registered in 2014, technology is going back to 1990s with $1
- 5. Brian Dawson Senior Director, Process Development 25 years experience in development & commercialization of HbO2’s products
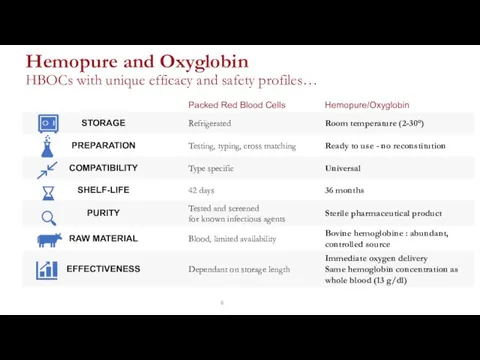
- 6. Hemopure and Oxyglobin HBOCs with unique efficacy and safety profiles…
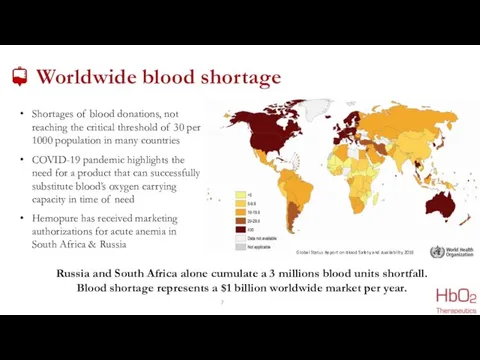
- 7. Worldwide blood shortage COVID-19 pandemic highlights the need for a product that can successfully substitute blood’s
- 8. Strategic National Stockpiles Out-of-hospital / Ambulance services Remote locations / Military battlefield use Prehospital trauma (military
- 9. Blood Is Not An Option Blood disorders including Sickle Cell disease, Hemolytic Anemia, etc. Rare blood
- 10. Blood shortage, a similar issue in veterinary markets 2 million canine transfusions are needed annually in
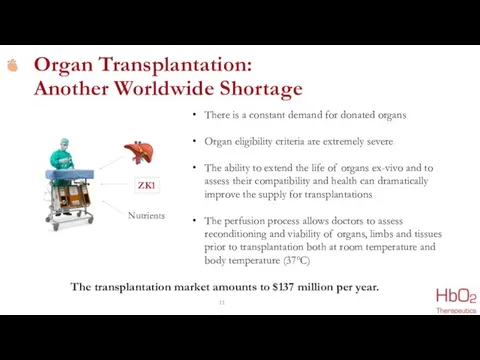
- 11. ZK1 Nutrients There is a constant demand for donated organs Organ eligibility criteria are extremely severe
- 12. Additional indications include… Ischemia indications including Minimization of infarct size (STEMI - ST-Elevation myocardial infarction) Resuscitation
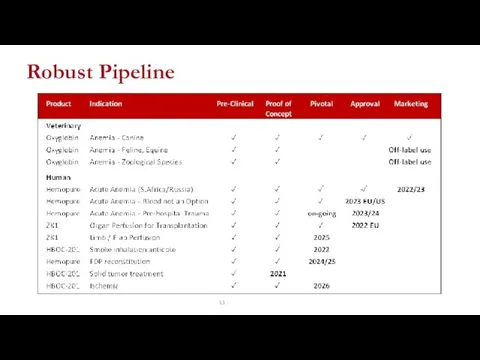
- 13. Robust Pipeline
- 14. Strong value creating recent progress US Department of Defense signed and sponsored Hemopure trial for pre-hospital
- 15. Strong value creating milestones FDA submission for Hemopure Phase 3 pivotal BNO clinical trial Start of
- 16. Major academic & health centers collaborations
- 17. Expanded access program hospitals
- 18. Over 300 peer-reviewed publications
- 19. Characteristics of Hemopure Hb concentration 13 g/dL Plasma half-life ~ 19 hours Size = ~ 1
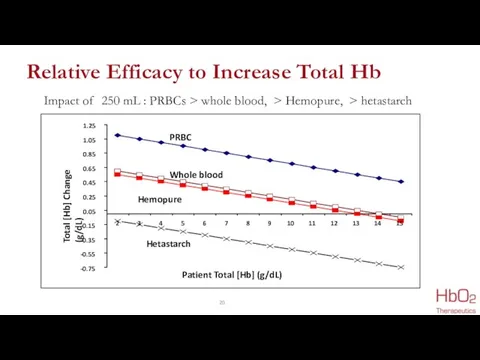
- 20. Relative Efficacy to Increase Total Hb Impact of 250 mL : PRBCs > whole blood, >
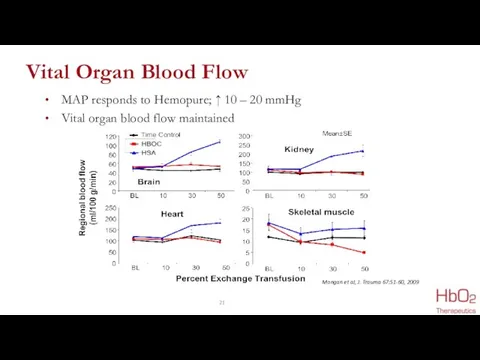
