Содержание
- 2. Learning objectives understand the origin of, and how to calculate relative atomic, molecular and formula mass
- 3. Counting atoms and molecules When conducting a chemical reaction, it is often important to mix reactants
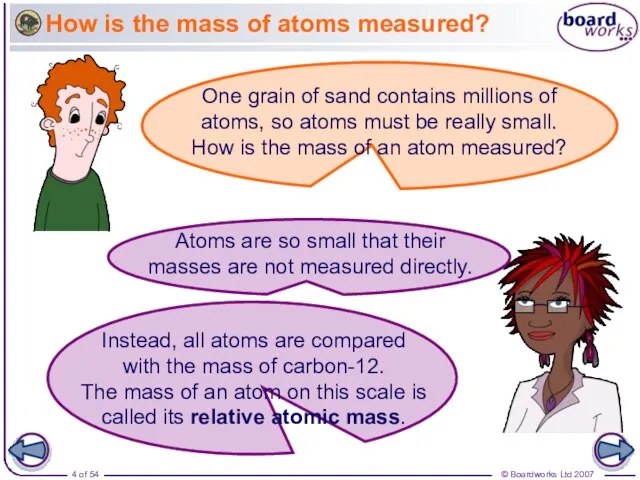
- 4. How is the mass of atoms measured?
- 5. What is relative atomic mass?
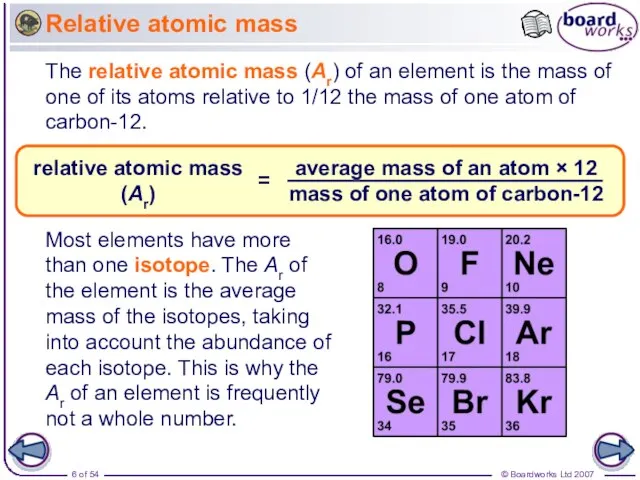
- 6. Relative atomic mass The relative atomic mass (Ar) of an element is the mass of one
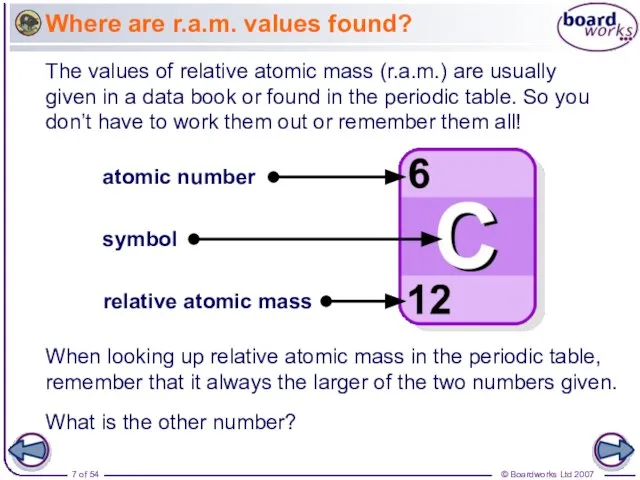
- 7. Where are r.a.m. values found? The values of relative atomic mass (r.a.m.) are usually given in
- 8. Identifying relative atomic mass
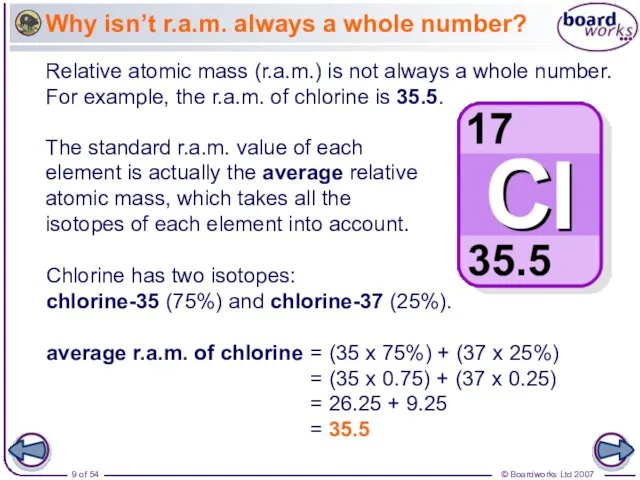
