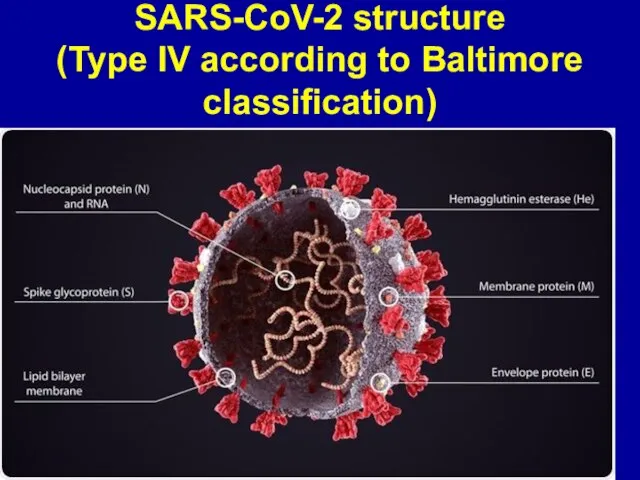
Слайд 2SARS-CoV-2 structure
(Type IV according to Baltimore classification)
Слайд 3SARS-CoV-2 genome
Genome: single stranded RNA messenger 29.9kb long, encoding 13 ORFs.
Coronavirus genomes
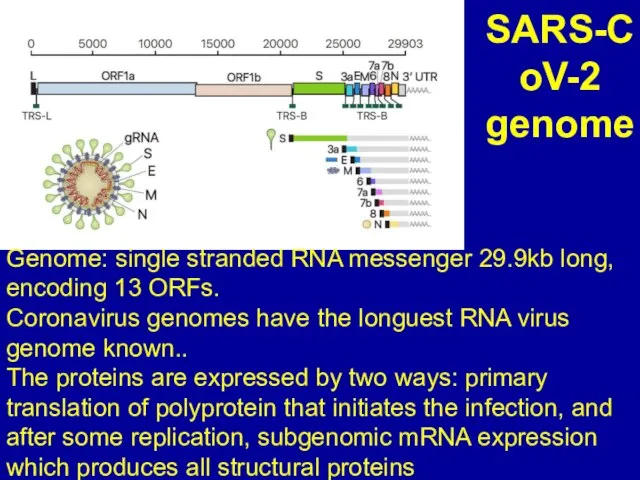
have the longuest RNA virus genome known..
The proteins are expressed by two ways: primary translation of polyprotein that initiates the infection, and after some replication, subgenomic mRNA expression which produces all structural proteins
Слайд 4SARS-CoV-2 causes an infectious causes COVID-19
Most people infected with the COVID-19

virus will experience mild to moderate respiratory illness and recover without requiring special treatment. Older people, some children and those with underlying medical problems like cardiovascular disease, diabetes, chronic respiratory disease, and cancer are more likely to develop serious illness.
Слайд 5There are basically three ways to stop the Covid-19 disease
Extraordinary restrictions on

free movement and assembly, as well as aggressive testing, to interrupt its transmission entirely;
Just wait until enough people get infected and develop NATURAL ACQUIRED ACTIVE immunity (herd immunity);
A vaccine that could protect everyone by developing ARTIFICIAL ACQUIRED ACTIVE immunity (also herd immunity)
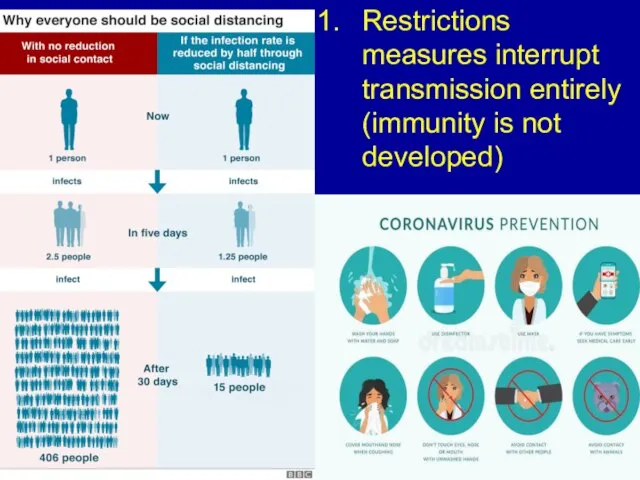
Слайд 6Restrictions measures interrupt transmission entirely (immunity is not developed)
Слайд 72. Get infected - another way for a herd immunity, aside from

vaccines. Some die, and the rest develop antibodies and/or cell-mediated immunity.
