Содержание
- 2. Goal The goal of this tutorial is to provide a background in cryogenics suitable for workers
- 3. Outline Part 1: Catching Cold Introduction To Cryogenics Basic refrigeration processes Isenthalpic (Joule-Thomson) Isentropic expansion Carnot
- 4. Outline Part 2: Keeping Cold Cryogenic Safety Oxygen Deficiency Hazards Pressure safety High Level Guidelines Cryostats
- 5. What is Cryogenics ? Cryogenics is the science & engineering of phenomena that occur at temperatures
- 6. Some Examples
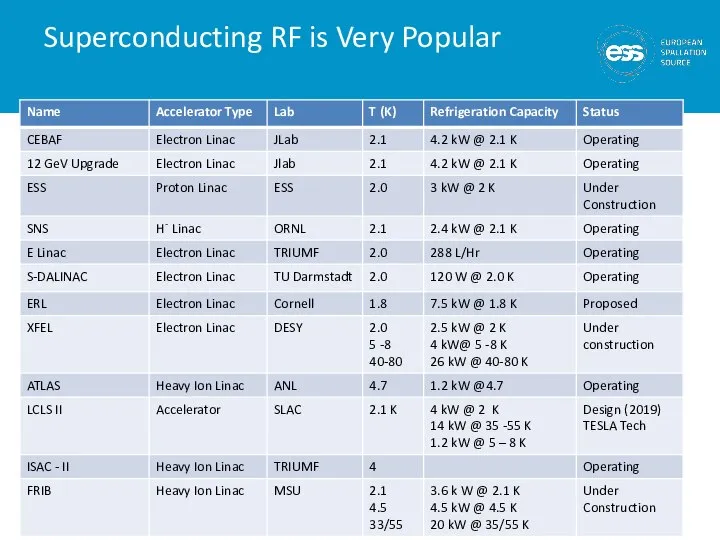
- 7. Superconducting RF is Very Popular
- 8. Catching Cold Before we get involved in thermodynamic cycles, let’s go over the basics There are
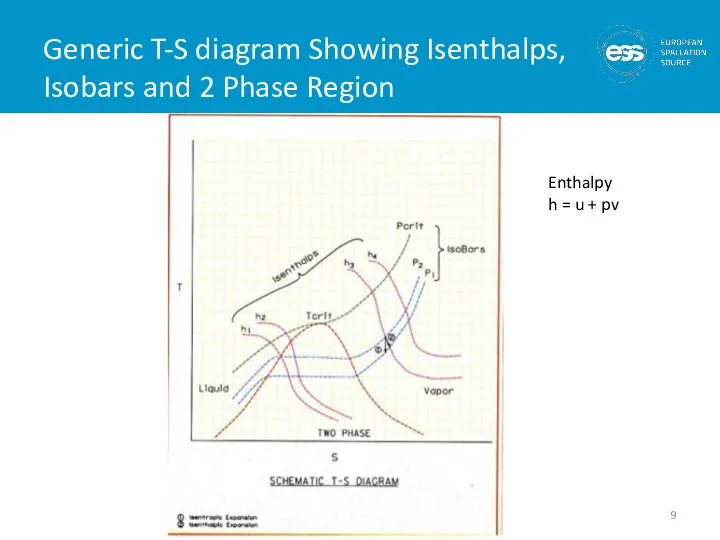
- 9. Generic T-S diagram Showing Isenthalps, Isobars and 2 Phase Region Enthalpy h = u + pv
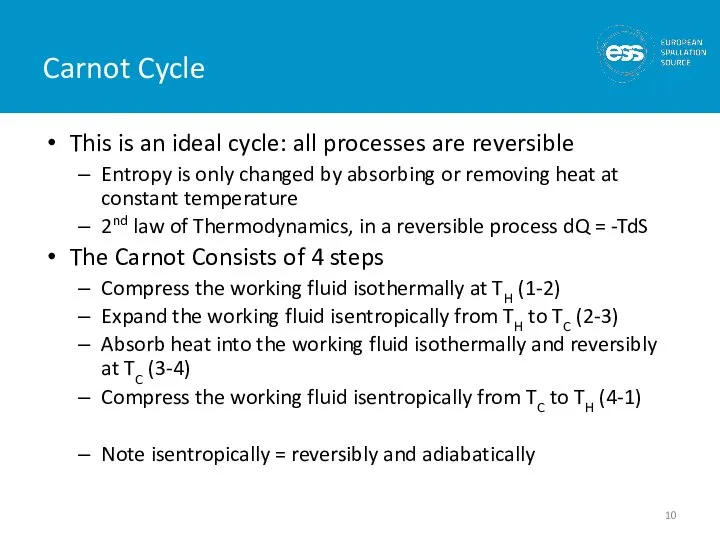
- 10. Carnot Cycle This is an ideal cycle: all processes are reversible Entropy is only changed by
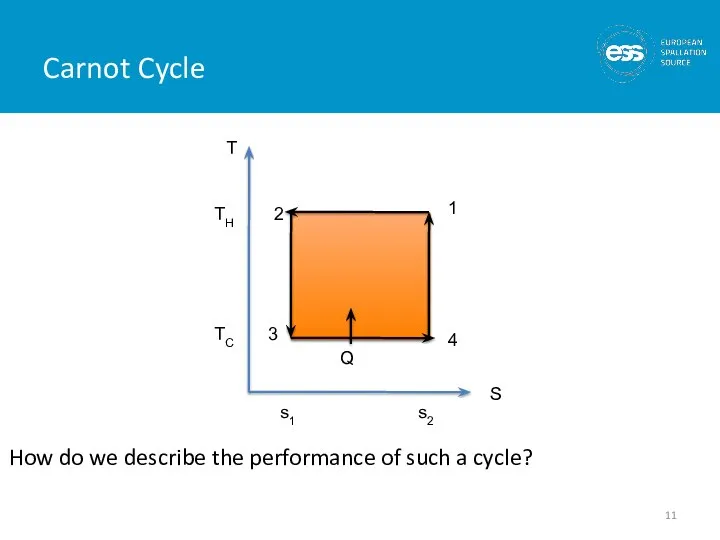
- 11. Carnot Cycle How do we describe the performance of such a cycle?
