Содержание
- 2. * Isotropic Wet Etching The most common group of silicon isotropic wet etchants is HNA, also
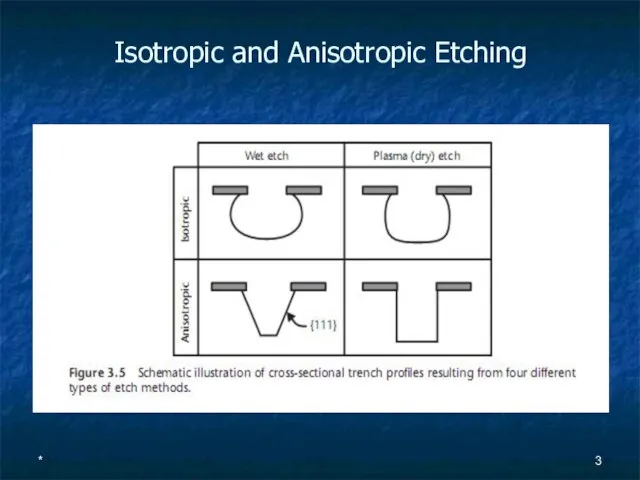
- 3. * Isotropic and Anisotropic Etching
- 4. * Anisotropic wet etchants Anisotropic wet etchants are also known as orientation-dependent etchants (ODEs) because their
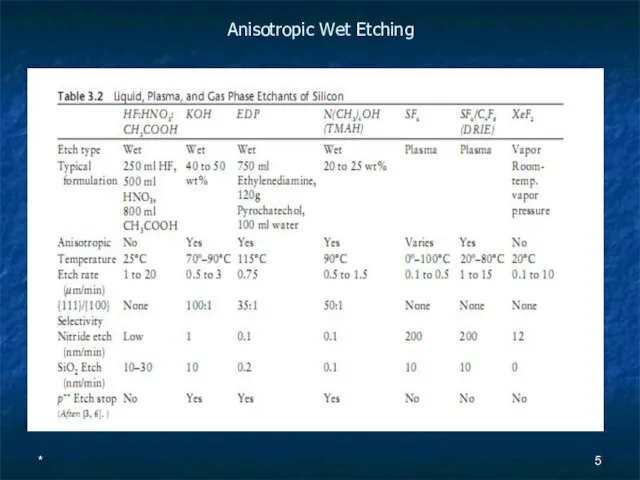
- 5. * Anisotropic Wet Etching
- 6. * KOH is by far the most common ODE Etch rates are typically given in the
- 7. * Anisotropic Etching of Crystalline Silicon in Alkaline Solutions I. Orientation Dependence and Behavior of Passivation
- 8. * In an oxidation step, four hydroxide ions react with one surface silicon atom, leading to
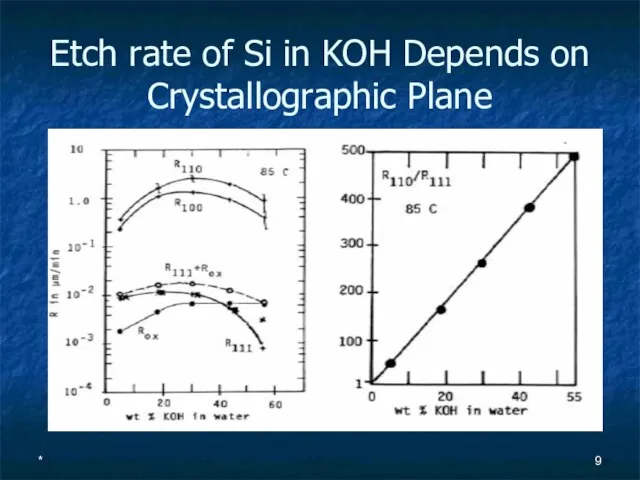
- 9. * Etch rate of Si in KOH Depends on Crystallographic Plane
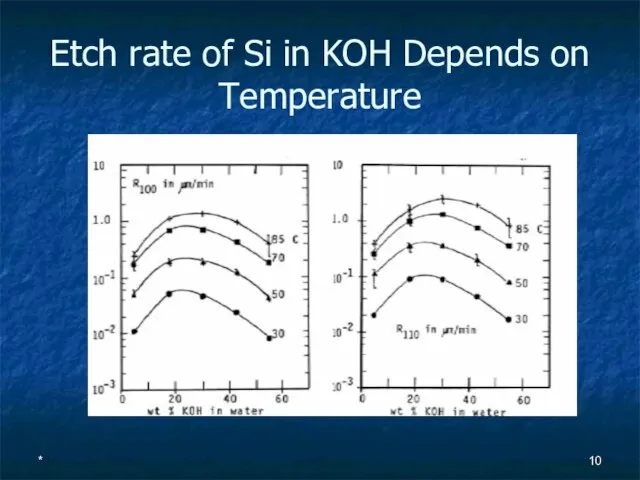
- 10. * Etch rate of Si in KOH Depends on Temperature
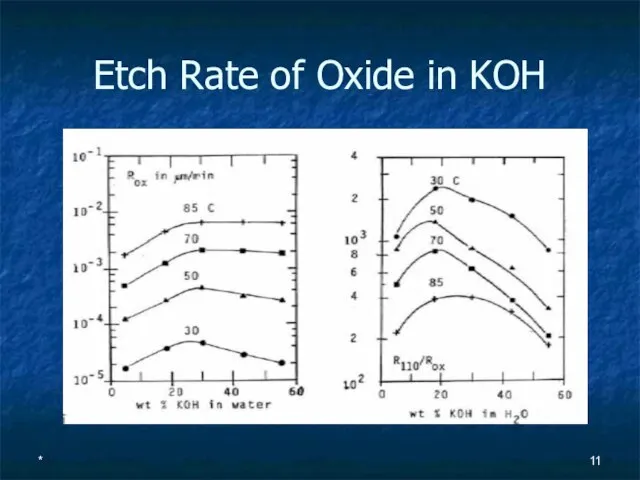
- 11. * Etch Rate of Oxide in KOH
