Содержание
- 3. The aim of the study: oligoperoxide based routes of tailored synthesis and functionalization of luminescent and
- 4. Talk outline . I. Functional surface-active oligoperoxides and derived oligoelectrolyte and nonionic surfactants of block, comb-like
- 5. The main approaches of synthesis of functional oligoperoxide and derived polymeric surfactants
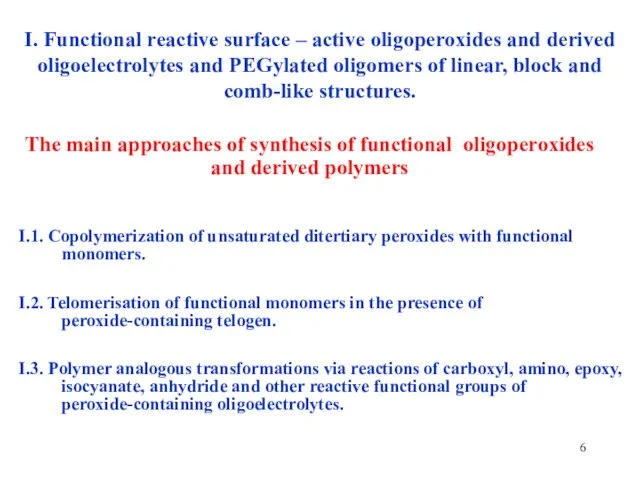
- 6. I. Functional reactive surface – active oligoperoxides and derived oligoelectrolytes and PEGylated oligomers of linear, block
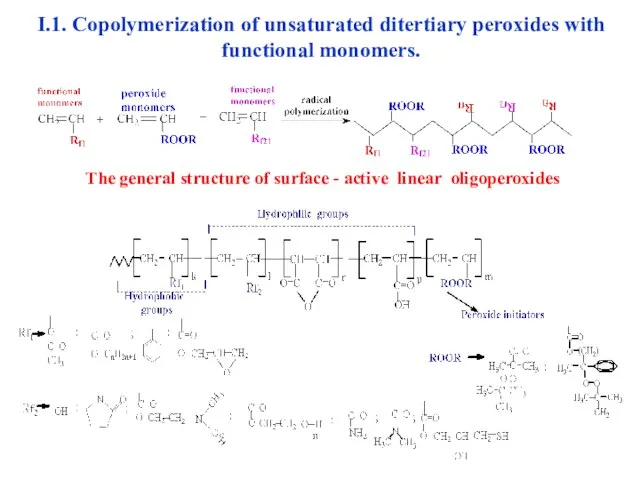
- 7. The general structure of surface - active linear oligoperoxides I.1. Copolymerization of unsaturated ditertiary peroxides with
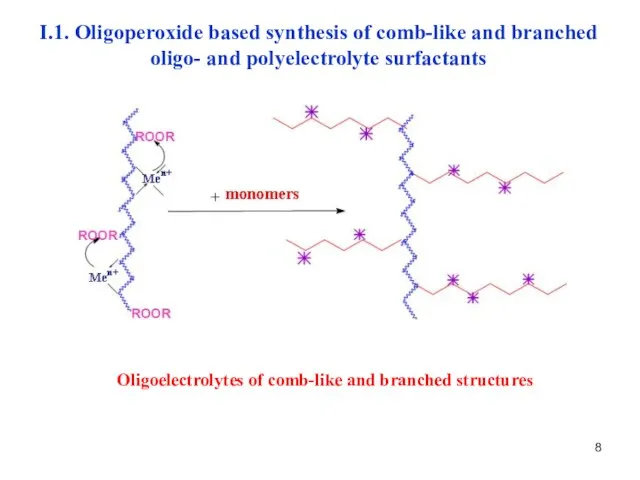
- 8. Oligoelectrolytes of comb-like and branched structures I.1. Oligoperoxide based synthesis of comb-like and branched oligo- and
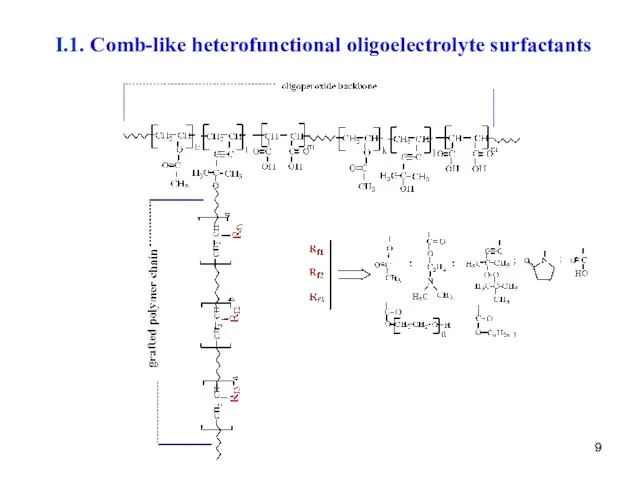
- 9. I.1. Comb-like heterofunctional oligoelectrolyte surfactants
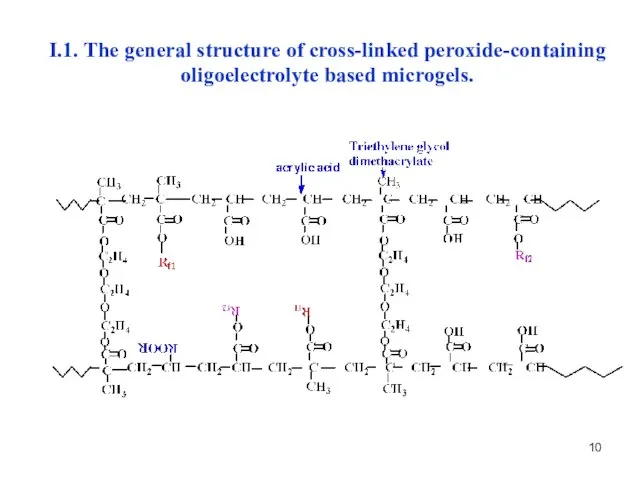
- 10. I.1. The general structure of cross-linked peroxide-containing oligoelectrolyte based microgels.