There have been two killer coronaviruses before:
SARS-CoV infected only 8,000 people, killing 774 (about 10%), and was contained in 7½ months.
MERS has never stopped but is rare. Since arising in 2012 it’s infected 2,519 people, killing 35% of them (866 deaths so far).
A novel SARS-CoV-2 by now infects more than 5,6 mln people, kills more then 351,000 of them, and is not going to stop.
Слайд 82. Herd immunity is an epidemiological concept that describes the state where
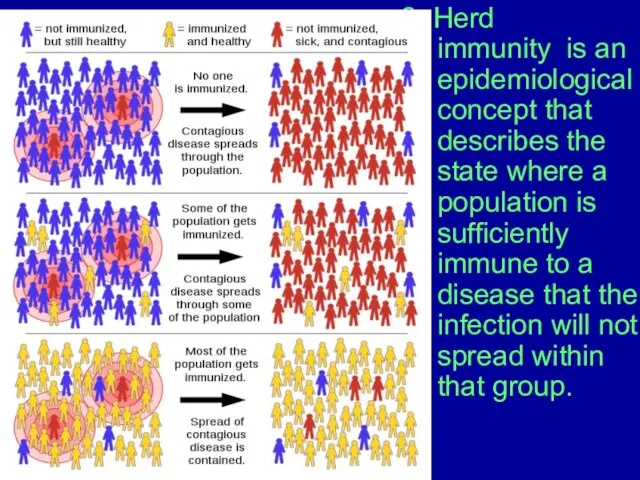
a population is sufficiently immune to a disease that the infection will not spread within that group.
.
Слайд 92. How many people should be infected to develop herd immunity?
For mumps,

92 percent of the population should be immune for the disease to stop spreading entirely. This is what's known as the herd immunity threshold.