- 12. * The etch rate of KOH and other alkaline etchants also slows greatly for heavily doped
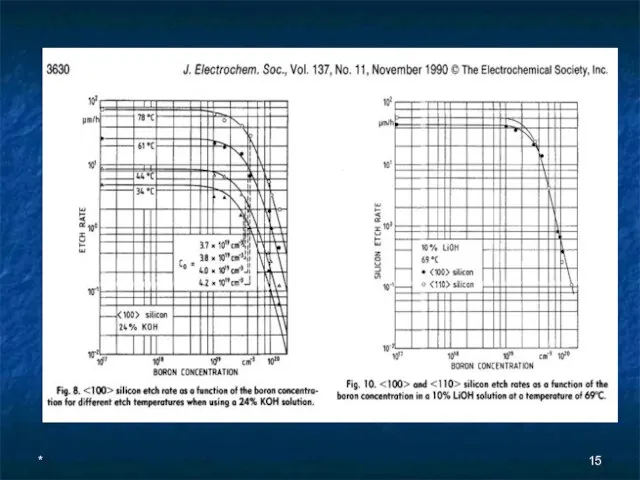
- 13. * Anisotropic Etching of Crystalline Silicon in Alkaline Solutions II. Influence of Dopants J. Electrochem. Soc.,
- 14. * On the basis of these results, a model is proposed attributing the etch stop phenomenon
- 15. *
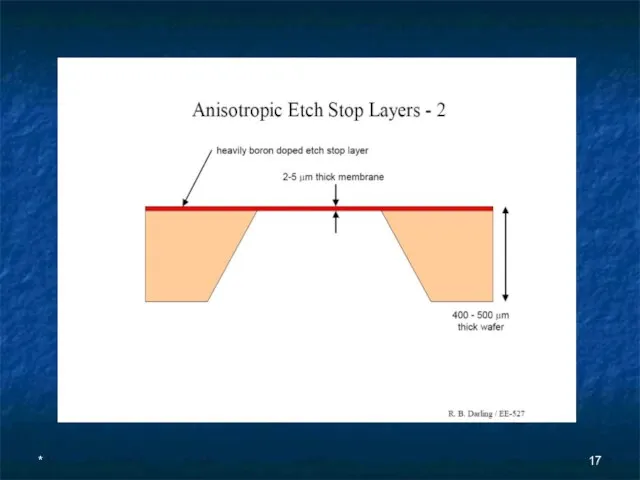
- 16. * Anisotropic Etch Stop Layers - 1 Controlling the absolute depth of an etch is often
- 17. *
- 18. * Oblique [эблик] = скошенный
- 19. * Alkali hydroxides are extremely corrosive; aluminum bond pads inadvertently exposed to KOH are quickly damaged.
- 20. * In the category of ammonium hydroxides, tetramethyl ammonium hydroxide (TMAH, N(CH3)4OH) exhibits similar properties to
- 21. * Both silicon dioxide and silicon nitride remain virtually unetched in TMAH and hence can be
- 22. * EDP is another wet etchant with selectivity to {111} planes and to heavily p-doped silicon.