- 9. Why isn’t r.a.m. always a whole number? Relative atomic mass (r.a.m.) is not always a whole
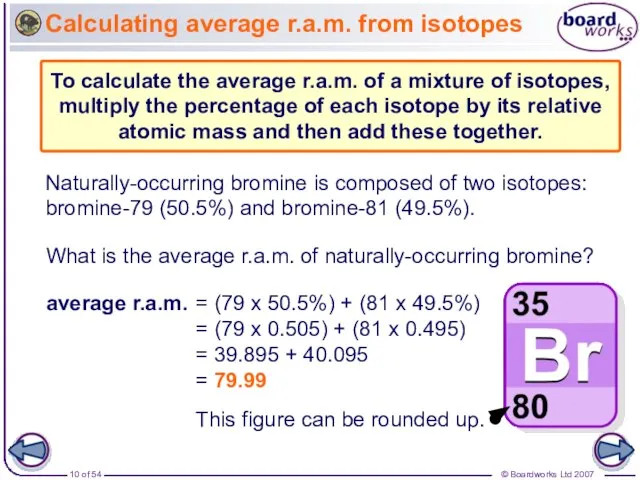
- 10. Calculating average r.a.m. from isotopes What is the average r.a.m. of naturally-occurring bromine? Naturally-occurring bromine is
- 11. What about the mass of compounds?
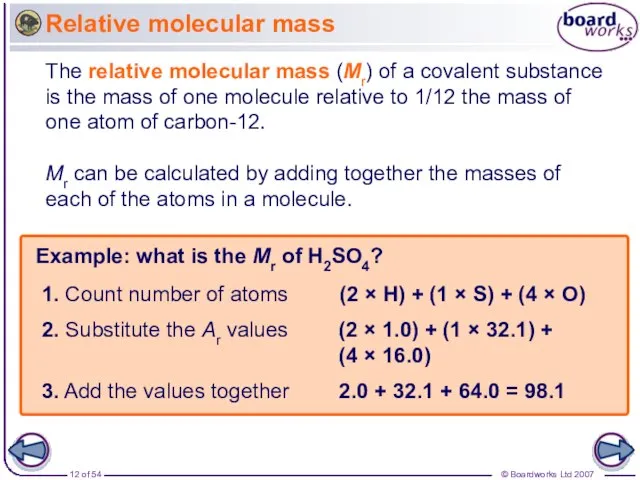
- 12. Relative molecular mass Example: what is the Mr of H2SO4? (2 × H) + (1 ×
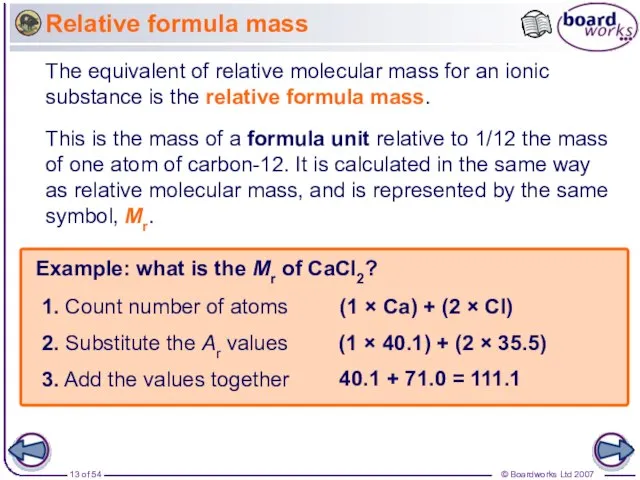
- 13. Relative formula mass The equivalent of relative molecular mass for an ionic substance is the relative
- 14. Calculating relative formula mass
- 15. Relative atomic mass – true or false?
