Содержание
- 2. * Deposition of Silicon Nitrides Stoichiometric silicon nitride (Si3N4) is deposited at atmospheric pressure by reacting
- 3. * Deposition of Silicon Nitrides CVD and LPCVD silicon nitride films generally exhibit large tensile stresses
- 4. * Deposition of Silicon Nitrides For deposition below 400ºC, nonstoichiometric silicon nitride (SixNy) is obtained by
- 5. * Deposition of Silicon Nitrides A high value in the range is indicative of excess silicon,
- 6. * Spin-On Methods Spin-on is a process to put down layers of dielectric insulators and organic
- 7. * Photoresists and polyimides are common organic materials that can be spun on a wafer with
- 8. * Spin-On Methods Thick (5–100 µm) spin-on glass (SOG) has the ability to uniformly coat surfaces
- 9. * There are two basic types of SOG: siloxane-based organic SOG and silicate-based inorganic SOG. Spin
- 10. * A siloxane A siloxane is any chemical compound composed of units of the form R2SiSiOSiO,
- 11. * Silicate-based SOG
- 12. * Lithography The mask itself consists of a patterned opaque chromium (the most common), emulsion, or
- 13. * Soda-lime glass Soda-lime glass, also called soda-lime-silica glass, is the most prevalent type of glass,
- 14. Soda-lime glass is divided technically into glass used for windows, called flat glass, and glass for
- 15. * Coefficient of restitution (glass sphere vs. glass wall): 0.97 ± 0.01 Thermal conductivity: 0.9-1.3 W/m.K
- 16. * Lime is a general term for calcium is a general term for calcium-containing inorganic materials
- 17. * Lithography Positive photoresist is an organic resin material containing a sensitizer. It is spin-coated on
- 18. The sensitizer prevents the dissolution of unexposed resist during immersion in the developer solution. Exposure to
- 19. * Lithography Resolution, defined as the minimum feature the optical system can resolve, is seldom a
- 20. * Lithography Depth of focus, however, is amore severe constraint on lithography, especially in light of
- 21. * Projection lithography is clearly a superior approach, but an optical projection system can cost significantly
- 22. * Thick Resist Patterned thick resist is normally used as a protective masking layer for the
- 23. * Thick Resist Exposing resist thicker than 5 µm often degrades the minimum resolvable feature size
- 24. * Topographical Height Variations Changes in topography on the surface of the wafer, such as deep
- 25. * Topographical Height Variations Exposing a pattern on a surface with height variations in excess of
- 26. * Topographical Height Variations
- 27. * Double-Sided Lithography Often, lithographic patterns on both sides of a wafer need to be aligned
- 28. * Double-Sided Lithography
- 29. * ЭМ-5086 УСТАНОВКА ДВУСТОРОННЕГО СОВМЕЩЕНИЯ И ЭКСПОНИРОВАНИЯ ЗНАКОВ СОВМЕЩЕНИЯ Установка предназначена для нанесения знаков совмещения на
- 30. *
- 31. *
- 32. * ЭМ-5026Б УСТАНОВКА ДВУСТОРОННЕГО СОВМЕЩЕНИЯ И ЭКСПОНИРОВАНИЯ Установка ЭМ–5026Б выполняет контактным (в зазоре) способом экспонирование верхней
- 33. *
- 34. *
- 35. * Large Field of View (Большая область экспонирования) The field of view is the extent of
- 36. * In projection systems, the field of view is often less than 1 × 1cm2. The
- 37. * Etching
- 38. * Оборудование фирмы SCR (Чехия) участка химического травления кремниевых пластин
- 39. * Isotropic Wet Etching The most common group of silicon isotropic wet etchants is HNA, also
- 40. * Isotropic and Anisotropic Etching
- 41. * Anisotropic wet etchants Anisotropic wet etchants are also known as orientation-dependent etchants (ODEs) because their
- 42. * Anisotropic Wet Etching
- 43. * KOH is by far the most common ODE Etch rates are typically given in the
- 45. Скачать презентацию
Слайд 2*
Deposition of Silicon Nitrides
Stoichiometric silicon nitride (Si3N4) is deposited at atmospheric pressure
*
Deposition of Silicon Nitrides
Stoichiometric silicon nitride (Si3N4) is deposited at atmospheric pressure

The deposition temperature for either method is between 700º and 900ºC. Both reactions generate hydrogen as a byproduct, some of which is incorporated in the deposited film.
Слайд 3*
Deposition of Silicon Nitrides
CVD and LPCVD silicon nitride films generally exhibit large
*
Deposition of Silicon Nitrides
CVD and LPCVD silicon nitride films generally exhibit large