COVID-19 is less infectious than mumps, with the proportion of people who need to be infected is lower but still high, sitting at around 70 percent of the entire population.
And what happens if 70 percent of an entire population gets sick, and due to fatality rate around 0,5-1% , how many of them will die?
It is a catastrophic outcome, and is a nonsense, but not preventive measure.
.
Слайд 103. Vaccination-the only way for the herd immunity.
How we develop immunity against
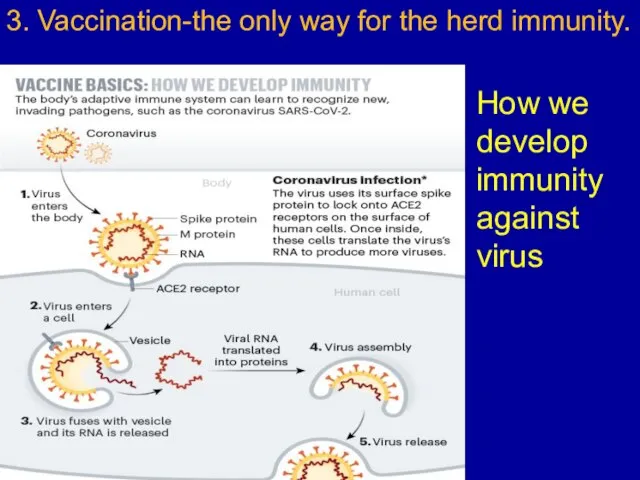
virus
Слайд 11How we develop immunity against virus
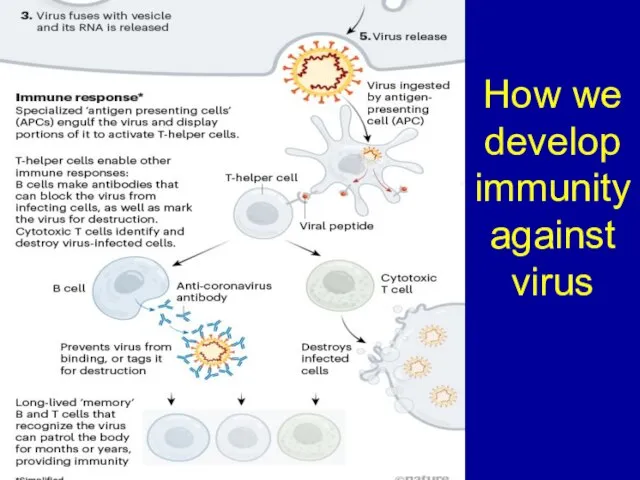
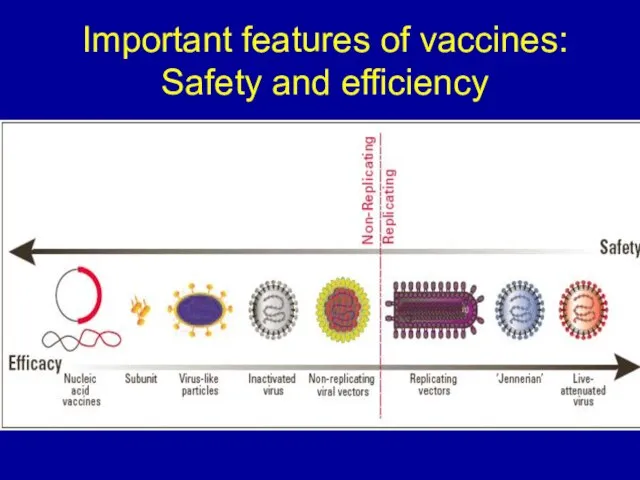
Слайд 13Important features of vaccines:
Safety and efficiency
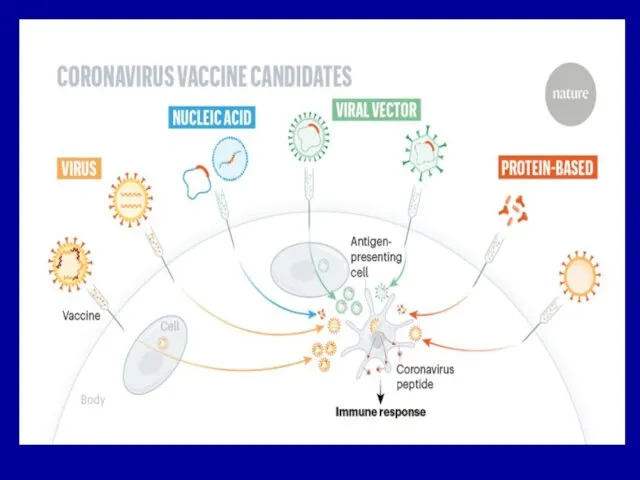