- 11. I.1. TEM images of oligoelectrolyte based nanogels
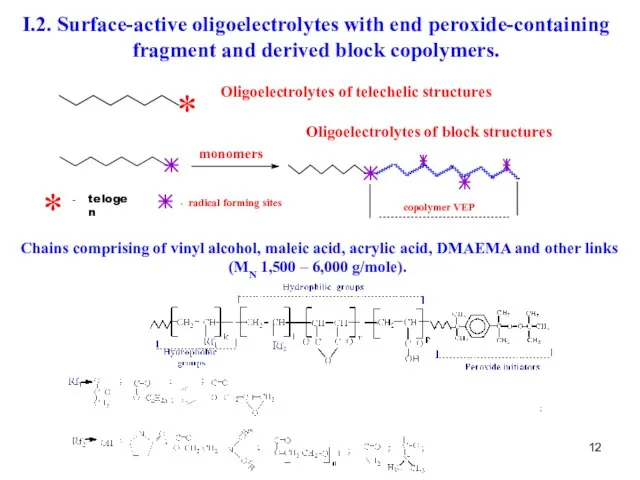
- 12. I.2. Surface-active oligoelectrolytes with end peroxide-containing fragment and derived block copolymers. Chains comprising of vinyl alcohol,
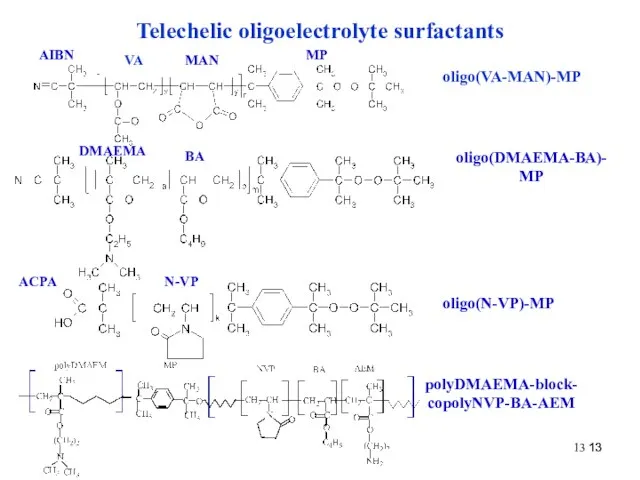
- 13. Telechelic oligoelectrolyte surfactants VA MAN MP oligo(VA-MAN)-MP AIBN DMAEMA BA oligo(DMAEMA-BA)-MP N-VP oligo(N-VP)-MP polyDMAEMA-block-copolyNVP-BA-AEM ACPA
- 14. O O R R f R f O O R + f O O R O
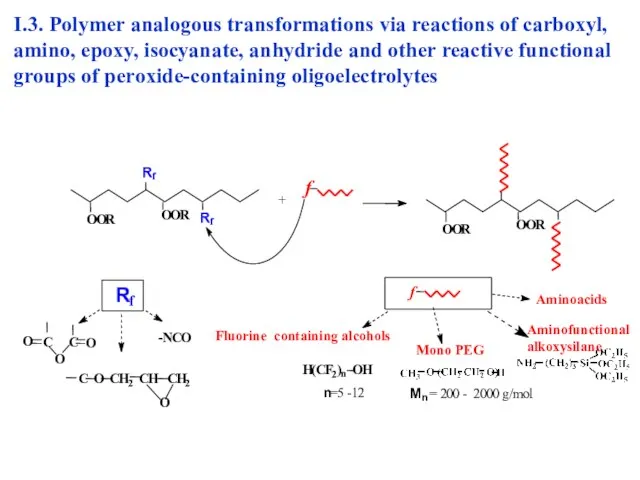
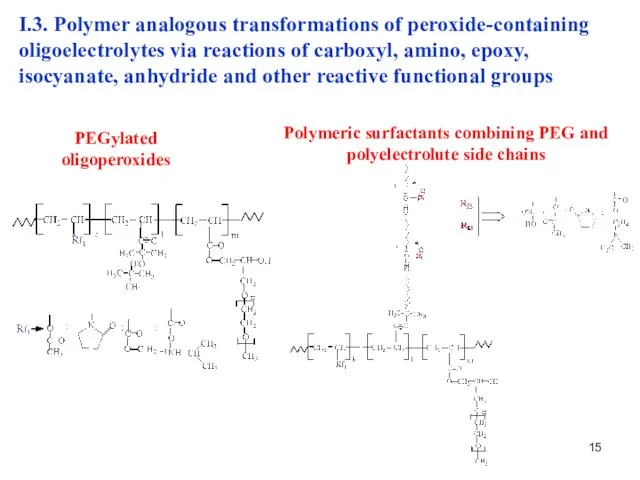
- 15. I.3. Polymer analogous transformations of peroxide-containing oligoelectrolytes via reactions of carboxyl, amino, epoxy, isocyanate, anhydride and
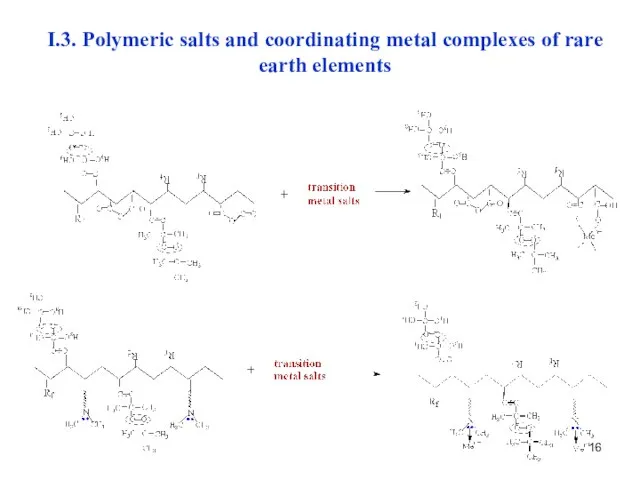
- 16. I.3. Polymeric salts and coordinating metal complexes of rare earth elements