However, if LPCVD silicon nitride is deposited at 800º–850ºC and is silicon-rich (an excess of silicon in the film) due to a greatly increased dichlorosilane flow rate, the stress can be below 100 MPa—a level acceptable for most micromachining applications.
Стехиометрия (от др.-греч. (от др.-греч. στοιχειον «элемент» + μετρειν «измерять») — раздел химии (от др.-греч. στοιχειον «элемент» + μετρειν «измерять») — раздел химии о соотношениях реагентов в химических реакциях.
Позволяет теоретически вычислять необходимые массы и объёмы реагентов.
Слайд 4*
Deposition of Silicon Nitrides
For deposition below 400ºC, nonstoichiometric silicon nitride (SixNy) is
*
Deposition of Silicon Nitrides
For deposition below 400ºC, nonstoichiometric silicon nitride (SixNy) is

Hydrogen is also a by product of this reaction and is incorporated in elevated concentrations (20%–25%) in the film.
The refractive index is an indirect measure of the stoichiometry of the silicon nitride film. The refractive index for stoichiometric LPCVD silicon nitride is 2.01 and ranges between 1.8 and 2.5 for PECVD films.
Слайд 5*
Deposition of Silicon Nitrides
A high value in the range is indicative of
*
Deposition of Silicon Nitrides
A high value in the range is indicative of

One of the key advantages of PECVD nitride is the ability to control stress during deposition.
Silicon nitride deposited at a plasma excitation frequency of 13.56 MHz exhibits tensile stress of about 400 MPa, whereas a film deposited at a frequency of 50 kHz has a compressive stress of 200 MPa. By alternating frequencies during deposition, one may obtain lower-stress films.
Слайд 6*
Spin-On Methods
Spin-on is a process to put down layers of dielectric insulators
*
Spin-On Methods
Spin-on is a process to put down layers of dielectric insulators

The equipment is simple, requiring a variable-speed spinning table with appropriate safety screens.
A nozzle dispenses the material as a liquid solution in the center of the wafer. Spinning the substrate at speeds of 500 to 5000 rpm for 30 to 60 seconds spreads the material to a uniform thickness.
Слайд 7*
Photoresists and polyimides are common organic materials that can be spun on
*
Photoresists and polyimides are common organic materials that can be spun on

The organic polymer is normally in suspension in a solvent solution; subsequent baking causes the solvent to evaporate, forming a firm film.
Spin-On Methods
Слайд 8*
Spin-On Methods
Thick (5–100 µm) spin-on glass (SOG) has the ability to uniformly
*
Spin-On Methods
Thick (5–100 µm) spin-on glass (SOG) has the ability to uniformly

Thin (0.1–0.5 µm) SOG was heavily investigated in the integrated circuit industry as an interlayer dielectric between metals for high-speed electrical interconnects; however, its electrical properties are considered poor compared to thermal or CVD silicon oxides.
Spin-on glass is commercially available in different forms, commonly siloxane- or silicate-based. The latter type allows water absorption into the film, resulting in a higher relative dielectric constant and a tendency to crack.
After deposition, the layer is typically densified at a temperature between 300º and 500ºC.
Measured film stress is approximately 200 MPa in tension but decreases substantially with increasing anneal temperatures.
Слайд 9*
There are two basic types of SOG: siloxane-based organic SOG and silicate-based
*
There are two basic types of SOG: siloxane-based organic SOG and silicate-based

Spin on glass (SOG) is a mixture of SiO2 and dopants (either boron or phosphorous) that is suspended in a solvent solution. The SOG is applied to a clean silicon wafer by spin-coating just like photoresist.
Слайд 10*
A siloxane
A siloxane is any chemical compound composed of units of the
*
A siloxane
A siloxane is any chemical compound composed of units of the