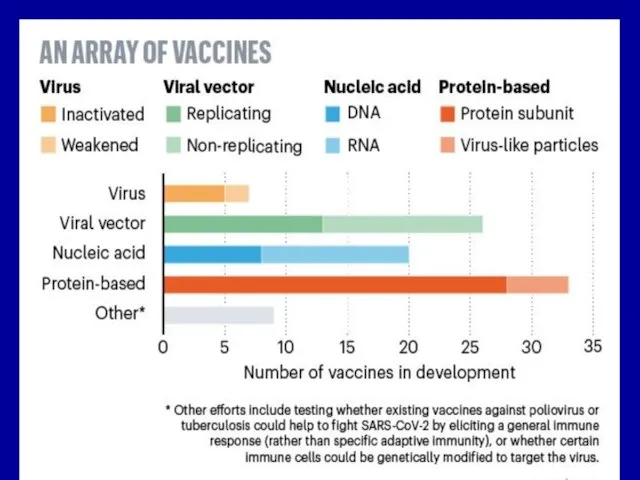
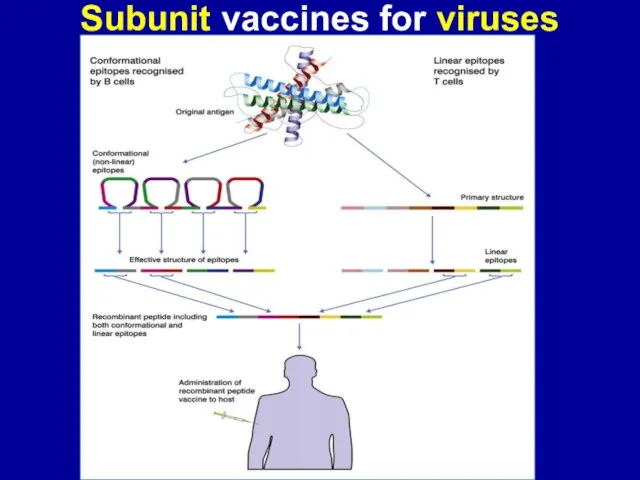
Слайд 17Subunit vaccines for viruses
They can be developed after identification of the

microbial components, that elicit a protec-tive immune response–protective antigens (S for SARS).
Immunogenic component may be isolated from viruses:
(1) by biochemical means (chemical vaccines) or
(2) by genetic engineering (recombinant vaccines) involving the expression of cloned viral genes in bacteria or eukariotic cell.
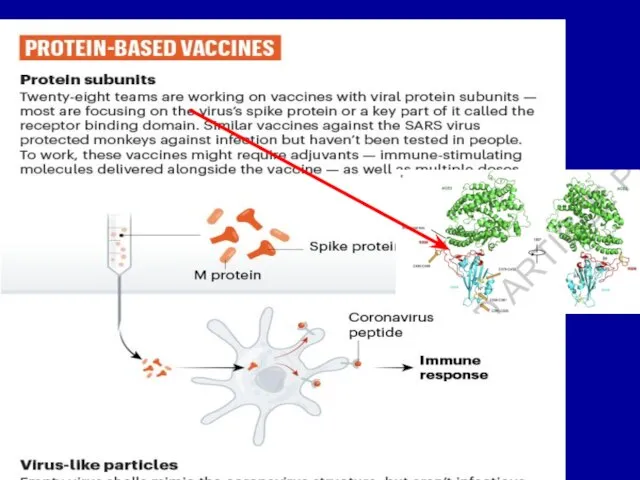
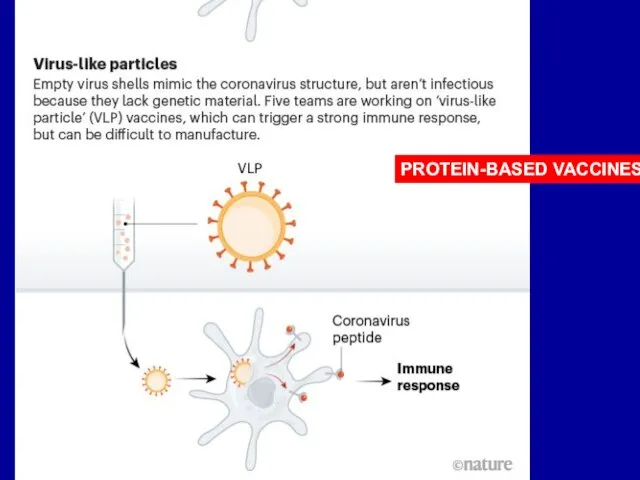
Слайд 19PROTEIN-BASED VACCINES disdvantages
Subunit VNs are poor immunogens and need to be administered
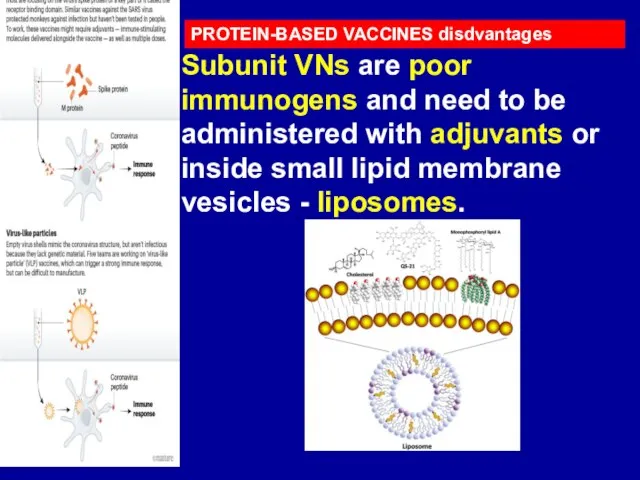
with adjuvants or inside small lipid membrane vesicles - liposomes.