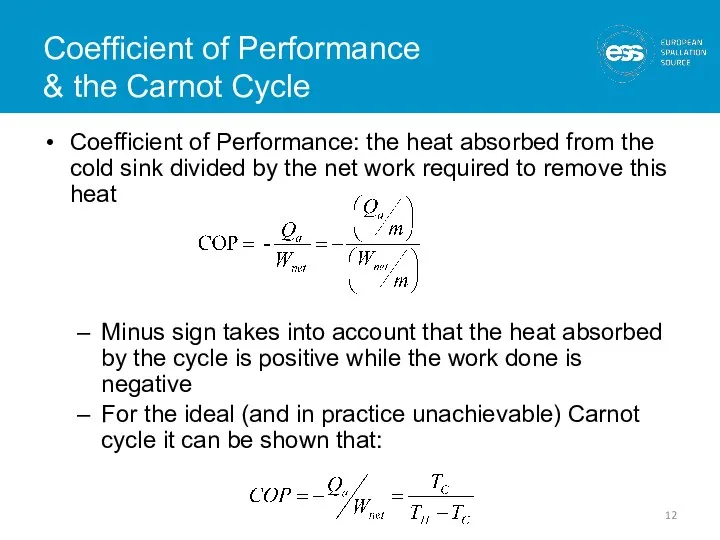
- 12. Coefficient of Performance & the Carnot Cycle Coefficient of Performance: the heat absorbed from the cold
- 13. Coefficient of Performance & the Carnot Cycle For a plant operating between room 300 K and
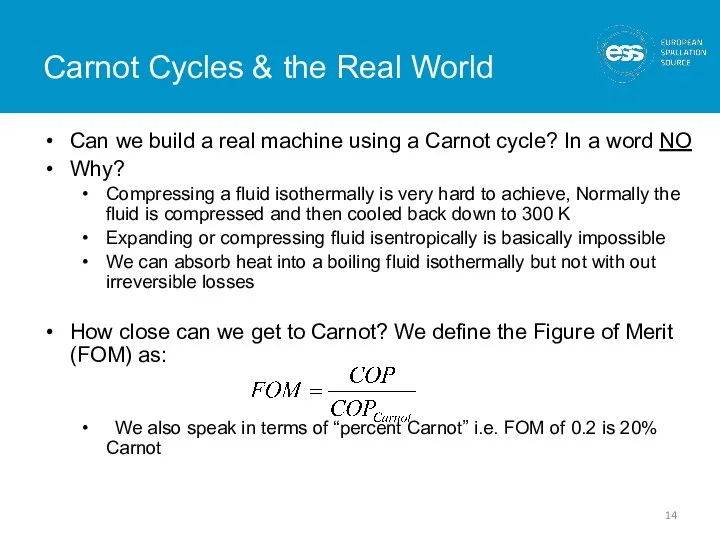
- 14. Carnot Cycles & the Real World Can we build a real machine using a Carnot cycle?
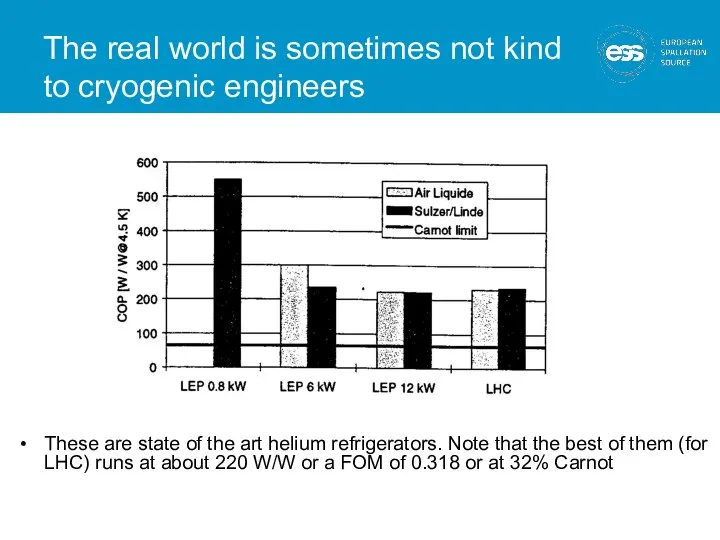
- 15. The real world is sometimes not kind to cryogenic engineers These are state of the art
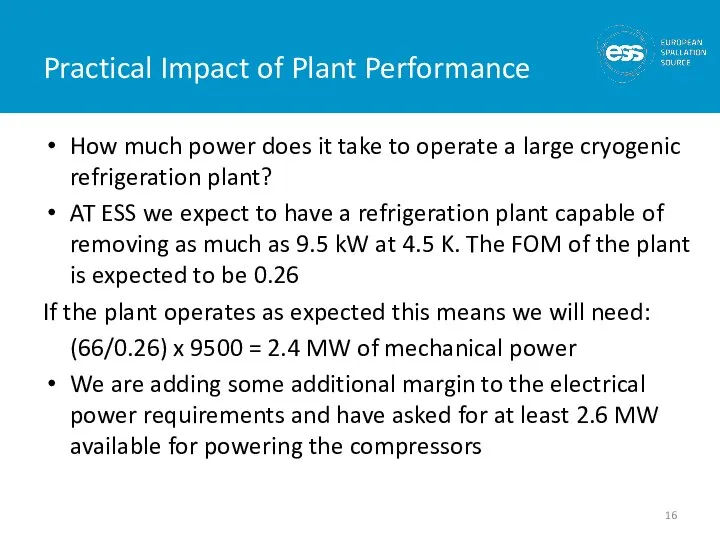
- 16. Practical Impact of Plant Performance How much power does it take to operate a large cryogenic
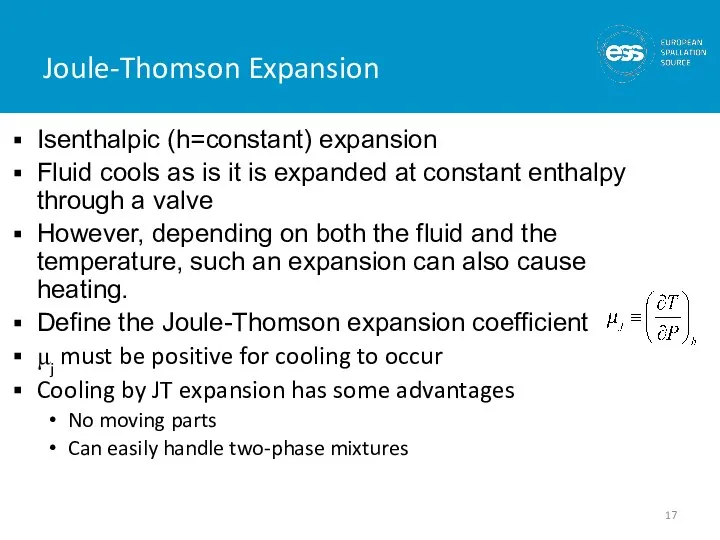
- 17. Joule-Thomson Expansion Isenthalpic (h=constant) expansion Fluid cools as is it is expanded at constant enthalpy through
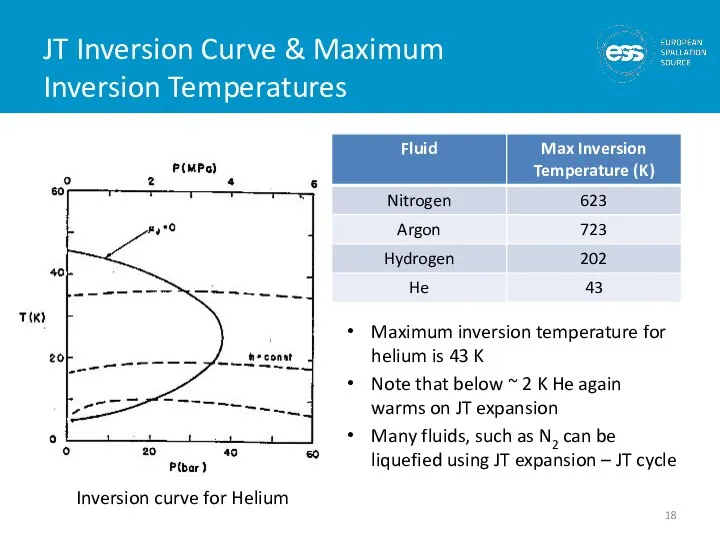
- 18. JT Inversion Curve & Maximum Inversion Temperatures Maximum inversion temperature for helium is 43 K Note
- 19. Practical Large Scale Helium Refrigerators Modern large scale Helium refrigerators/liquefiers use a variation of the Claude