- 21. Vital Organ Blood Flow MAP responds to Hemopure; ↑ 10 – 20 mmHg Vital organ blood
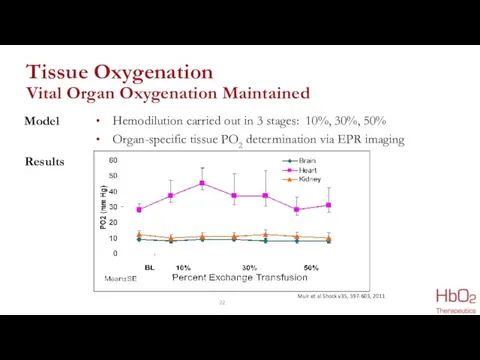
- 22. Tissue Oxygenation Vital Organ Oxygenation Maintained Hemodilution carried out in 3 stages: 10%, 30%, 50% Organ-specific
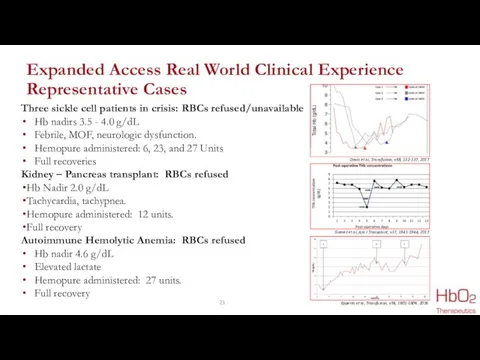
- 23. Expanded Access Real World Clinical Experience Representative Cases Three sickle cell patients in crisis: RBCs refused/unavailable
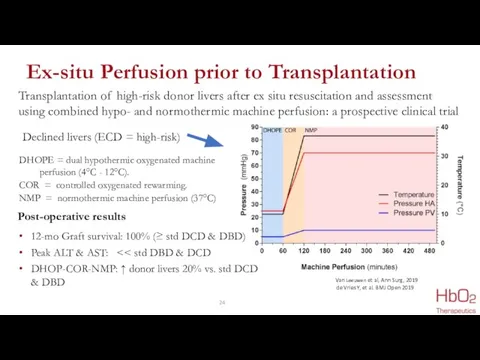
- 24. Ex-situ Perfusion prior to Transplantation Transplantation of high-risk donor livers after ex situ resuscitation and assessment
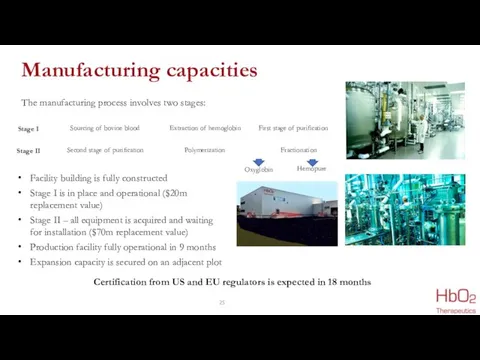
- 25. Manufacturing capacities Facility building is fully constructed Stage I is in place and operational ($20m replacement
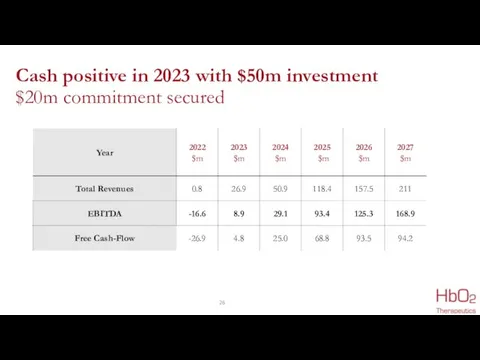
- 26. Cash positive in 2023 with $50m investment $20m commitment secured
- 28. Скачать презентацию














 Алгоритм обследования больного с механической желтухой
Алгоритм обследования больного с механической желтухой Варикозная болезнь
Варикозная болезнь Информационные технологии в медицине
Информационные технологии в медицине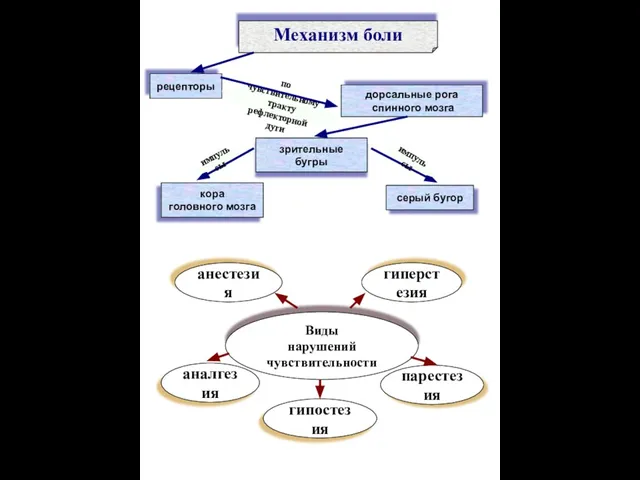 Механизм боли
Механизм боли Магниторезонансное обнаружение контралатерального рака молочной железы
Магниторезонансное обнаружение контралатерального рака молочной железы Возбудители протозойных кишечных инфекций. (Лекция 15)
Возбудители протозойных кишечных инфекций. (Лекция 15) Аллергические реакции и токсическое воздействие частичных съемных протезов
Аллергические реакции и токсическое воздействие частичных съемных протезов Эмболизация маточных артерий как альтернатива гистерэктомии при лечении миомы матки