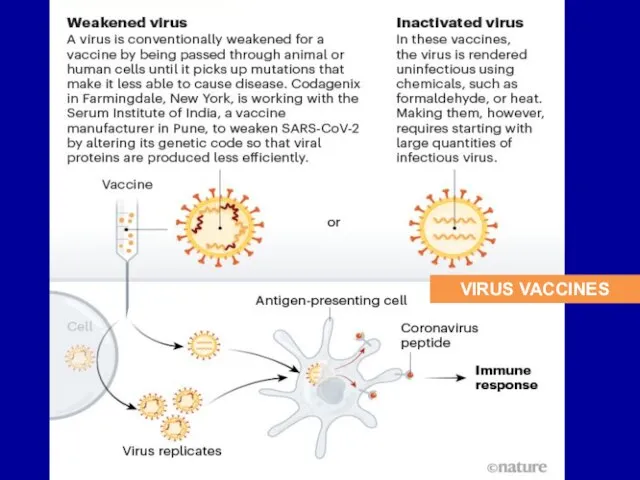
Слайд 21Live vaccines: The advantages
(1) The immunity is long live, and mimics the

normal immune responses.
(2) When vaccine is administrated orally, SIgA is secreted in the gut and oropharynx to protect the mucous.
This prevents the establishing of carrier state and facilitates the eradication of the virus from the community.
(3) Live vaccines are administrated in low doses. Basically one single administration is enough for protection because organisms multiply in a body.
Слайд 22Live vaccines: The disadvantages
(1) they may cause disease in immunosuppressed individuals;
(2)

the vaccine may revert to virulent form;
(3) the COVID – 19 vaccine may cause the effect of the virus (cytokine “storm”)
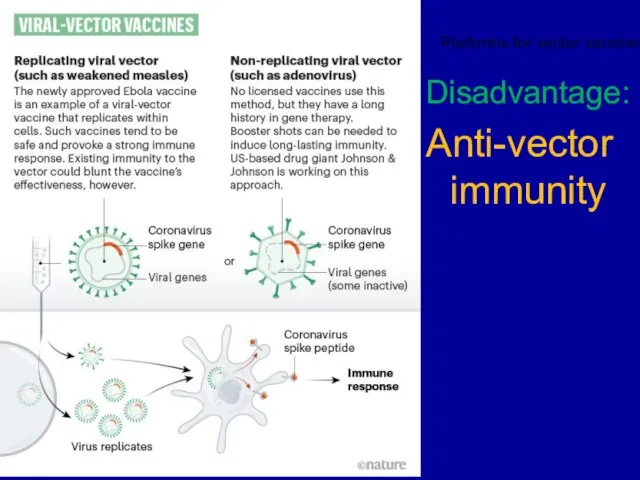
Слайд 23Disadvantage:
Anti-vector immunity
Platforms for vector vaccines:
Слайд 25Mechanisms of immune activation by vaccine vector particles (VVP) through two pathways
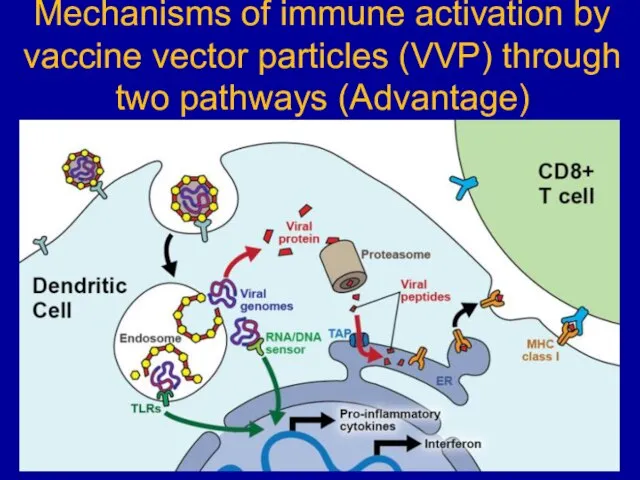
(Advantage)
Слайд 26VVP infect DCs of a vaccinee, taken up by receptor-mediated endocytosis and