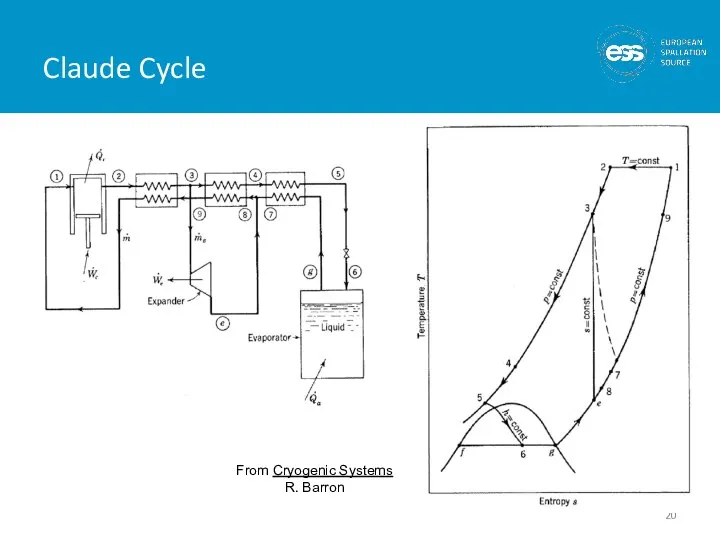
- 20. Claude Cycle From Cryogenic Systems R. Barron
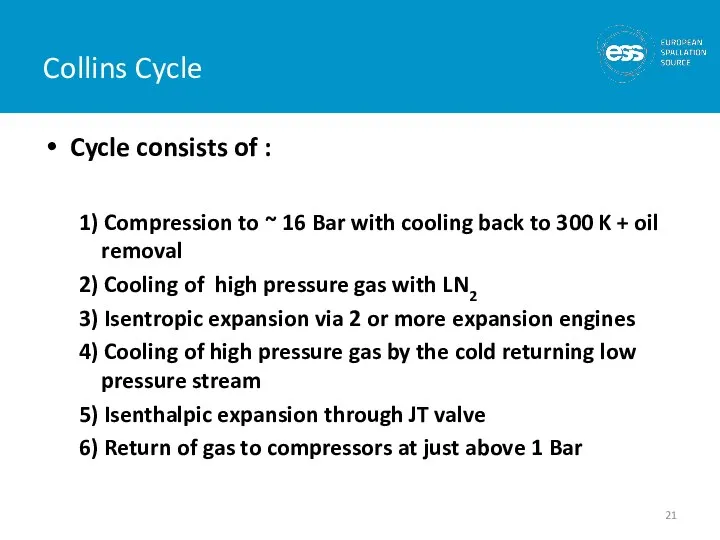
- 21. Cycle consists of : 1) Compression to ~ 16 Bar with cooling back to 300 K
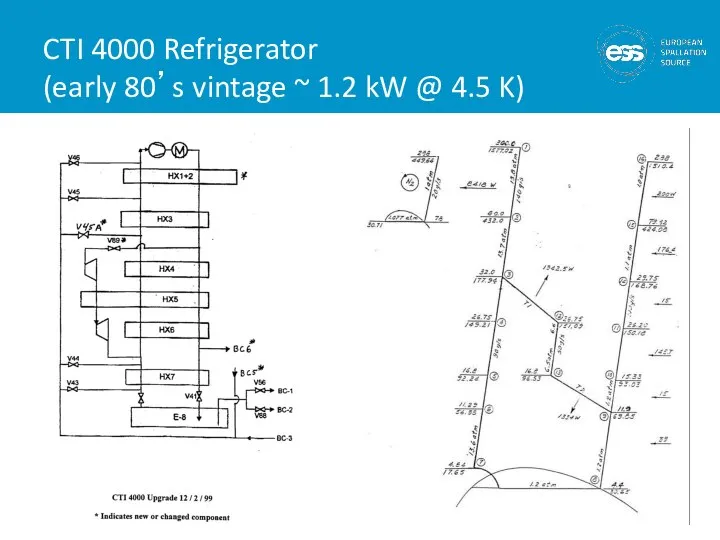
- 22. CTI 4000 Refrigerator (early 80’s vintage ~ 1.2 kW @ 4.5 K)
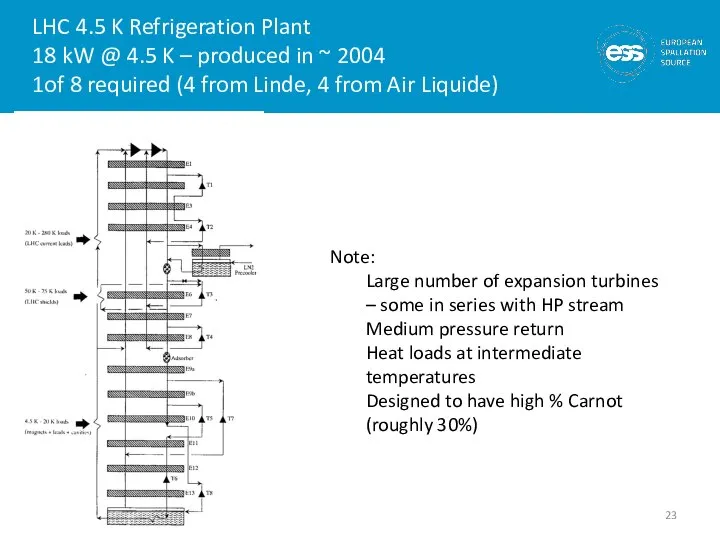
- 23. LHC 4.5 K Refrigeration Plant 18 kW @ 4.5 K – produced in ~ 2004 1of
- 24. Refrigerators vs. Liquefiers Refrigerators are closed cycle systems They provide cooling and can create liquids but
- 25. Refrigerators vs. Liquefiers In practice, this distinction is less clear cut Modern cryogenic plants can operate
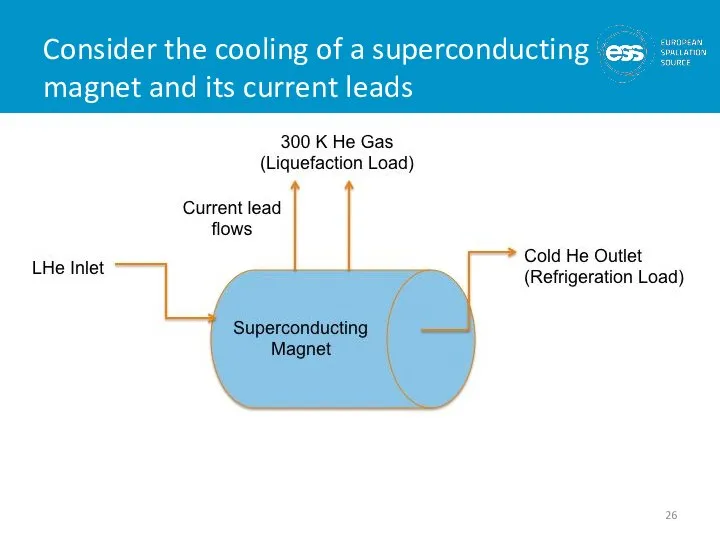
- 26. Consider the cooling of a superconducting magnet and its current leads
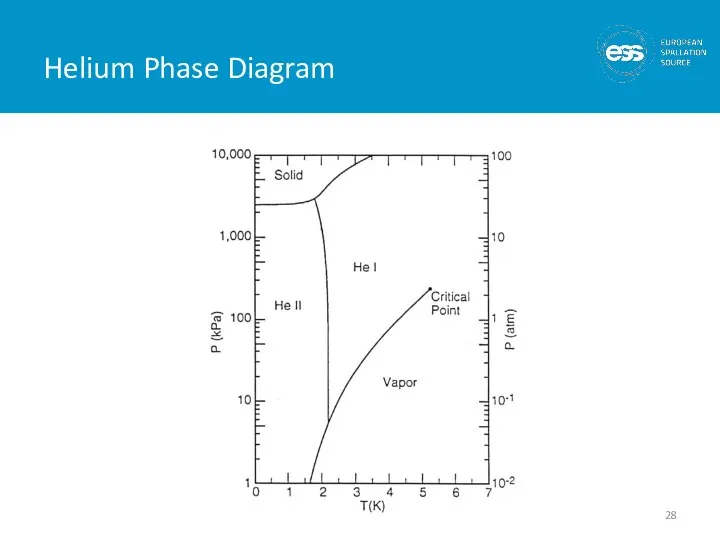
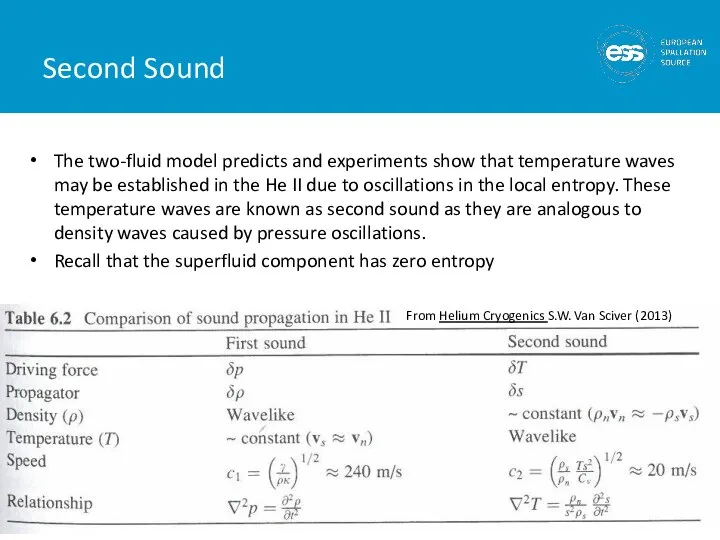
- 27. He II (Superfluid Helium) 2nd liquid phase of helium (hence He II) Phase transition is 2nd
- 28. Helium Phase Diagram