Эмболизация маточных артерий как альтернатива гистерэктомии при лечении миомы матки Балансирующая система ClearScienceClearScience Балансирующая система ClearScience Простой уход, сочетающий в себе все преимущества ClearScience. Сочетан
Балансирующая система ClearScienceClearScience Балансирующая система ClearScience Простой уход, сочетающий в себе все преимущества ClearScience. Сочетан Медикаментозное прерывание беременности
Медикаментозное прерывание беременности Понятие эффективной и эквивалентной доз. Порядок доз при рентгенологических исследованиях
Понятие эффективной и эквивалентной доз. Порядок доз при рентгенологических исследованиях Интеллектуальная недостаточность или умственная отсталость
Интеллектуальная недостаточность или умственная отсталость Палочка Коха
Палочка Коха Классификация ИБС
Классификация ИБС Сестринский уход за больными в послеоперационном периоде
Сестринский уход за больными в послеоперационном периоде lec 10
lec 10 Графические методы наименований лс
Графические методы наименований лс Общественная организация Общее дело. Алкоголь и табак убивают Россию
Общественная организация Общее дело. Алкоголь и табак убивают Россию Теории развития дисфункции плаценты
Теории развития дисфункции плаценты Тромбоэмболия легочной артерии: современные стандарты диагностики, лечения и профилактики. (Новые рекомендации ESC)
Тромбоэмболия легочной артерии: современные стандарты диагностики, лечения и профилактики. (Новые рекомендации ESC) Реабилитация при ПМК
Реабилитация при ПМК Внутриутробные инфекции
Внутриутробные инфекции Экстренная лучевая диагностика
Экстренная лучевая диагностика Денсаулық сақтаудағы қаржыландыру үлгіл
Денсаулық сақтаудағы қаржыландыру үлгіл Профилактика деменций
Профилактика деменций Предстерилизационная очистка (ПСО) изделий медицинского назначения
Предстерилизационная очистка (ПСО) изделий медицинского назначения Дефекты зрения
Дефекты зрения Хронически панкреатит
Хронически панкреатит