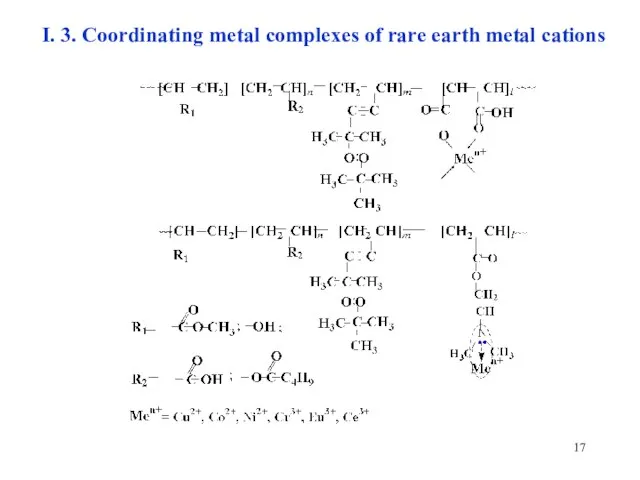
- 17. I. 3. Coordinating metal complexes of rare earth metal cations
- 18. controlled design of a structure controlled molecular weight (1,000 – 30,000g/mole) narrowed molecular weight distribution controlled
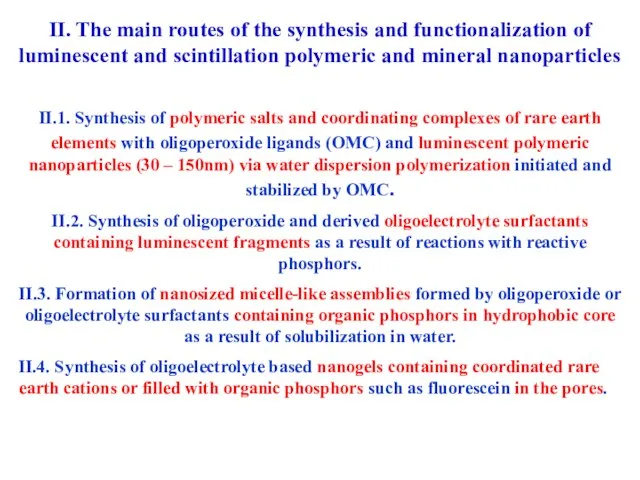
- 19. II. The main routes of the synthesis and functionalization of luminescent and scintillation polymeric and mineral
- 20. II. The main routes of the synthesis and functionalization of luminescent and scintillation polymeric and mineral
- 21. II. The main routes of the synthesis and functionalization of luminescent and scintillation polymeric and mineral
- 22. II.1. Coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and polymeric nanoparticles synthesized via
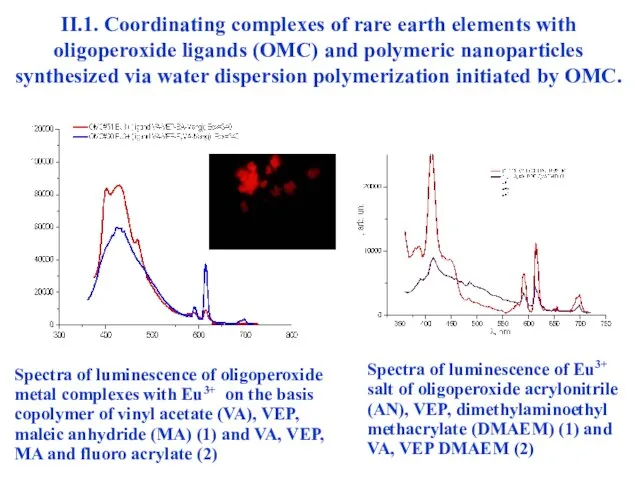