An examples are: [SiO(CH3)2]n (polydimethylsiloxane)
and [SiO(C6H5)2]n (polydiphenylsiloxane).
Слайд 11*
Silicate-based SOG
*
Silicate-based SOG
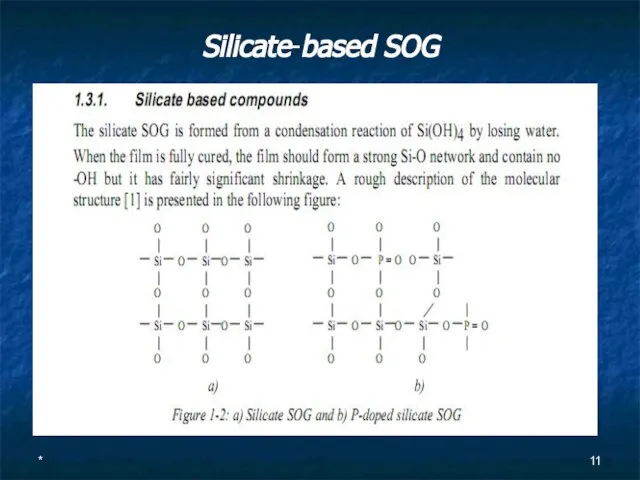
Слайд 12*
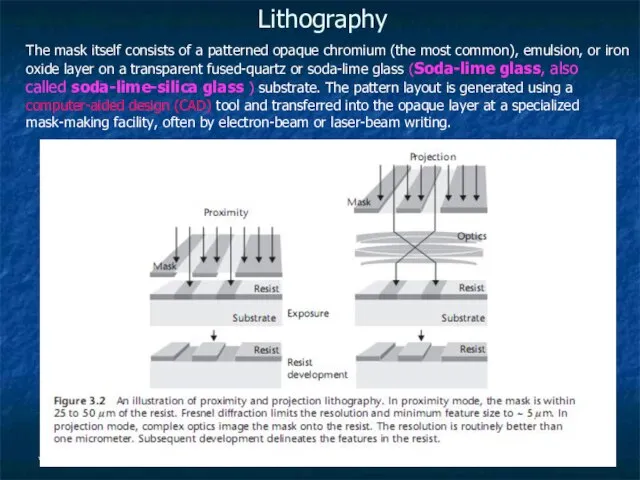
Lithography
The mask itself consists of a patterned opaque chromium (the most common),
*
Lithography
The mask itself consists of a patterned opaque chromium (the most common),
Слайд 13*
Soda-lime glass
Soda-lime glass, also called soda-lime-silica glass, is the most prevalent type of glass,
*
Soda-lime glass
Soda-lime glass, also called soda-lime-silica glass, is the most prevalent type of glass,

Soda-lime glass is prepared by melting the raw materialsSoda-lime glass is prepared by melting the raw materials, such as sodium carbonateSoda-lime glass is prepared by melting the raw materials, such as sodium carbonate (soda), limeSoda-lime glass is prepared by melting the raw materials, such as sodium carbonate (soda), lime, dolomiteSoda-lime glass is prepared by melting the raw materials, such as sodium carbonate (soda), lime, dolomite, silicon dioxideSoda-lime glass is prepared by melting the raw materials, such as sodium carbonate (soda), lime, dolomite, silicon dioxide (silica), aluminium oxideSoda-lime glass is prepared by melting the raw materials, such as sodium carbonate (soda), lime, dolomite, silicon dioxide (silica), aluminium oxide (alumina), and small quantities of fining agents (e.g., sodium sulfateSoda-lime glass is prepared by melting the raw materials, such as sodium carbonate (soda), lime, dolomite, silicon dioxide (silica), aluminium oxide (alumina), and small quantities of fining agents (e.g., sodium sulfate, sodium chlorideSoda-lime glass is prepared by melting the raw materials, such as sodium carbonate (soda), lime, dolomite, silicon dioxide (silica), aluminium oxide (alumina), and small quantities of fining agents (e.g., sodium sulfate, sodium chloride) in a glass furnace at temperatures locally up to 1675 °C.
Lime (известь) is a calcium (известь) is a calcium-containing inorganic (известь) is a calcium-containing inorganic material in which carbonates (известь) is a calcium-containing inorganic material in which carbonates, oxides (известь) is a calcium-containing inorganic material in which carbonates, oxides and hydroxides (известь) is a calcium-containing inorganic material in which carbonates, oxides and hydroxides predominate. Strictly speaking, lime is calcium oxide (известь) is a calcium-containing inorganic material in which carbonates, oxides and hydroxides predominate. Strictly speaking, lime is calcium oxide or calcium hydroxide (известь) is a calcium-containing inorganic material in which carbonates, oxides and hydroxides predominate. Strictly speaking, lime is calcium oxide or calcium hydroxide. It is also the name of the natural mineral (известь) is a calcium-containing inorganic material in which carbonates, oxides and hydroxides predominate. Strictly speaking, lime is calcium oxide or calcium hydroxide. It is also the name of the natural mineral (native lime) CaO which occurs as a product of coal seam fires (известь) is a calcium-containing inorganic material in which carbonates, oxides and hydroxides predominate. Strictly speaking, lime is calcium oxide or calcium hydroxide. It is also the name of the natural mineral (native lime) CaO which occurs as a product of coal seam fires and in altered limestone (известь) is a calcium-containing inorganic material in which carbonates, oxides and hydroxides predominate. Strictly speaking, lime is calcium oxide or calcium hydroxide. It is also the name of the natural mineral (native lime) CaO which occurs as a product of coal seam fires and in altered limestone xenoliths (известь) is a calcium-containing inorganic material in which carbonates, oxides and hydroxides predominate. Strictly speaking, lime is calcium oxide or calcium hydroxide. It is also the name of the natural mineral (native lime) CaO which occurs as a product of coal seam fires and in altered limestone xenoliths in volcanic ejecta.
The word "lime" originates with its earliest use as building mortar and has the sense of "sticking or adhering.
Слайд 14Soda-lime glass is divided technically into glass used for windows, called flat glass,
Soda-lime glass is divided technically into glass used for windows, called flat glass,