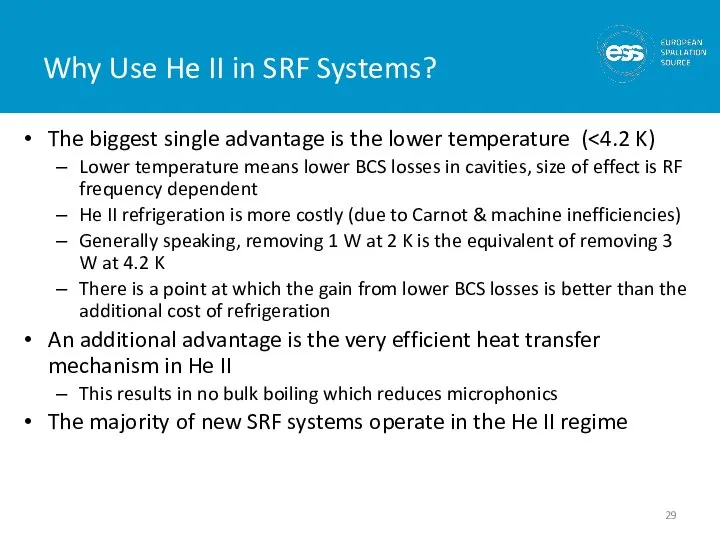
- 29. Why Use He II in SRF Systems? The biggest single advantage is the lower temperature (
- 30. What is He II ? A “Bose – Einstein like” Condensate A fraction of atoms in
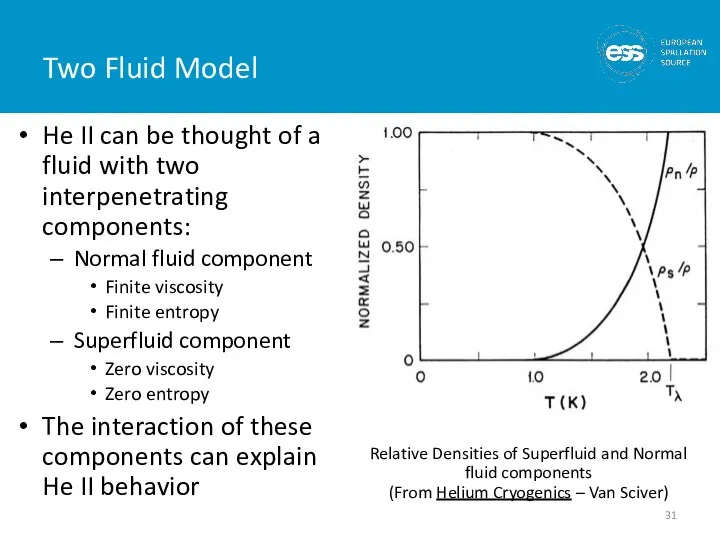
- 31. Two Fluid Model He II can be thought of a fluid with two interpenetrating components: Normal
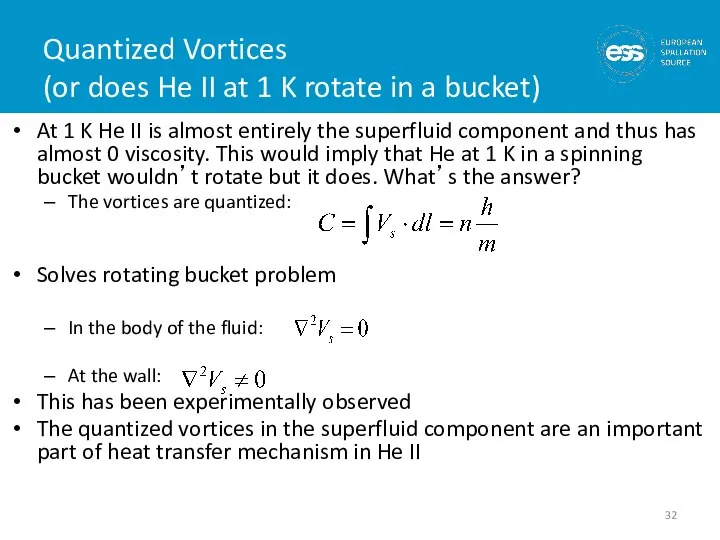
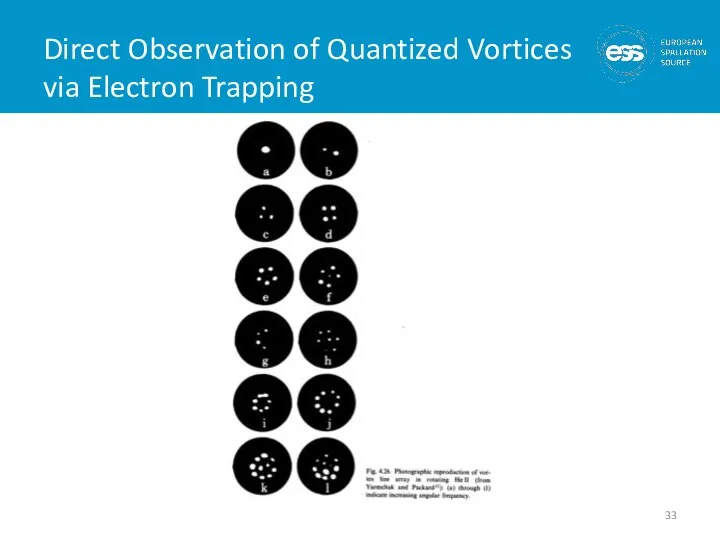
- 32. Quantized Vortices (or does He II at 1 K rotate in a bucket) At 1 K
- 33. Direct Observation of Quantized Vortices via Electron Trapping
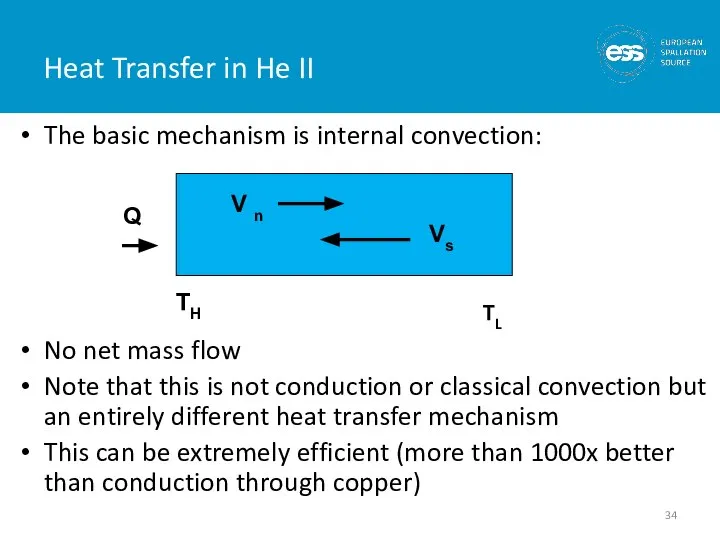
- 34. Heat Transfer in He II The basic mechanism is internal convection: No net mass flow Note
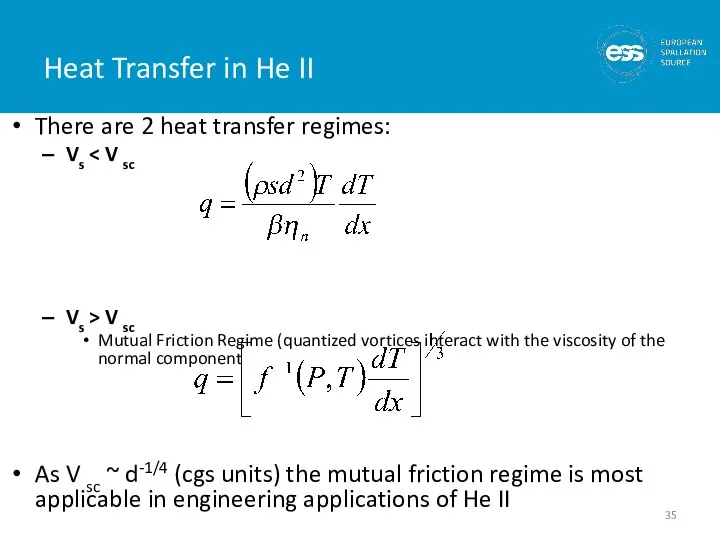
- 35. Heat Transfer in He II There are 2 heat transfer regimes: Vs Vs > V sc
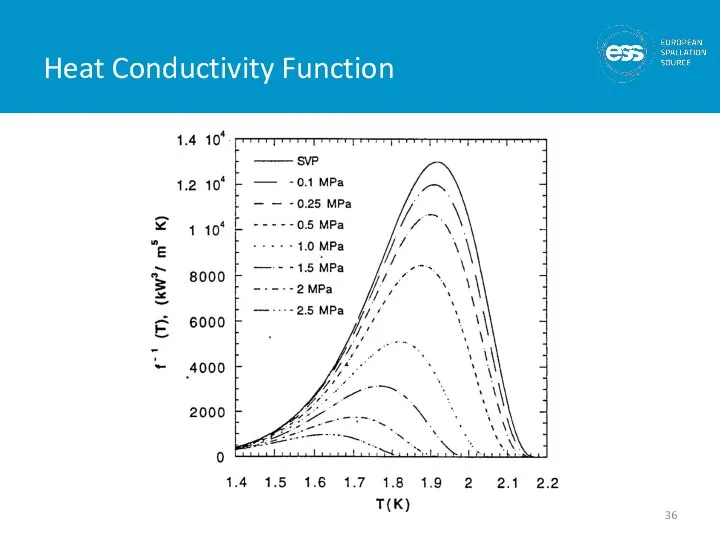
- 36. Heat Conductivity Function
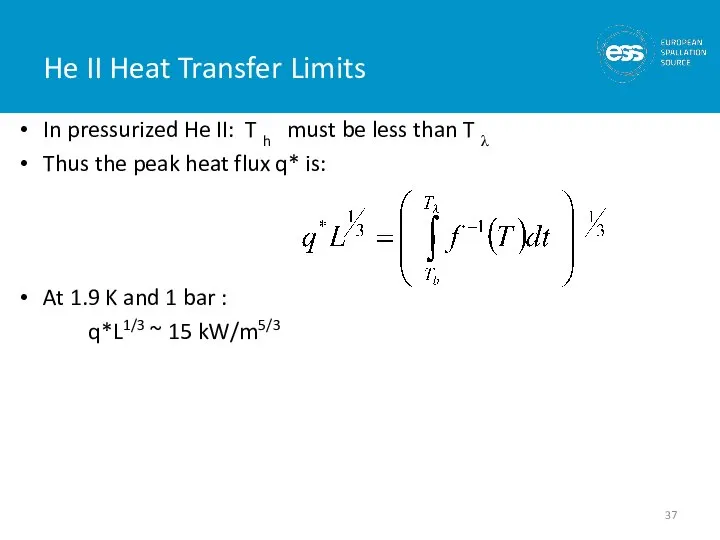
- 37. He II Heat Transfer Limits In pressurized He II: T h must be less than T
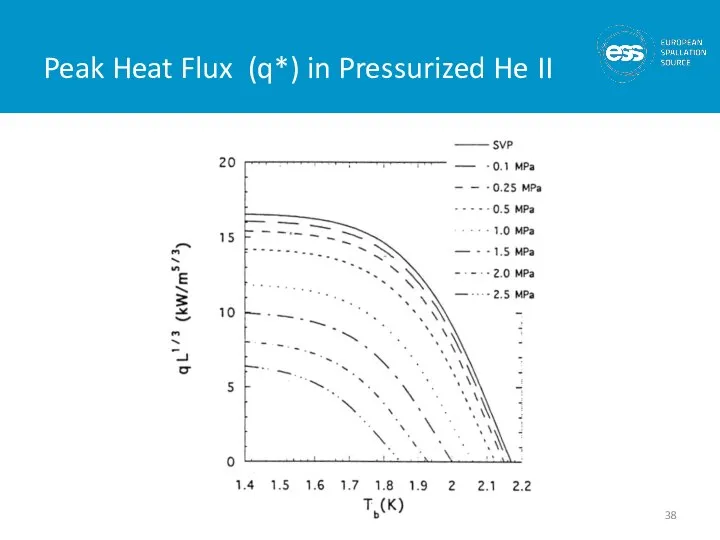