- 23. II.1. Coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and polymeric nanoparticles synthesized via
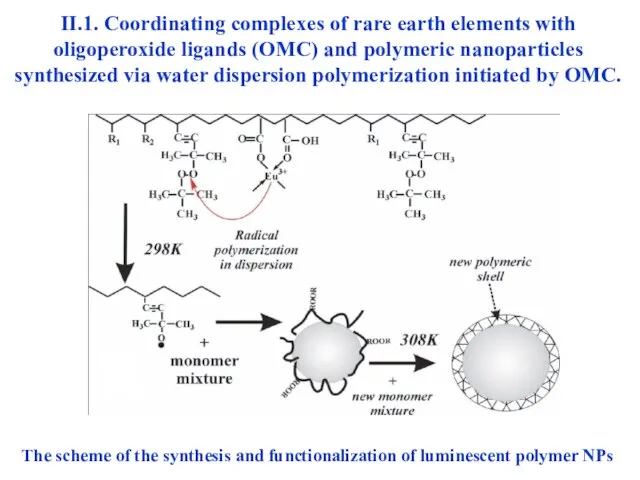
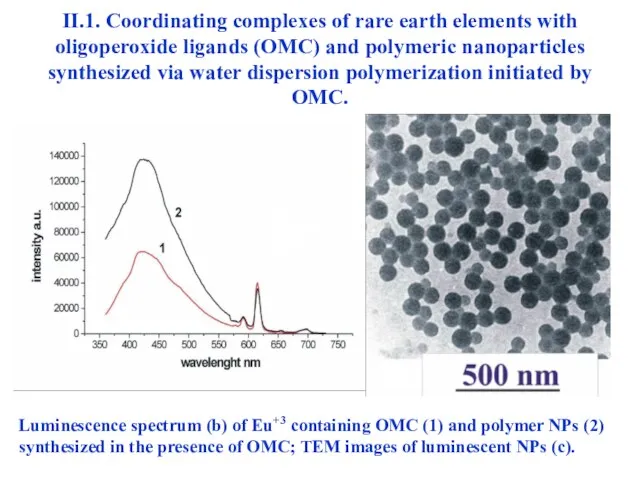
- 24. II.1. Coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and polymeric nanoparticles synthesized via
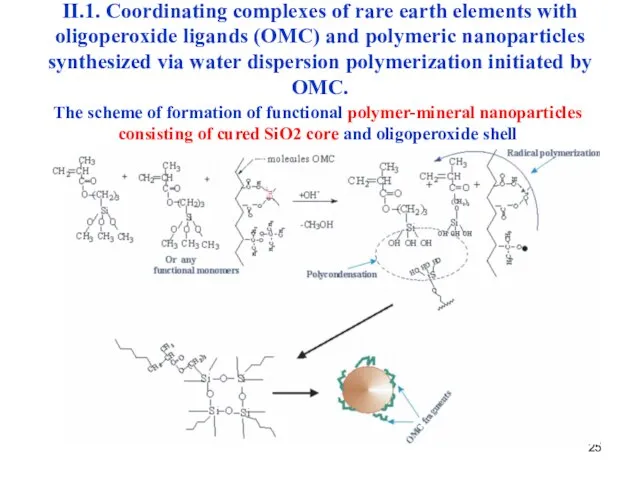
- 25. The scheme of formation of functional polymer-mineral nanoparticles consisting of cured SiO2 core and oligoperoxide shell
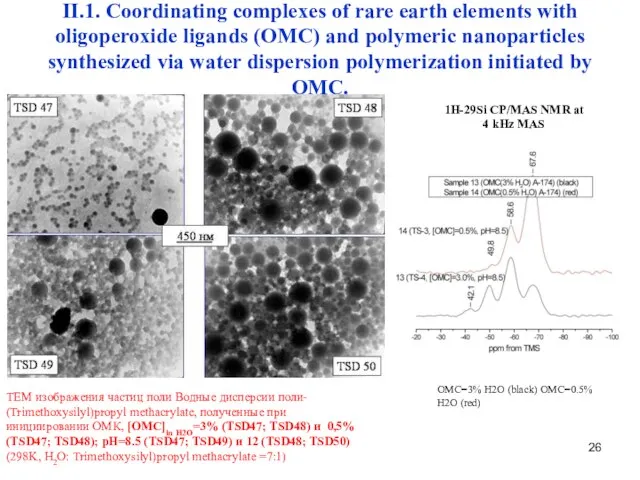
- 26. II.1. Coordinating complexes of rare earth elements with oligoperoxide ligands (OMC) and polymeric nanoparticles synthesized via
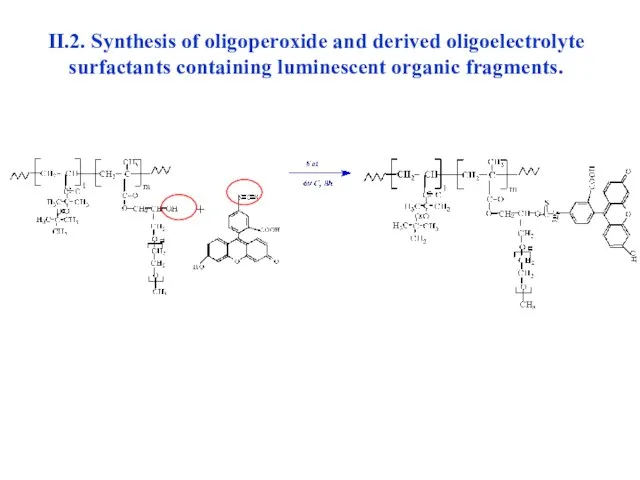
- 27. II.2. Synthesis of oligoperoxide and derived oligoelectrolyte surfactants containing luminescent organic fragments.