*
Слайд 15*
Coefficient of restitution (glass sphere vs. glass wall):
0.97 ± 0.01
Thermal conductivity:
0.9-1.3
*
Coefficient of restitution (glass sphere vs. glass wall):
0.97 ± 0.01
Thermal conductivity:
0.9-1.3
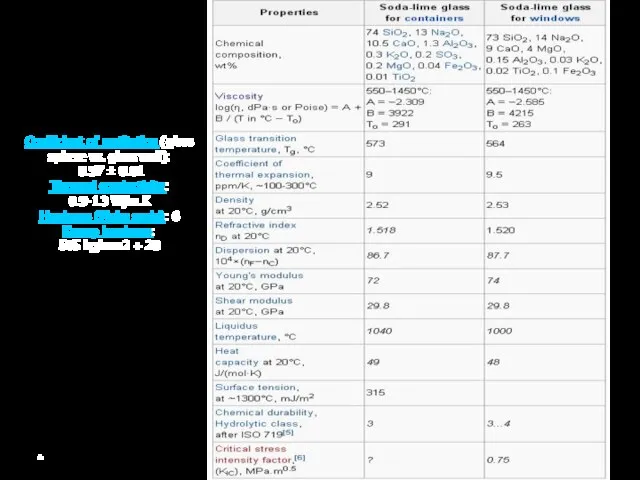
Hardness (Mohs scale): 6
Knoop hardness: 585 kg/mm2 + 20
Слайд 16*
Lime is a general term for calcium is a general term for calcium-containing inorganic materials
Lime is a general term
*
Lime is a general term for calcium is a general term for calcium-containing inorganic materials
Lime is a general term

Strictly speaking, lime is calcium oxideStrictly speaking, lime is calcium oxide or calcium hydroxideStrictly speaking, lime is calcium oxide or calcium hydroxide. It is also the name of the natural mineralStrictly speaking, lime is calcium oxide or calcium hydroxide. It is also the name of the natural mineral (native lime) of the CaO composition which occurs as a product of coal seam firesStrictly speaking, lime is calcium oxide or calcium hydroxide. It is also the name of the natural mineral (native lime) of the CaO composition which occurs as a product of coal seam fires and in altered limestoneStrictly speaking, lime is calcium oxide or calcium hydroxide. It is also the name of the natural mineral (native lime) of the CaO composition which occurs as a product of coal seam fires and in altered limestone xenolithsStrictly speaking, lime is calcium oxide or calcium hydroxide. It is also the name of the natural mineral (native lime) of the CaO composition which occurs as a product of coal seam fires and in altered limestone xenoliths involcanic ejecta. The word "lime" originates with its earliest use as building mortar and has the sense of "sticking or adhering."
These materials are still used in large quantities as building and engineering materials (including limestoneThese materials are still used in large quantities as building and engineering materials (including limestone products, concreteThese materials are still used in large quantities as building and engineering materials (including limestone products, concrete and mortarThese materials are still used in large quantities as building and engineering materials (including limestone products, concrete and mortar) and as chemicalThese materials are still used in large quantities as building and engineering materials (including limestone products, concrete and mortar) and as chemical feedstocks, among other uses. Lime industries and the use of many of the resulting products date from prehistoricThese materials are still used in large quantities as building and engineering materials (including limestone products, concrete and mortar) and as chemical feedstocks, among other uses. Lime industries and the use of many of the resulting products date from prehistoric periods in both the Old WorldThese materials are still used in large quantities as building and engineering materials (including limestone products, concrete and mortar) and as chemical feedstocks, among other uses. Lime industries and the use of many of the resulting products date from prehistoric periods in both the Old World and the New WorldThese materials are still used in large quantities as building and engineering materials (including limestone products, concrete and mortar) and as chemical feedstocks, among other uses. Lime industries and the use of many of the resulting products date from prehistoric periods in both the Old World and the New World. Lime is used extensively forwaste water treatmentThese materials are still used in large quantities as building and engineering materials (including limestone products, concrete and mortar) and as chemical feedstocks, among other uses. Lime industries and the use of many of the resulting products date from prehistoric periods in both the Old World and the New World. Lime is used extensively forwaste water treatment with ferrous sulfate.
The rocks and minerals from which these materials are derived, typically limestoneThe rocks and minerals from which these materials are derived, typically limestone or chalkThe rocks and minerals from which these materials are derived, typically limestone or chalk, are composed primarily of calcium carbonateThe rocks and minerals from which these materials are derived, typically limestone or chalk, are composed primarily of calcium carbonate. They may be cut, crushed or pulverized and chemically altered. "Burning" (calcinationThe rocks and minerals from which these materials are derived, typically limestone or chalk, are composed primarily of calcium carbonate. They may be cut, crushed or pulverized and chemically altered. "Burning" (calcination) converts them into the highly caustic material quicklime (calcium oxide, CaO) and, through subsequent addition of water, into the less caustic (but still strongly alkaline) slaked lime or hydrated lime (calcium hydroxide, Ca(OH)2), the process of which is called slaking of lime.
When the term is encountered in an agricultural context, it probably refers to agricultural limeWhen the term is encountered in an agricultural context, it probably refers to agricultural lime. Otherwise it most commonly means slaked limeWhen the term is encountered in an agricultural context, it probably refers to agricultural lime. Otherwise it most commonly means slaked lime, as the more dangerous form is usually described more specifically as quicklime or burnt lime.
Слайд 17*
Lithography
Positive photoresist is an organic resin material containing a sensitizer. It is
*
Lithography
Positive photoresist is an organic resin material containing a sensitizer. It is