- 38. Peak Heat Flux (q*) in Pressurized He II
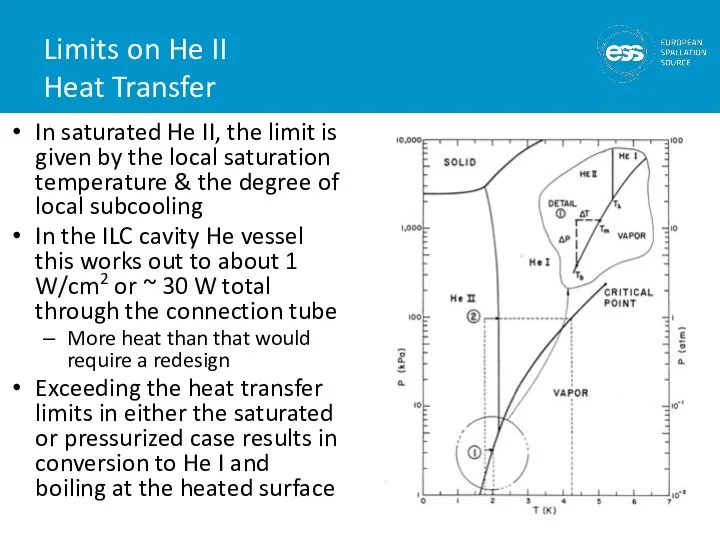
- 39. Limits on He II Heat Transfer In saturated He II, the limit is given by the
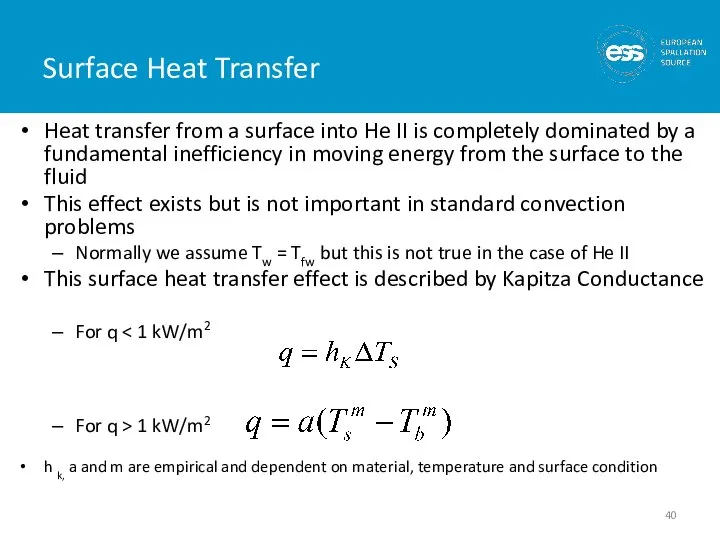
- 40. Surface Heat Transfer Heat transfer from a surface into He II is completely dominated by a
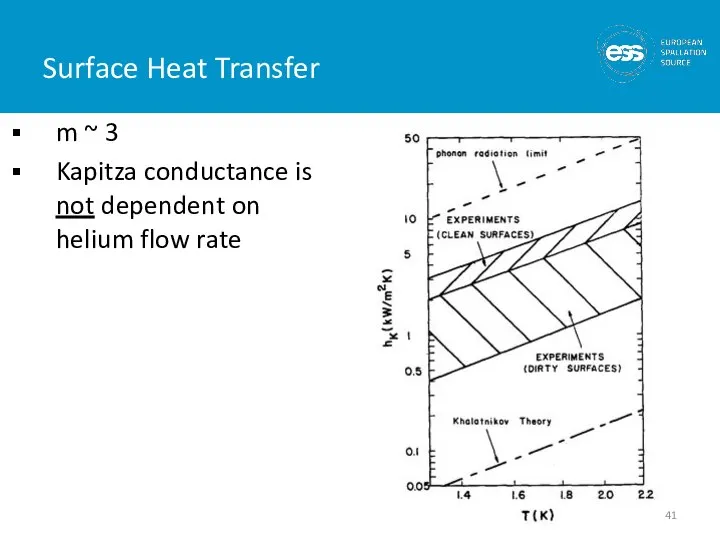
- 41. Surface Heat Transfer m ~ 3 Kapitza conductance is not dependent on helium flow rate
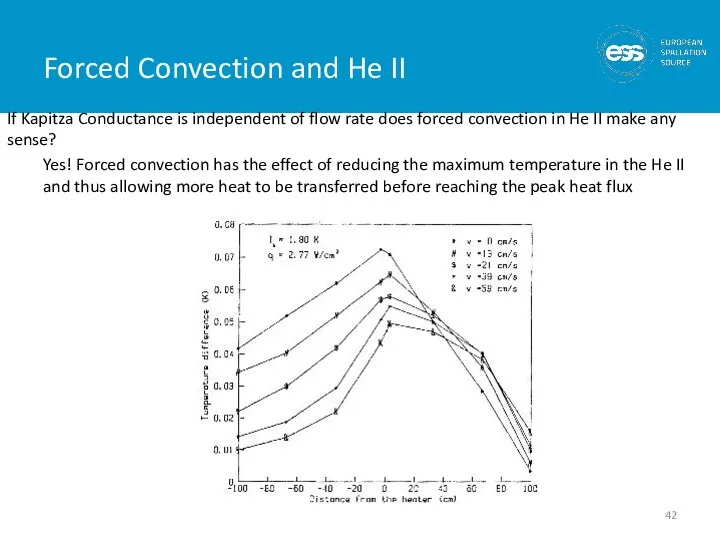
- 42. Forced Convection and He II If Kapitza Conductance is independent of flow rate does forced convection
- 43. He II Fluid Dynamics Despite the presence of the superfluid component, in almost all engineering applications
- 44. He II Fluid Dynamics He II does behave differently in cases of: Film flow Porous plugs
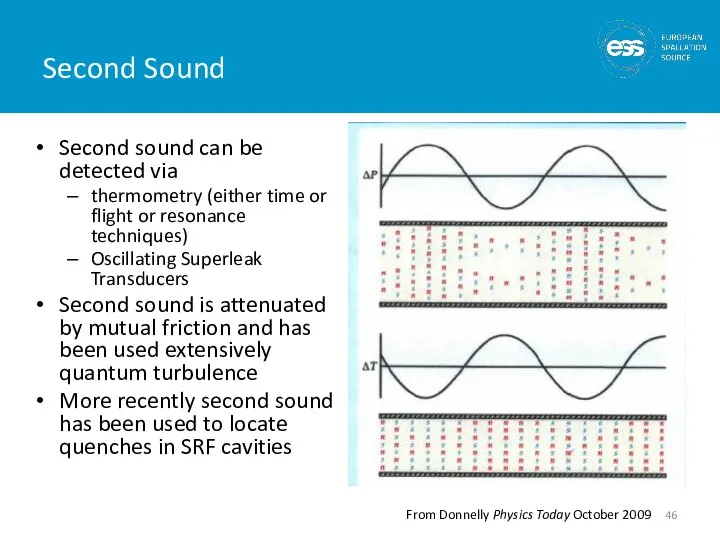
- 45. Second Sound The two-fluid model predicts and experiments show that temperature waves may be established in
- 46. Second Sound Second sound can be detected via thermometry (either time or flight or resonance techniques)