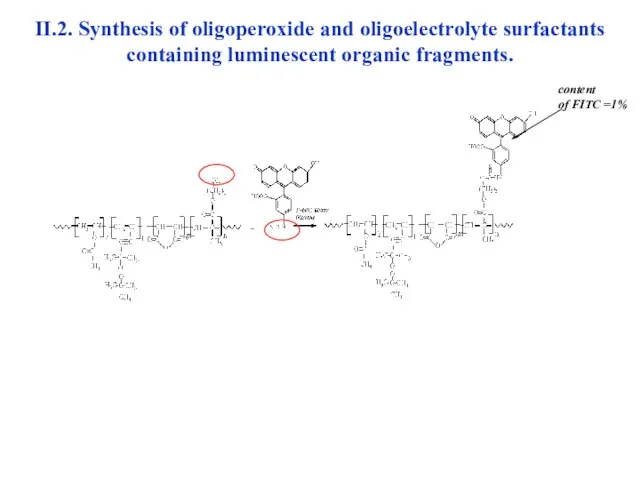
- 28. II.2. Synthesis of oligoperoxide and oligoelectrolyte surfactants containing luminescent organic fragments. content of FITC =1%
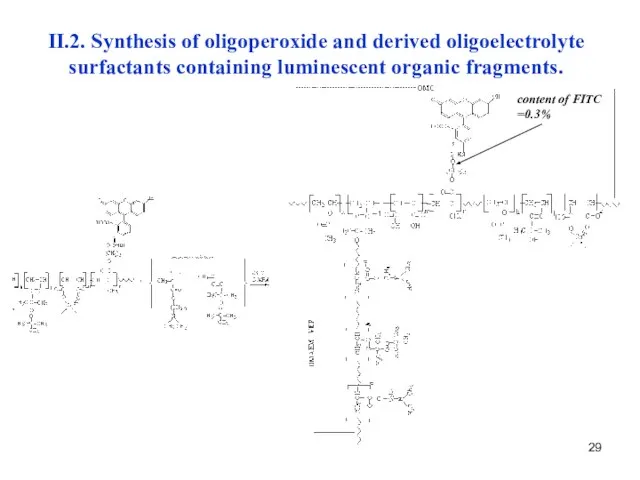
- 29. II.2. Synthesis of oligoperoxide and derived oligoelectrolyte surfactants containing luminescent organic fragments. content of FITC =0.3%
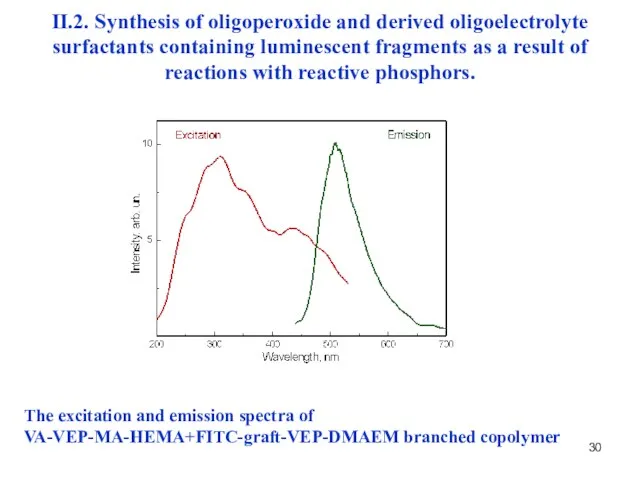
- 30. The excitation and emission spectra of VA-VEP-MA-HEMA+FITC-graft-VEP-DMAEM branched copolymer II.2. Synthesis of oligoperoxide and derived oligoelectrolyte
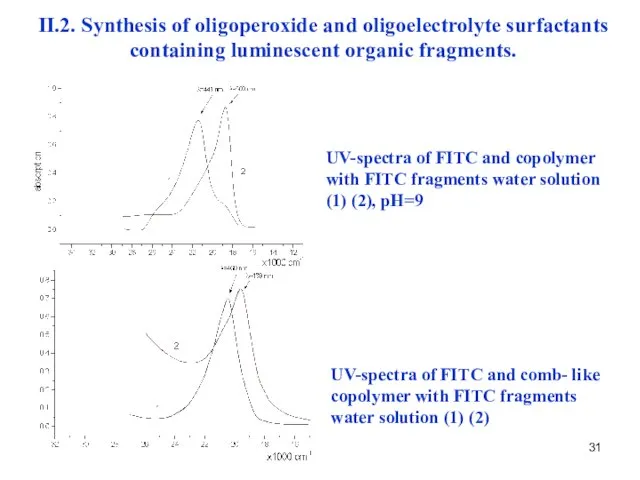
- 31. UV-spectra of FITC and copolymer with FITC fragments water solution (1) (2), рН=9 UV-spectra of FITC
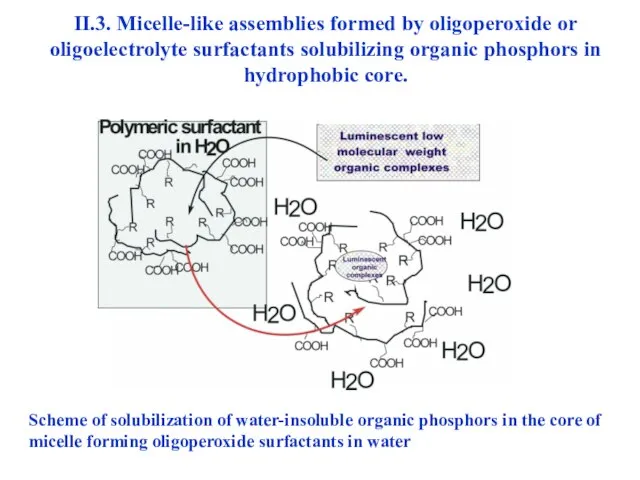