Special types of resists can be spun to thicknesses of over 200 µm, but the large thickness poses significant challenges to exposing and defining features below 25 µm in size.
Слайд 18The sensitizer prevents the dissolution of unexposed resist during immersion in the
The sensitizer prevents the dissolution of unexposed resist during immersion in the

The exact opposite process happens in negative resists—exposed areas remain and unexposed areas dissolve in the developer.
*
Слайд 19*
Lithography
Resolution, defined as the minimum feature the optical system can resolve, is
*
Lithography
Resolution, defined as the minimum feature the optical system can resolve, is

For projection systems, it is given by 0.5×λ⁄NA where λ is the wavelength (~ 400 nm) and NA is the numerical aperture of the optics (~ 0.25 for steppers used in MEMS). Resolution in projection lithography is routinely better than one micrometer.
Слайд 20*
Lithography
Depth of focus, however, is amore severe constraint on lithography, especially in
*
Lithography
Depth of focus, however, is amore severe constraint on lithography, especially in

Depth of focus for contact and proximity systems is poor, also limited by Fresnel diffraction.
In projection systems, the image plane can be moved by adjusting the focus settings, but once it is fixed, the depth of focus about that plane is limited to ±0.5×λ/NA2. Depth of focus is typically limited to few microns.
Слайд 21*
Projection lithography is clearly a superior approach, but an optical projection system
*
Projection lithography is clearly a superior approach, but an optical projection system

Long-term cost of ownership plays a critical role in the decision to acquire a particular lithographic tool.
While resolution of most lithographic systems is not a limitation for MEMS, lithography can be challenging depending on the nature of the application; examples include exposure of thick resist, topographical height variations, front to back side pattern alignment, and large fields of view.
Lithography
Слайд 22*
Thick Resist
Patterned thick resist is normally used as a protective masking layer
*
Thick Resist
Patterned thick resist is normally used as a protective masking layer

Coating substrates with thick resist is achieved either by multiple spin-coating applications (up to a total of 20 µm) or by spinning special viscous resist solutions at slower speeds (up to 100 µm).
Maintaining thickness control and uniformity across the wafer becomes difficult with increasing resist thickness.
Слайд 23*
Thick Resist
Exposing resist thicker than 5 µm often degrades the minimum resolvable
*
Thick Resist
Exposing resist thicker than 5 µm often degrades the minimum resolvable

As a general guideline, the maximum aspect ratio (ratio of resist thickness to minimum feature dimension) is approximately three—in other words, the minimum achievable feature size (e.g., line width or spacing between lines) is larger than one third of the resist thickness. This limitation may be overcome using special exposure methods, but their value in a manufacturing environment remains questionable.
Слайд 24*
Topographical Height Variations
Changes in topography on the surface of the wafer, such
*
Topographical Height Variations
Changes in topography on the surface of the wafer, such
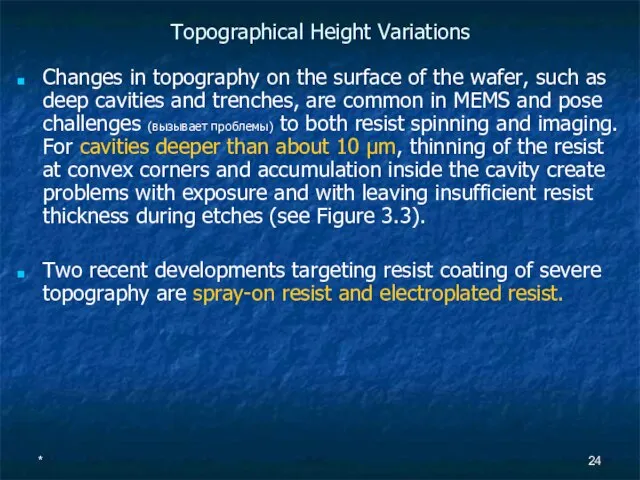
Two recent developments targeting resist coating of severe topography are spray-on resist and electroplated resist.
Слайд 25*
Topographical Height Variations
Exposing a pattern on a surface with height variations in
*
Topographical Height Variations
Exposing a pattern on a surface with height variations in