release their genome into the cytoplasm of the DC. TLRs sense it in endosome or/and by cytoplasmic sensors of viral nucleic acids (“RNA/DNA sensor”). Both pathways signal through common pathways such as the NFκB and MAPK pathways, resulting in the transcriptional activation of pro-inflammatory cytokines but also in type I interferon production. These events lead to functional activation of the DCs dendritic cell as APCs. Simultaneously, the viral genomic information will be expressed, leading to synthesis of viral proteins and endosomal processing. Viral peptides are loaded onto MHC class I molecules, which are then exported to the cell surface for presentation to virus-specific CD8+ T cells
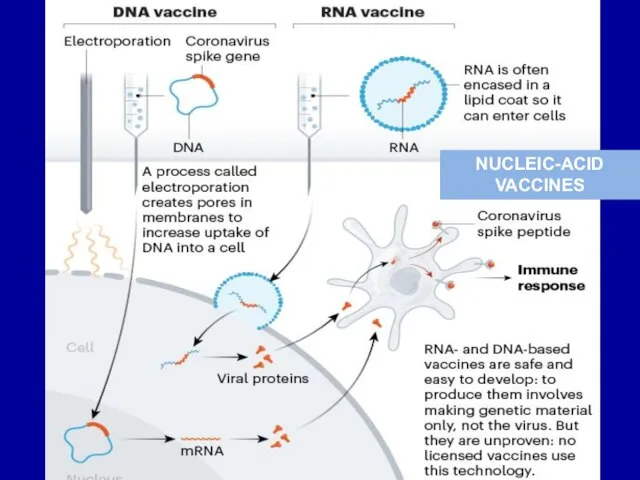
Слайд 28DNA vaccines
DNA vaccines consist of naked DNA code for a gene for

vaccinal protective antigen. This construct is produced
by cloning gene, code for
protective antigen, into
a bacterial plasmid.
The use of DNA vaccines makes possible developing vaccines against infectious agents such as HIV, herpes virus, malaria, and others, which require not only humoral but also cellular immune responses for protection.
Слайд 29Plasmid DNA for gene vaccination
has two major units:
(1) A transcription
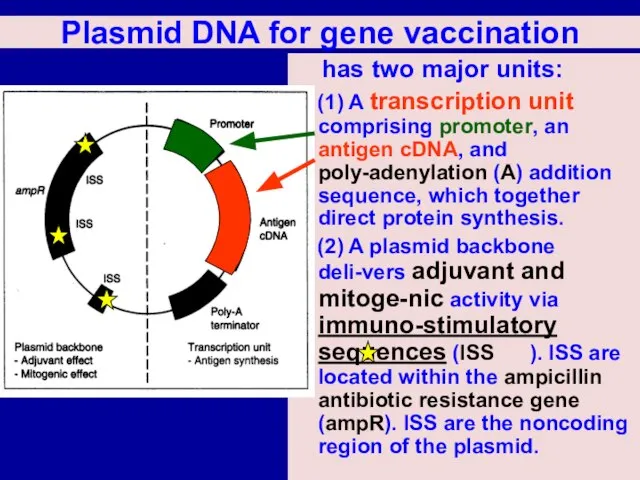
unit comprising promoter, an antigen cDNA, and poly-adenylation (A) addition sequence, which together direct protein synthesis.