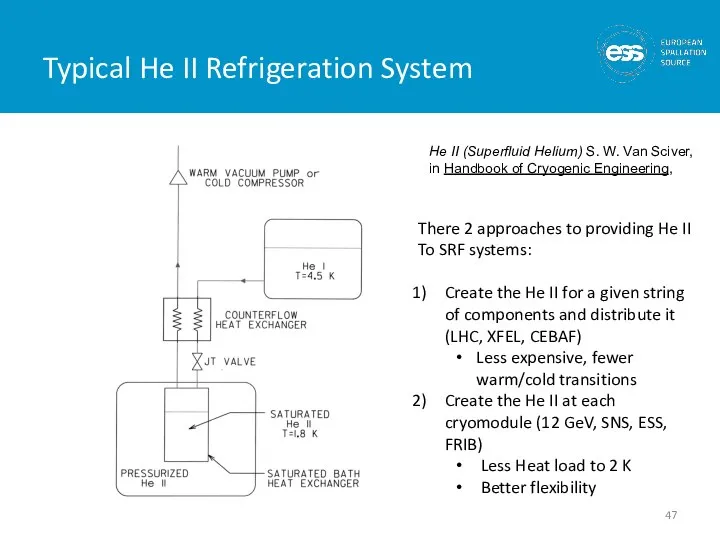
- 47. Typical He II Refrigeration System He II (Superfluid Helium) S. W. Van Sciver, in Handbook of
- 49. Скачать презентацию







 Положение о системе общественного наблюдения при проведении государственной (итоговой) аттестации обучающихся, освоивших образ
Положение о системе общественного наблюдения при проведении государственной (итоговой) аттестации обучающихся, освоивших образ Употребление многозначных глаголов в речи (6 класс)
Употребление многозначных глаголов в речи (6 класс) Методический материал к уроку русского языка в 6 классе на тему: «Фразеологизмы».
Методический материал к уроку русского языка в 6 классе на тему: «Фразеологизмы». Западная философия 20-го века
Западная философия 20-го века Лот 5, г. Хабаровск, ул. Сысоева, 21, кв. 14
Лот 5, г. Хабаровск, ул. Сысоева, 21, кв. 14 Барометрия. Задачи барометрии
Барометрия. Задачи барометрии Прямоугольный параллелепипед. Куб (5 класс)
Прямоугольный параллелепипед. Куб (5 класс) Образ современного подростка в произведениях А. Иванова «Географ глобус пропил» и «Общага-на-крови»