Contact and proximity tools are not suitable for this task unless a significant loss of resolution is tolerable.
Under certain circumstances where the number of height levels is limited (say, less than three), one may use a projection lithography tool to perform an exposure with a corresponding focus adjustment at each of these height levels. Naturally, this is costly because the number of masks and exposures increases linearly with the number of height levels.
Слайд 26*
Topographical Height Variations
*
Topographical Height Variations
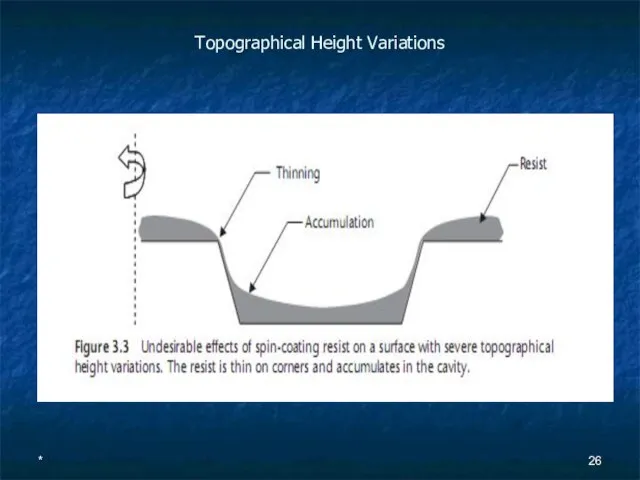
Слайд 27*
Double-Sided Lithography
Often, lithographic patterns on both sides of a wafer need to
*
Double-Sided Lithography
Often, lithographic patterns on both sides of a wafer need to
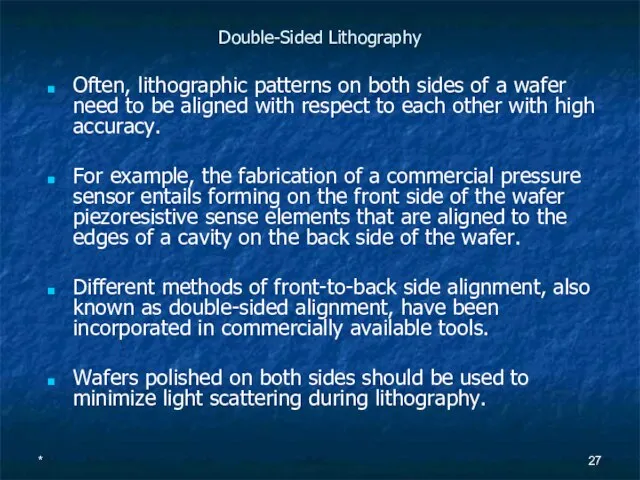
For example, the fabrication of a commercial pressure sensor entails forming on the front side of the wafer piezoresistive sense elements that are aligned to the edges of a cavity on the back side of the wafer.
Different methods of front-to-back side alignment, also known as double-sided alignment, have been incorporated in commercially available tools.
Wafers polished on both sides should be used to minimize light scattering during lithography.
Слайд 28*
Double-Sided Lithography
*
Double-Sided Lithography
Слайд 29*
ЭМ-5086 УСТАНОВКА ДВУСТОРОННЕГО СОВМЕЩЕНИЯ И ЭКСПОНИРОВАНИЯ ЗНАКОВ СОВМЕЩЕНИЯ
Установка предназначена для нанесения знаков
*
ЭМ-5086 УСТАНОВКА ДВУСТОРОННЕГО СОВМЕЩЕНИЯ И ЭКСПОНИРОВАНИЯ ЗНАКОВ СОВМЕЩЕНИЯ
Установка предназначена для нанесения знаков