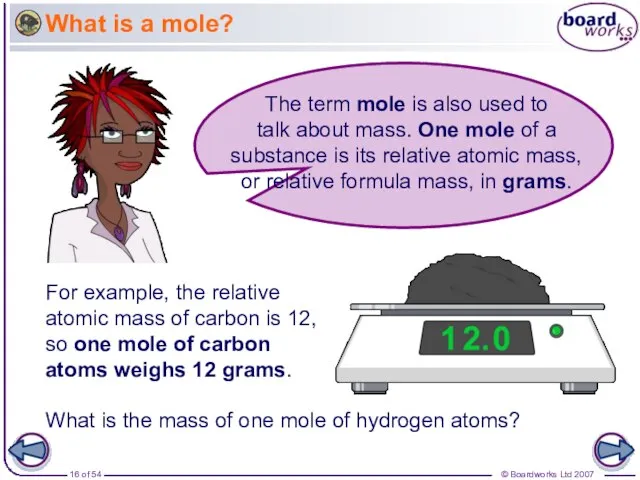
- 16. What is a mole? For example, the relative atomic mass of carbon is 12, so one
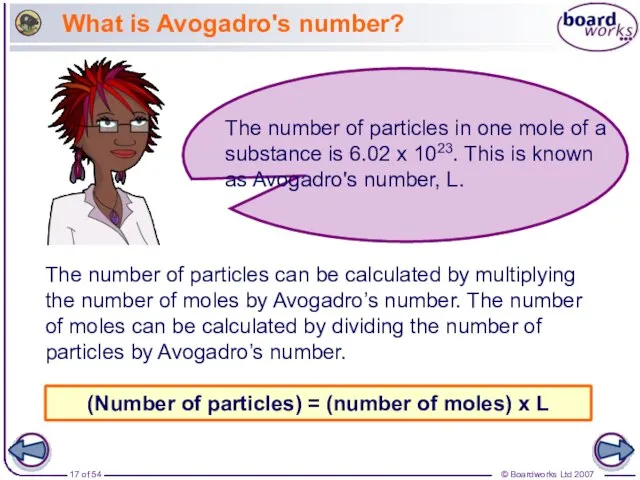
- 17. What is Avogadro's number? The number of particles can be calculated by multiplying the number of
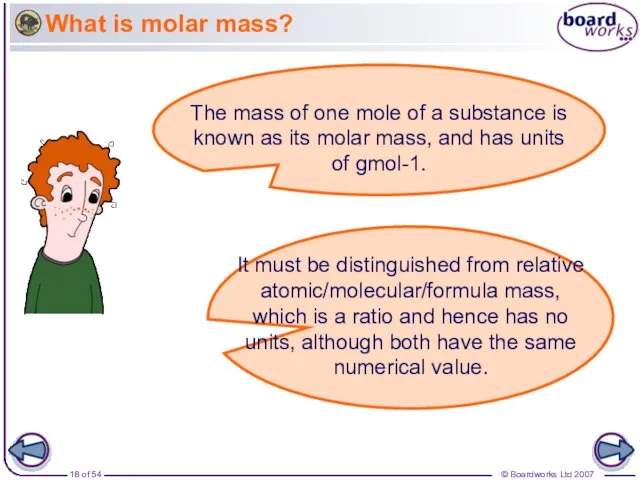
- 18. What is molar mass? The mass of one mole of a substance is known as its
- 19. What is the mass of one mole?
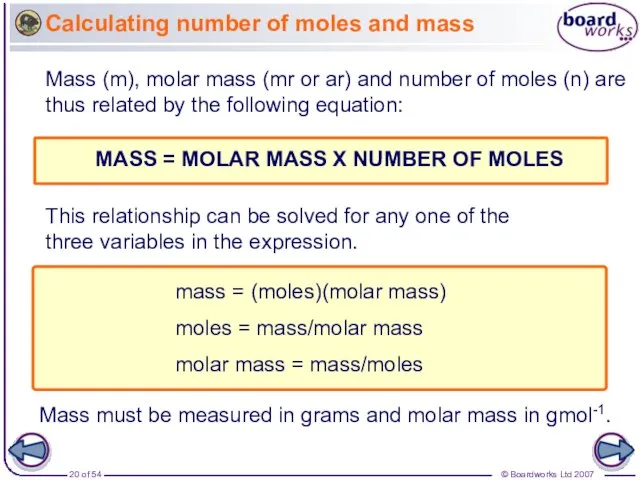
- 20. Calculating number of moles and mass Mass (m), molar mass (mr or ar) and number of
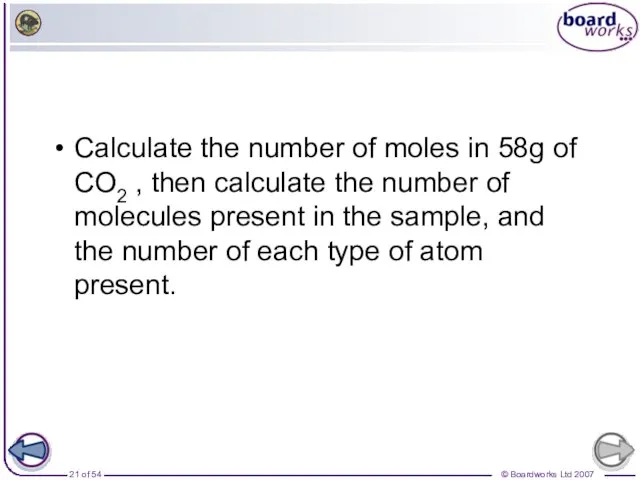
- 21. Calculate the number of moles in 58g of CO2 , then calculate the number of molecules
- 22. How can we predict the amount of substance that will be created in a reaction?