(2) A plasmid backbone deli-vers adjuvant and mitoge-nic activity via immuno-stimulatory sequences (ISS ). ISS are located within the ampicillin antibiotic resistance gene (ampR). ISS are the noncoding region of the plasmid.
Слайд 30Immune responses elicited by DNA vaccines
The DNA plasmid is injected into
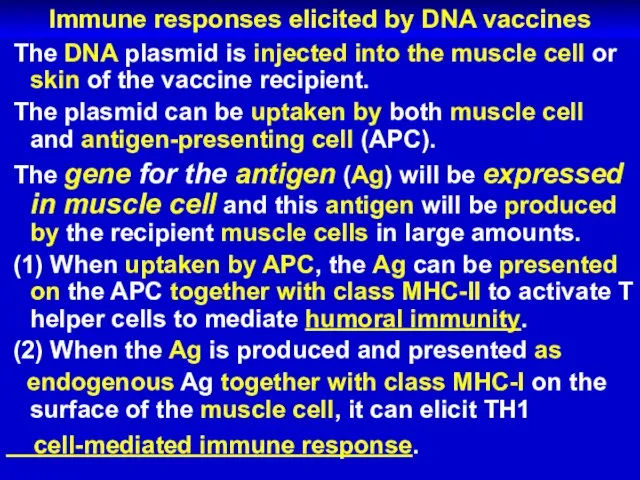
the muscle cell or skin of the vaccine recipient.
The plasmid can be uptaken by both muscle cell and antigen-presenting cell (APC).
The gene for the antigen (Ag) will be expressed in muscle cell and this antigen will be produced by the recipient muscle cells in large amounts.
(1) When uptaken by APC, the Ag can be presented on the APC together with class MHC-II to activate T helper cells to mediate humoral immunity.
(2) When the Ag is produced and presented as
endogenous Ag together with class MHC-I on the surface of the muscle cell, it can elicit TH1
cell-mediated immune response.





















 ВИЧ-инфекция и особенности В-клеточного иммунитета
ВИЧ-инфекция и особенности В-клеточного иммунитета Использование VR-технологий в терапии пациентов с психическими расстройствами
Использование VR-технологий в терапии пациентов с психическими расстройствами  Правила проведения функциональных исследований
Правила проведения функциональных исследований Понятие о предболезни, выявление предболезни надгрузочнымыми пробами
Понятие о предболезни, выявление предболезни надгрузочнымыми пробами Введение в лучевую диагностику
Введение в лучевую диагностику Иммунитет при туберкулезе
Иммунитет при туберкулезе Пневмония. Классификация
Пневмония. Классификация Хронический гепатит
Хронический гепатит Miokard Infarkti
Miokard Infarkti Психопатологическая семиотика. Психопатологическая синдромология. Первая помощь при эпилепсии