- 23. * Etching using anisotropic aqueous solutions results in three-dimensional faceted structures formed by intersecting {111} planes
- 24. * The easiest structures to visualize are V-shaped cavities etched in (100)-oriented wafers. The etch front
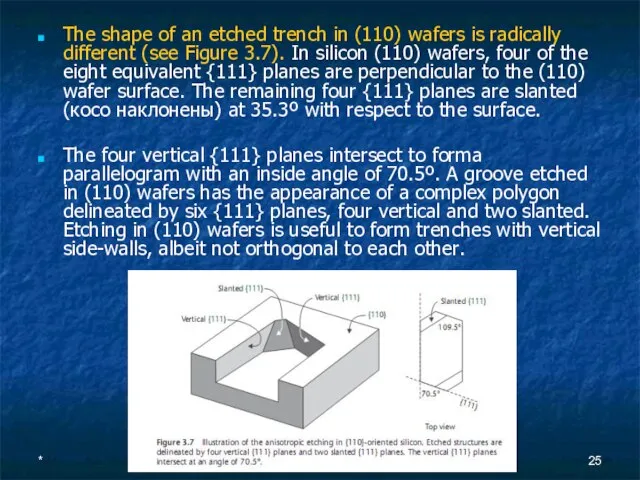
- 25. * The shape of an etched trench in (110) wafers is radically different (see Figure 3.7).
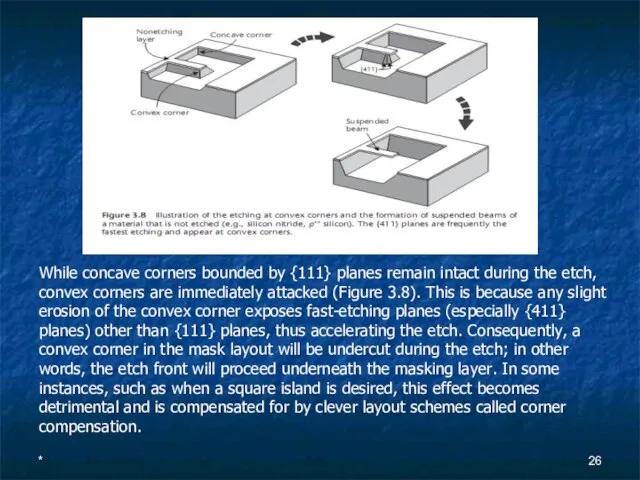
- 26. * While concave corners bounded by {111} planes remain intact during the etch, convex corners are
- 27. * Often, however, the effect is intentionally used to form beams suspended over cavities (see Figure
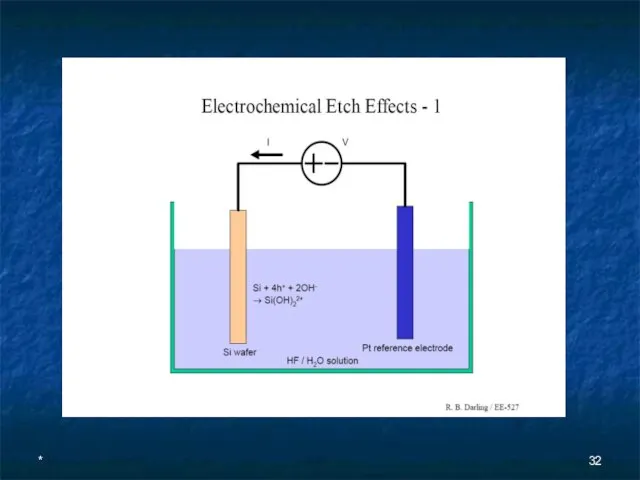
- 28. * Electrochemical Etching (ECE) The relatively large etch rates of anisotropic wet etchants (>0.5 µm/min) make
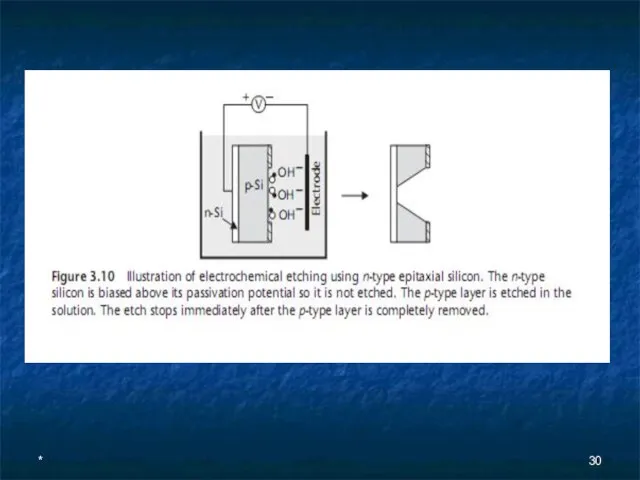
- 29. * An n-type epitaxial layer grown on a p-type wafer forms a p-n junction diode that
- 30. *
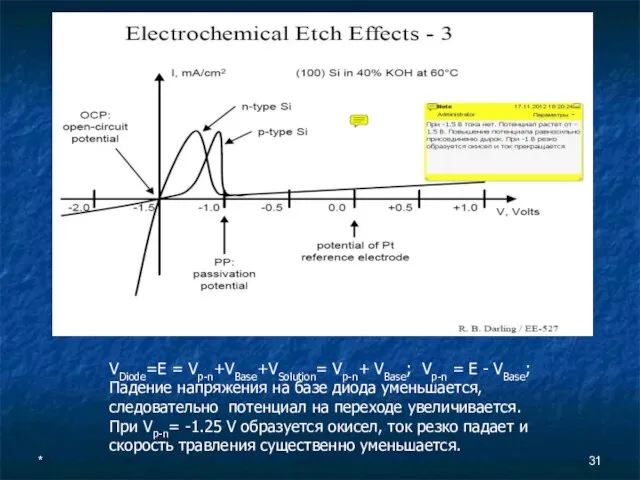
- 31. * VDiode=E = Vp-n+VBase+VSolution= Vp-n+ VBase; Vp-n = E - VBase; Падение напряжения на базе диода
- 32. *
- 33. *
- 35. Скачать презентацию









![* Oblique [эблик] = скошенный](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/378458/slide-17.jpg)










 Презентация на тему Заповедник Остров Врангеля
Презентация на тему Заповедник Остров Врангеля Презентация на тему Общие сведения о животном мире
Презентация на тему Общие сведения о животном мире Связь поколений
Связь поколений Учебные трудности пятиклассников
Учебные трудности пятиклассников Harry Potter
Harry Potter Мастер-класс по развитию творческих способностей учащихся
Мастер-класс по развитию творческих способностей учащихся Мои клиенты - K.A. Planner
Мои клиенты - K.A. Planner РЕЗИСТЕНТНОСТЬ ВОЗБУДИТЕЛЕЙ ИНФЕКЦИЙ МОЧЕВЫХ ПУТЕЙ К ФТОРХИНОЛОНАМ: МИФ ИЛИ РЕАЛЬНОСТЬ?
РЕЗИСТЕНТНОСТЬ ВОЗБУДИТЕЛЕЙ ИНФЕКЦИЙ МОЧЕВЫХ ПУТЕЙ К ФТОРХИНОЛОНАМ: МИФ ИЛИ РЕАЛЬНОСТЬ? Типологические особенности студентов. (Тема 5)