- 32. II.3. Micelle-like assemblies formed by oligoperoxide or oligoelectrolyte surfactants solubilizing organic phosphors in hydrophobic core. Scheme
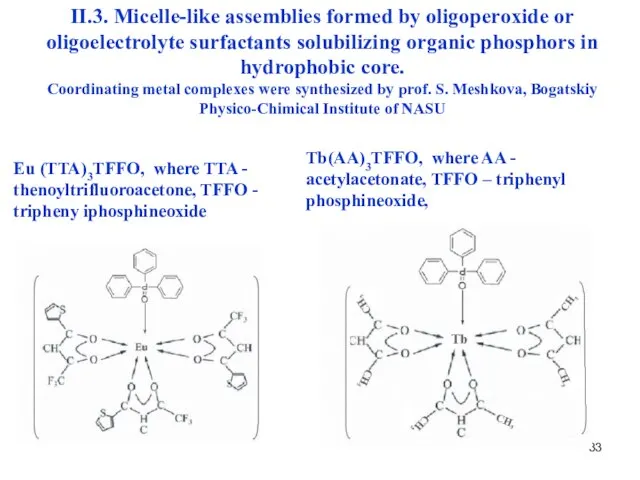
- 33. II.3. Micelle-like assemblies formed by oligoperoxide or oligoelectrolyte surfactants solubilizing organic phosphors in hydrophobic core. Coordinating
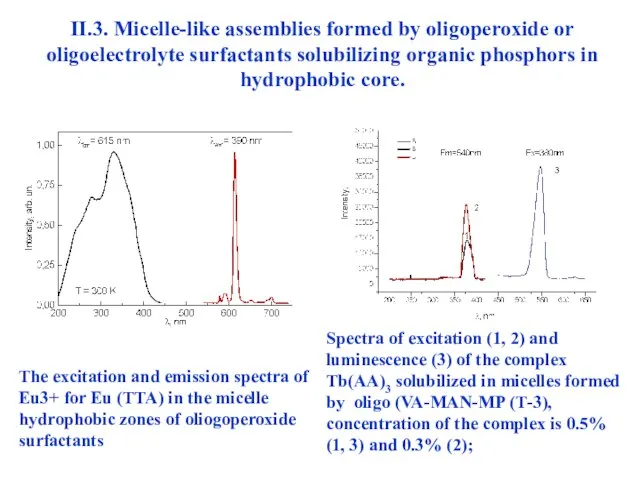
- 34. II.3. Micelle-like assemblies formed by oligoperoxide or oligoelectrolyte surfactants solubilizing organic phosphors in hydrophobic core. The
- 35. II.4. Oligoelectrolyte based nanogels containing coordinating rare earth cations or organic phosphors in the pores. The
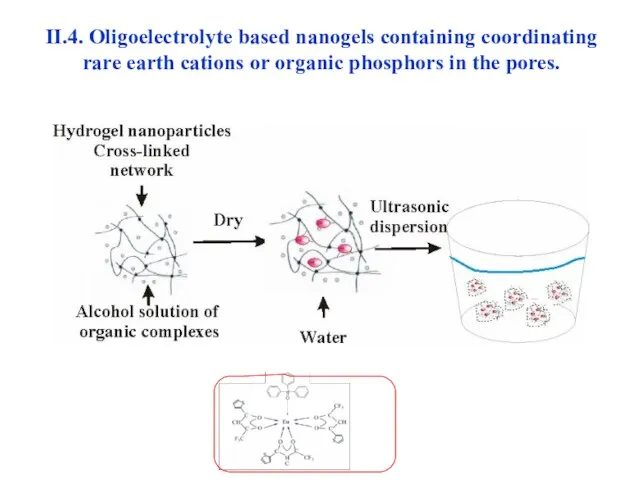
- 36. II.4. Oligoelectrolyte based nanogels containing coordinating rare earth cations or organic phosphors in the pores.