Психопатологическая семиотика. Психопатологическая синдромология. Первая помощь при эпилепсии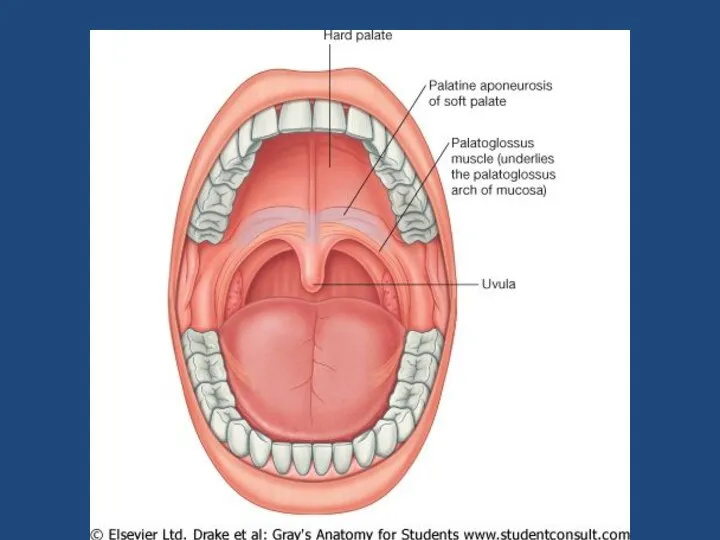 Пищеварительная система. Врожденные дефекты лица
Пищеварительная система. Врожденные дефекты лица Уход и наблюдение за детьми с заболеваниями ССС
Уход и наблюдение за детьми с заболеваниями ССС Иммуноферментный анализ (ИФА)
Иммуноферментный анализ (ИФА) Энтероцеллюлярная мальабсорбция с нарушением мембранного пищеварения и всасывания
Энтероцеллюлярная мальабсорбция с нарушением мембранного пищеварения и всасывания Сальмонеллёзы
Сальмонеллёзы Правила и принципы безопасной и эффективной Гимнастики для лица
Правила и принципы безопасной и эффективной Гимнастики для лица Частная психология
Частная психология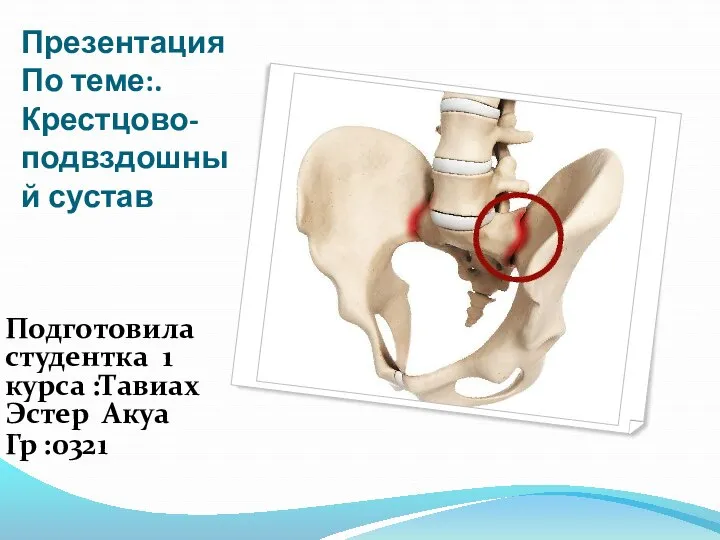 Крестцово-подвздошный сустав
Крестцово-подвздошный сустав Лечебно-охранительный режим ЛПУ и его значение для пациента
Лечебно-охранительный режим ЛПУ и его значение для пациента Рентгенодиагностика неопухолевых заболеваний пищевода
Рентгенодиагностика неопухолевых заболеваний пищевода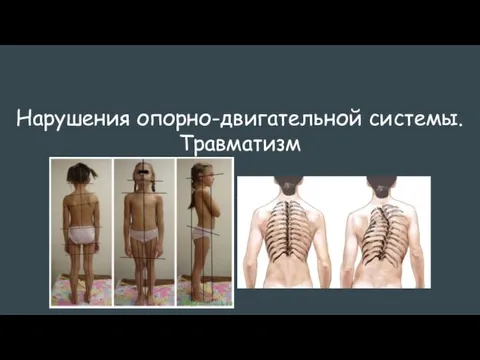 Нарушения опорно-двигательной системы. Травматизм
Нарушения опорно-двигательной системы. Травматизм Клапан уретры
Клапан уретры Методика регистрации ЭКГ. План расшифровки ЭКГ
Методика регистрации ЭКГ. План расшифровки ЭКГ Влажная уборка помещений, текущая и заключительная дезинфекция
Влажная уборка помещений, текущая и заключительная дезинфекция Синдром менингита
Синдром менингита Предмет и задачи гигиены и экологии человека
Предмет и задачи гигиены и экологии человека Роль функциональных методов обследования в диагностике ахроматопсии у детей
Роль функциональных методов обследования в диагностике ахроматопсии у детей Невынашивание и перенашивание беременности
Невынашивание и перенашивание беременности