Работа установки основана на проекционном переносе изображения знака совмещения на обратную сторону пластины. Знаки совмещения на нижней стороне пластины формируются точно напротив знаков совмещения на лицевой стороне пластины.
Установка применяется в фотолитографических процессах при изготовлении полупроводниковых, гибридных, оптических, оптоэлектронных, МЭМС, МОЭМС и других приборов.
Применение этой установки позволяет использовать оборудование для односторонней контактной или проекционной фотолитографии для двустороннего технологического процесса вместо использования установок для двусторонней фотолитографии.
Слайд 32*
ЭМ-5026Б УСТАНОВКА ДВУСТОРОННЕГО СОВМЕЩЕНИЯ И ЭКСПОНИРОВАНИЯ
Установка ЭМ–5026Б выполняет контактным (в зазоре) способом
*
ЭМ-5026Б УСТАНОВКА ДВУСТОРОННЕГО СОВМЕЩЕНИЯ И ЭКСПОНИРОВАНИЯ
Установка ЭМ–5026Б выполняет контактным (в зазоре) способом

Слайд 35*
Large Field of View (Большая область экспонирования)
The field of view is the
*
Large Field of View (Большая область экспонирования)
The field of view is the

Область проецирования это область, которая экспонируется в любой момент на пластину.
In proximity and contact lithography, it covers the entire wafer.
В проекционной и контактной литографии эта область покрывает всю пластину.
Слайд 36*
In projection systems, the field of view is often less than 1
*
In projection systems, the field of view is often less than 1

В проекционной литографии эта область часто меньше чем 1 × 1cm2. Поэтому вся пластина экспонируется пошагово малыми фрагментами, как двумерный массив.
In some applications, the device structure may span dimensions exceeding the field of view. A remedy to this is called field stitching, in which two or more different fields are exposed sequentially, with the edges of the fields overlapping.
В некоторых приборах структура может иметь размеры, превышающие область экспонирования. Для решения этой проблемы делают области сшивания, которые находятся по краям сопрягаемых областей.
Large Field of View (Большая область экспонирования)
Слайд 37*
Etching
*
Etching
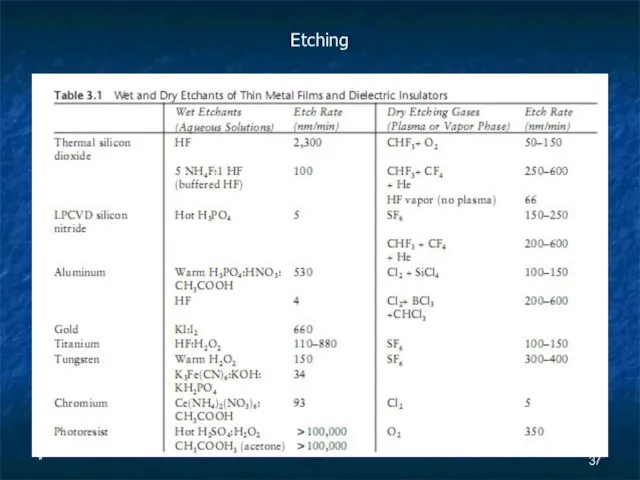
Слайд 38*
Оборудование фирмы SCR (Чехия) участка химического травления кремниевых пластин
*
Оборудование фирмы SCR (Чехия) участка химического травления кремниевых пластин

Слайд 39*
Isotropic Wet Etching
The most common group of silicon isotropic wet etchants is
*
Isotropic Wet Etching
The most common group of silicon isotropic wet etchants is

In the chemical reaction, the nitric acid oxidizes silicon, which is then etched by the hydrofluoric acid. The etch rate of silicon can vary from 0.1 to over 100 µm/min depending on the proportion of the acids in the mixture. Etch uniformity is normally difficult to control but is improved by stirring.
Слайд 40*
Isotropic and Anisotropic Etching
*
Isotropic and Anisotropic Etching
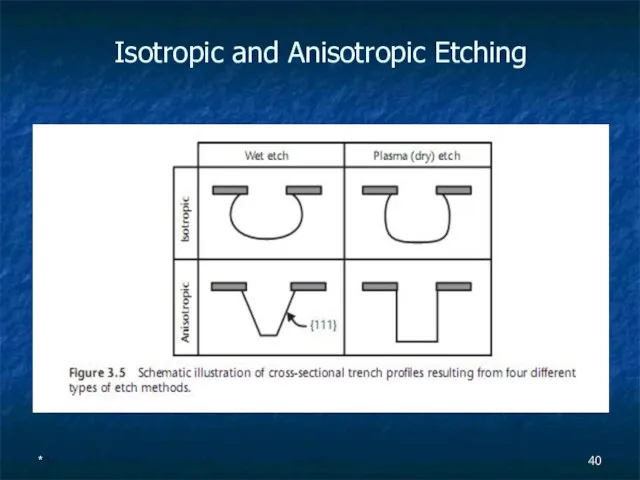
Слайд 41*
Anisotropic wet etchants
Anisotropic wet etchants are also known as orientation-dependent etchants (ODEs)
*
Anisotropic wet etchants
Anisotropic wet etchants are also known as orientation-dependent etchants (ODEs)