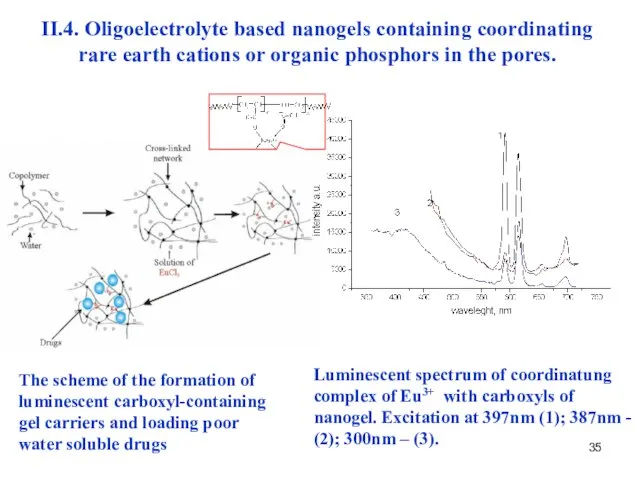
- 37. II.4. Oligoelectrolyte based nanogels containing coordinating rare earth cations or organic phosphors in the pores. The
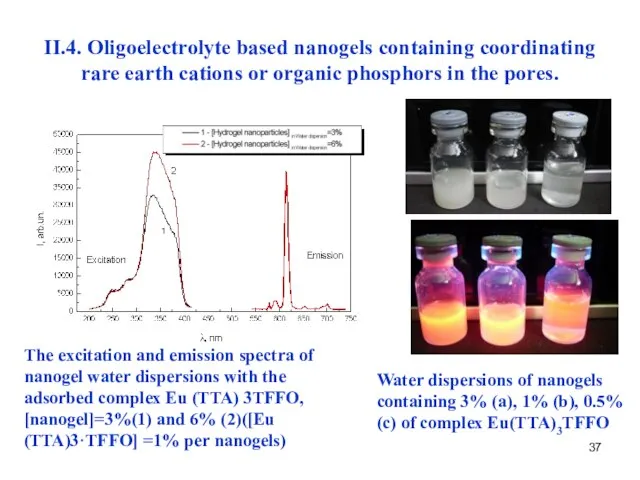
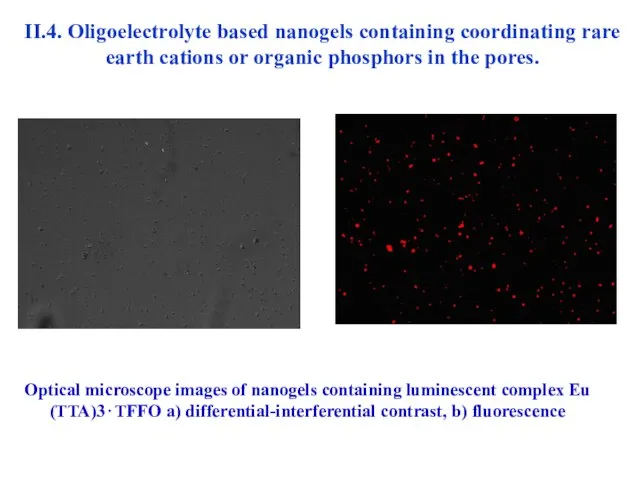
- 38. II.4. Oligoelectrolyte based nanogels containing coordinating rare earth cations or organic phosphors in the pores. Optical
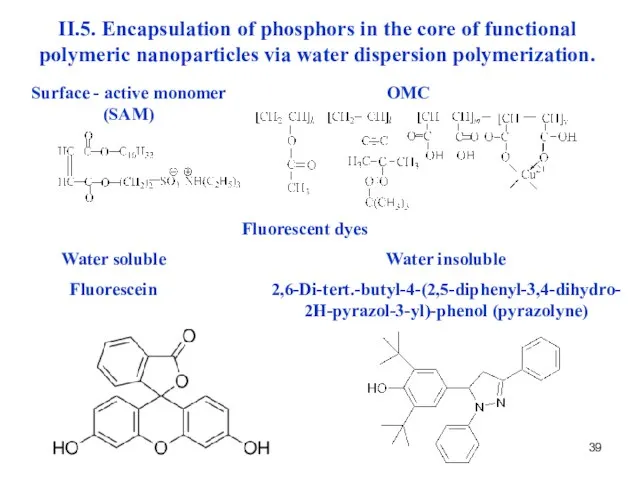
- 39. II.5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. Surface
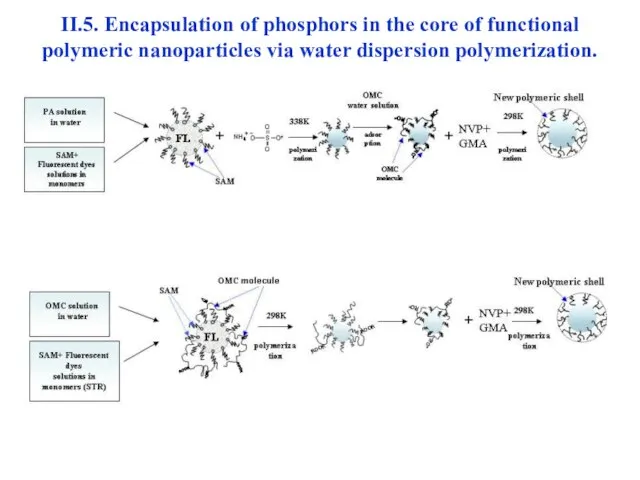
- 40. II.5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization.