Типологические особенности студентов. (Тема 5) Жители леса
Жители леса Фонетика (урок-повторение, 6 класс)
Фонетика (урок-повторение, 6 класс) ПРИЛОЖЕНИЯ К ТЕЗИСАМ по ПРЕДЛОЖЕНИЯМ по актуальным проблемам социально-экономической стратегии России на период до 2020 года в ча
ПРИЛОЖЕНИЯ К ТЕЗИСАМ по ПРЕДЛОЖЕНИЯМ по актуальным проблемам социально-экономической стратегии России на период до 2020 года в ча Презентация на тему Свойства корня n-й степени (11 класс)
Презентация на тему Свойства корня n-й степени (11 класс) ТАРИФНАЯ ПОЛИТИКА - 2012
ТАРИФНАЯ ПОЛИТИКА - 2012 По сказке К.Чуковского «Айболит»
По сказке К.Чуковского «Айболит» МОУ КСЛ «Эврика»
МОУ КСЛ «Эврика» Решение трудной математической проблемы можно сравнить с взятием крепости.Н.Я. Виленкин
Решение трудной математической проблемы можно сравнить с взятием крепости.Н.Я. Виленкин «Механизация сельского хозяйства»
«Механизация сельского хозяйства» Личные окончания глаголов настоящего времени
Личные окончания глаголов настоящего времени Abeilles (nombres)
Abeilles (nombres) Корпоративная культура как эффективный инструмент психолого – педагогического сопровождения образовательной среды
Корпоративная культура как эффективный инструмент психолого – педагогического сопровождения образовательной среды Должность Президента школы
Должность Президента школы Презентация на тему Азия
Презентация на тему Азия  Психология счастливой жизни
Психология счастливой жизни Возникновение отрицательных чисел
Возникновение отрицательных чисел Управление репутацией и репутационные риски в Интернете
Управление репутацией и репутационные риски в Интернете В прекрасном имени Мужчина Сложились мужество и стать, Уменье думать и мечтать, Быть вдохновенным без причины.
В прекрасном имени Мужчина Сложились мужество и стать, Уменье думать и мечтать, Быть вдохновенным без причины. Разработка методики организации и проведения практических работ
Разработка методики организации и проведения практических работ