The list of anisotropic wet etchants includes the hydroxides of alkali metals (e.g., NaOH, KOH, CsOH), simple and quaternary ammonium hydroxides (e.g., NH4OH, N(CH3)4OH), and ethylenediamine mixed with pyrochatechol (EDP) in water.
The solutions are typically heated to 70º–100ºC. A comparison of various silicon etchants is given in Table 3.2.
Слайд 42*
Anisotropic Wet Etching
*
Anisotropic Wet Etching
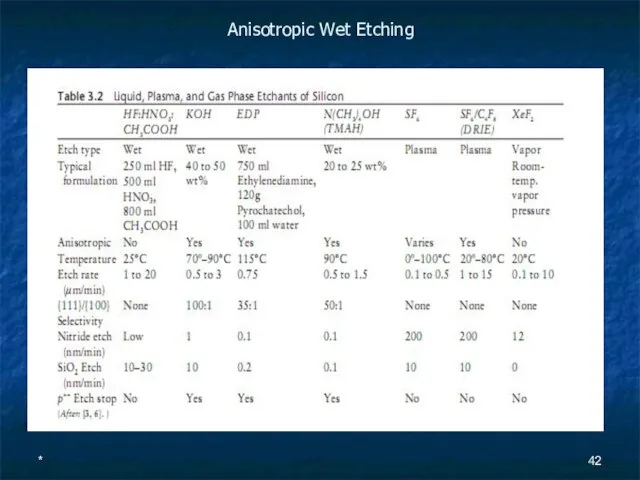
Слайд 43*
KOH is by far the most common ODE
Etch rates are typically given
*
KOH is by far the most common ODE
Etch rates are typically given

The {110} planes are etched in KOH about twice as rapidly as {100} planes.
While {111} planes are etched at a rate about 100 times slower than for {100} planes.


 Новая библиотека-читающая школа
Новая библиотека-читающая школа Как «вершки» и «корешки» помогают людям?
Как «вершки» и «корешки» помогают людям? Городецкая роспись по дереву
Городецкая роспись по дереву Товароведная характеристика, экспертиза качества и экономические условия реализации “йогуртов”
Товароведная характеристика, экспертиза качества и экономические условия реализации “йогуртов” Изменщик. Суд и остальное
Изменщик. Суд и остальное Портфолио педагога
Портфолио педагога Отражение изменений «зарплатного» законодательства 2011 года в прикладных решениях фирмы «1С»
Отражение изменений «зарплатного» законодательства 2011 года в прикладных решениях фирмы «1С»  Немного о формировании общественного мнения
Немного о формировании общественного мнения Заболевания ЛОР-органов в пожилом и старческом возрасте
Заболевания ЛОР-органов в пожилом и старческом возрасте Основы социального проектирования
Основы социального проектирования Модернизация в СССР: триумф и трагедия
Модернизация в СССР: триумф и трагедия Параметры областного бюджета Тверской области на 2008-2010 годы
Параметры областного бюджета Тверской области на 2008-2010 годы Государство. Признаки государства
Государство. Признаки государства Анализ и моделирование течений жидкостей и газовc использованием комплекса ANSYS CFX
Анализ и моделирование течений жидкостей и газовc использованием комплекса ANSYS CFX Презентация на тему Природа Казахстана
Презентация на тему Природа Казахстана Линии второго порядка в жизни
Линии второго порядка в жизни Гигиена кожи, одежды и обуви
Гигиена кожи, одежды и обуви МОУ СОШ №32
МОУ СОШ №32 Учимся читать
Учимся читать Тема урока: Экономическая система. Классификация.
Тема урока: Экономическая система. Классификация. Проектирование и расчет координатной защиты башенных кранов
Проектирование и расчет координатной защиты башенных кранов Учимся составлять и решать задачи
Учимся составлять и решать задачи Виды страховых правоотношений. Тема 5
Виды страховых правоотношений. Тема 5 Бактяева Галина Владимировна
Бактяева Галина Владимировна Самопрезентация "Моя гражданская позиция"
Самопрезентация "Моя гражданская позиция" Чебоксарское производственное объединение им.В.И.Чапаева
Чебоксарское производственное объединение им.В.И.Чапаева Подросток и закон
Подросток и закон ЛИСТАЯ СТАРЫЕ СТРАНИЦЫ...
ЛИСТАЯ СТАРЫЕ СТРАНИЦЫ...