Образ современного подростка в произведениях А. Иванова «Географ глобус пропил» и «Общага-на-крови» Финансовая компания Автомайн
Финансовая компания Автомайн Энциклопедия термина число
Энциклопедия термина число Своя игра
Своя игра Формування системи документування господарських операцій та документообігу (Тема №6). Організація документообігу на підприємстві (Лекція
Формування системи документування господарських операцій та документообігу (Тема №6). Організація документообігу на підприємстві (Лекція Лекция №2
Лекция №2 Розроблення технології пасти із насінням соняшника з підвищеною біологічною цінністю для людей конституції типу Пітта-Доша
Розроблення технології пасти із насінням соняшника з підвищеною біологічною цінністю для людей конституції типу Пітта-Доша Удельное сопротивление
Удельное сопротивление Народная сказка: история происхождения и её герои
Народная сказка: история происхождения и её герои Описание рекламных мест
Описание рекламных мест Типология организационных культур Герта Хофстеда
Типология организационных культур Герта Хофстеда Общая физическая подготовка на занятиях по волейболу
Общая физическая подготовка на занятиях по волейболу Значение мяса в питании человека. Виды мяса. Требования к качеству
Значение мяса в питании человека. Виды мяса. Требования к качеству КАСКО – Профи
КАСКО – Профи Socialization
Socialization Презентация по обществознанию
Презентация по обществознанию Формирование функции голосообразования у младших школьников в условиях школы второго вида
Формирование функции голосообразования у младших школьников в условиях школы второго вида Общая схема МЭМС. МЭМС с 3-х аксиальными гироскопами и акселерометрами различных компаний
Общая схема МЭМС. МЭМС с 3-х аксиальными гироскопами и акселерометрами различных компаний Зарядка для глаз
Зарядка для глаз Модель внимания Сергея Леонидовича Рубинштейна
Модель внимания Сергея Леонидовича Рубинштейна Проблемы экологии в России и Великобритании в 21 веке Андрианова Н.Г.
Проблемы экологии в России и Великобритании в 21 веке Андрианова Н.Г.