- 23. In the reaction 2 Mg + O2 MgO How much MgO will be produced if we
- 24. Glossary (1/2) relative atomic mass – The mass of one of its atoms relative to 1/12
- 26. Скачать презентацию







 Презентация на тему Электролиты
Презентация на тему Электролиты Поздравляем вас, наши любимые мужчины!
Поздравляем вас, наши любимые мужчины! Баш3орт милли аштары
Баш3орт милли аштары История ислама
История ислама «Товаропроводящая цепочка фармацевтических препаратов. Где тонко, там и рвется (а где тонко?)».
«Товаропроводящая цепочка фармацевтических препаратов. Где тонко, там и рвется (а где тонко?)». Основные концепции культуры
Основные концепции культуры Декоративно- прикладное творчество народов Поволжья
Декоративно- прикладное творчество народов Поволжья Синхронный электродвигатель
Синхронный электродвигатель Мероприятия по энергосбережению в электрических сетях электроосвещения объектов капитального строительства
Мероприятия по энергосбережению в электрических сетях электроосвещения объектов капитального строительства КОНСТРУИРОВАНИЕ
КОНСТРУИРОВАНИЕ Зона смешанных и широколиственных лесов 8 класс
Зона смешанных и широколиственных лесов 8 класс Выставка марийских национальных костюмов
Выставка марийских национальных костюмов Особенности празднования Нового года в России
Особенности празднования Нового года в России Детская площадка
Детская площадка The system of State bodies of India
The system of State bodies of India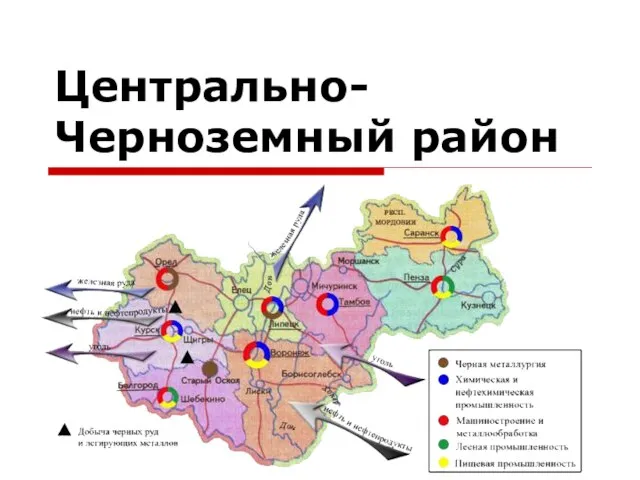 Центрально-Черноземный район
Центрально-Черноземный район Повторение. Чтение текста с изученными буквами
Повторение. Чтение текста с изученными буквами Конституционное право. Своя игра
Конституционное право. Своя игра Холодная прокатка труб (ХПТ).Краснов Денис
Холодная прокатка труб (ХПТ).Краснов Денис От кого же, мы всё же произошли?
От кого же, мы всё же произошли? Презентация_32
Презентация_32 Государственное общеобразовательное учреждение средняя общеобразовательная школа № 28 Василеостровского района
Государственное общеобразовательное учреждение средняя общеобразовательная школа № 28 Василеостровского района Туесок из стружки с геометрической резьбой
Туесок из стружки с геометрической резьбой Бизнес Мечты!
Бизнес Мечты! Typológia 3 - Letiská
Typológia 3 - Letiská Готовимся к
Готовимся к Апельсиновое настроение. Рисуем апельсины. Декоративный натюрморт в технике масляной живописи
Апельсиновое настроение. Рисуем апельсины. Декоративный натюрморт в технике масляной живописи Выпуск Эльгяйской СОШ 1972 года
Выпуск Эльгяйской СОШ 1972 года