Слайд 3Make a list of inferences about any properties of objects in the

box.
How could you learn more about the objects in the box without opening the box?
Scientist face these same questions as they try to learn more about atoms.
Слайд 4Quantum Numbers
Quantum numbers specify the address of each electron in an

atom. There are four types of quantum numbers:
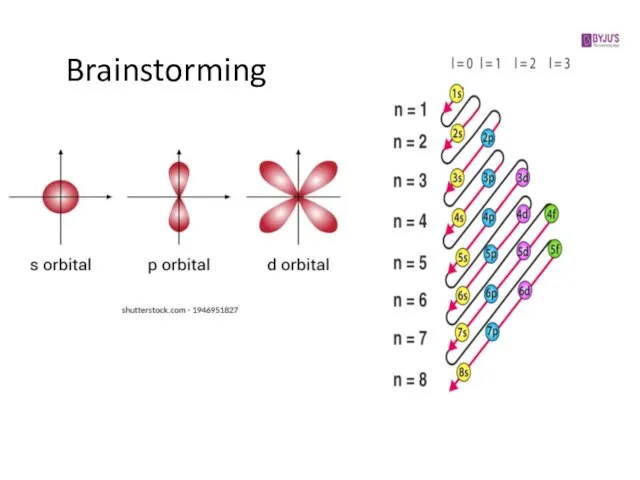
1. Principal quantum number, n → energy level (shell)
2. Secondary quantum number, l → subshell (s, p, d, f)
3. Magnetic quantum number, ml → orbital
4. Spin quantum number, ms → spin type of electro
There are no two electrons in an atom that can have the same four quantum numbers. Each electron has a unique address, like a family living in a flat. This is Pauli's Exclusion Principle.
Слайд 51. The principal quantum number, n
determines the size and energy of an

atom (larger n means bigger atoms and higher energy),
can take an integer value n = 1, 2, 3, 4 ... or (K, L, M, N...),
all electrons in an atom with the same value are said to belong to the same shell.
Слайд 62. Secondary quantum number, l
determines the overall shape of the orbital

within a shell
affects orbital energies (bigger l = higher energy)
all electrons in an atom with the same value of ‘l’ are said to belong to the same subshell
has integer values between 0 and n-1
may be called the “orbital angular momentum quantum number”
Слайд 73. Magnetic quantum number, ml
determines the orientation of orbitals within a subshell

does not affect orbital energy
has integer values between -I and +I
the number of ml values within a subshell is the number of orbitals within a subshell
s, p, d and f subshells includes 1, 3, 5 and 7 orbitals respectively.
Слайд 84. Spin quantum number, ms
each orbital may contain two electrons at most

several experimental observations can be explained by treating the electron as though it were spinning
spin affects the electron behave like a tiny magnet
spin can be clockwise (+1/2) or counterclockwise (-1/2)
Слайд 9Solving problems
Example 1
Find the values of quantum numbers for hydrogen atom.
Example
2
Show the values of possible quantum numbers for magnesium atom.( 12Mg)
Слайд 10Electron configuration
In 1925 Wolfgang Pauli stated his exclusion principle;
‘In the
same atom, two electrons may not have identical sets of all quantum numbers.’
According to this principle, the quantum numbers, n, l, ml, and ms, can never be identical for two electrons in an atom.
The Aufbau process
The Aufbau principle basically states that the lowest energy orbitals are filled first.
Hund’s rule states that;
the electrons are distributed among the orbitals of a subshell of the same energy in a way that gives the maximum number of unpaired electrons with parallel spin.








 Конкурентная стратегия
Конкурентная стратегия Экстрасенсорное общение
Экстрасенсорное общение Презентация без названия
Презентация без названия Дистанционный конкурс на лучший школьный инновационный проект «Инновации рождаются в школе» НП «Телешкола»
Дистанционный конкурс на лучший школьный инновационный проект «Инновации рождаются в школе» НП «Телешкола» Повторение понятий
Повторение понятий Михаил Врубель Ведь женщины
Михаил Врубель Ведь женщины Бережно ласкающий гель, обволакивающий аромат, заботливая мягкость. Что еще нужно, чтобы почувствовать легкость. Окунуться в мир б
Бережно ласкающий гель, обволакивающий аромат, заботливая мягкость. Что еще нужно, чтобы почувствовать легкость. Окунуться в мир б Базы данных EBSCO по бизнесу и экономике Андрей Соколов
Базы данных EBSCO по бизнесу и экономике Андрей Соколов Культура Древнего Египта
Культура Древнего Египта Путешествие в мир искусства
Путешествие в мир искусства Lektsia_1
Lektsia_1 Почему важно помнить героев, их подвиги
Почему важно помнить героев, их подвиги Литературное чтение
Литературное чтение Как подготовиться к ЭССЕ?
Как подготовиться к ЭССЕ? Акулы и человек
Акулы и человек Фильтры/кондиционеры охлаждающей жидкости
Фильтры/кондиционеры охлаждающей жидкости Портфолио методического объединения учителей информатики и ИКТ за 2011-2012 учебный год Руководитель МО – Заломина Е.Ю.
Портфолио методического объединения учителей информатики и ИКТ за 2011-2012 учебный год Руководитель МО – Заломина Е.Ю. Спинной мозг, структуры и функции
Спинной мозг, структуры и функции  Плавающие подсвечники
Плавающие подсвечники Проблема компенсации в специальной психологии
Проблема компенсации в специальной психологии Виктор Петрович Астафьев
Виктор Петрович Астафьев Презентация на тему Общие закономерности развития науки
Презентация на тему Общие закономерности развития науки  Презентация на тему Измерение пространства и времени
Презентация на тему Измерение пространства и времени Презентация на тему Простые вещества металлы 8 класс
Презентация на тему Простые вещества металлы 8 класс  Прибыль. Финансы и кредит
Прибыль. Финансы и кредит Международный день птиц
Международный день птиц Сертификация QA
Сертификация QA “Внутренний контроль качества образования. Поиск оптимальной модели. Опыт работы по организации внутришкольного контроля ”
“Внутренний контроль качества образования. Поиск оптимальной модели. Опыт работы по организации внутришкольного контроля ”