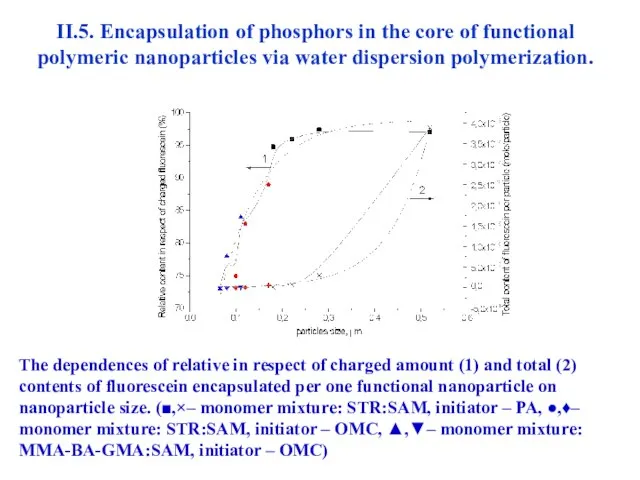
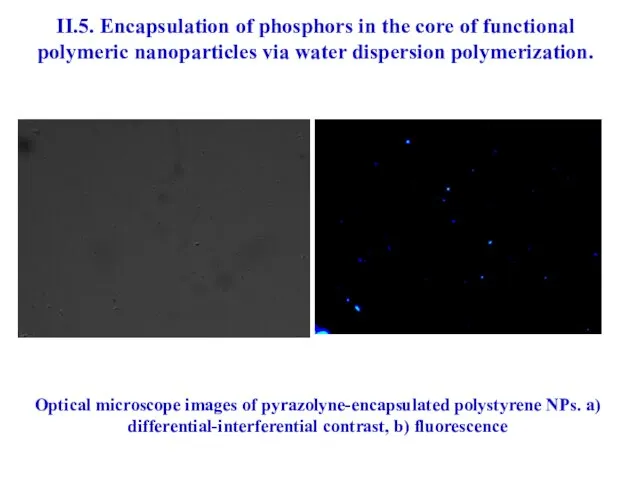
- 41. II.5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. The
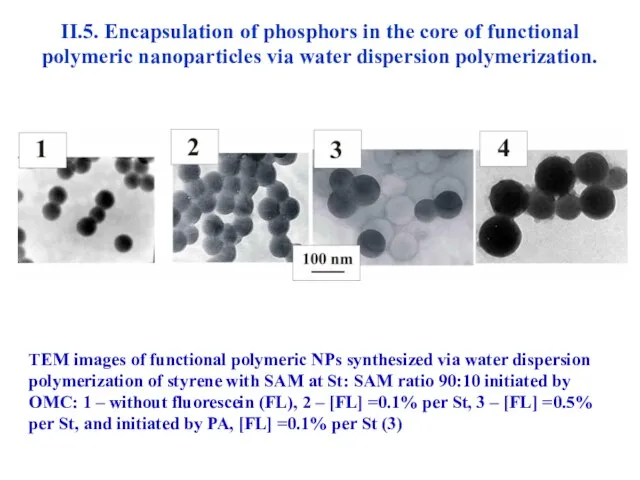
- 42. II.5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. TEM
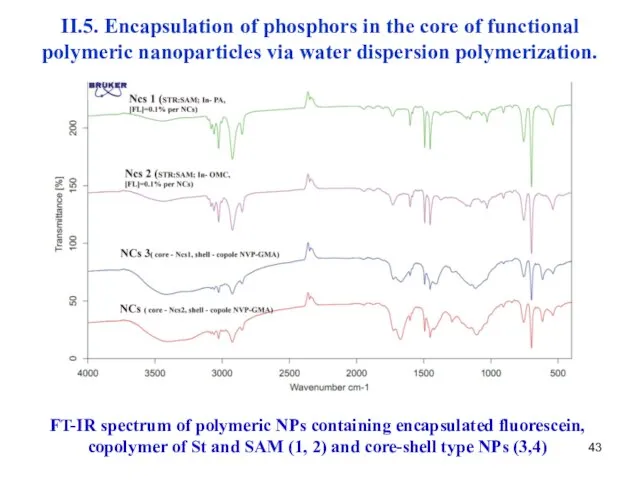
- 43. II.5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. FT-IR
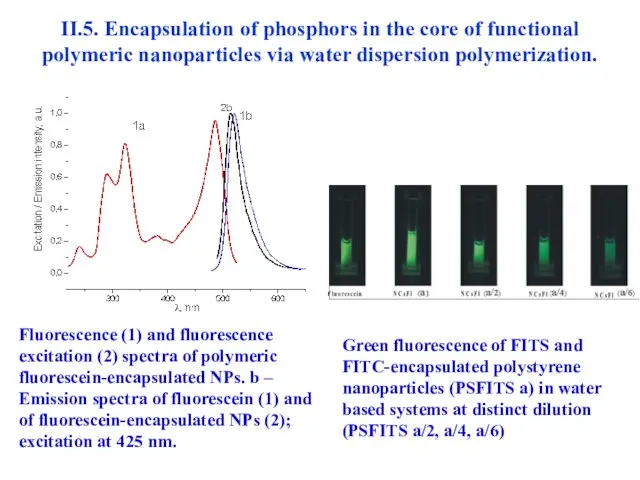
- 44. II.5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. Fluorescence
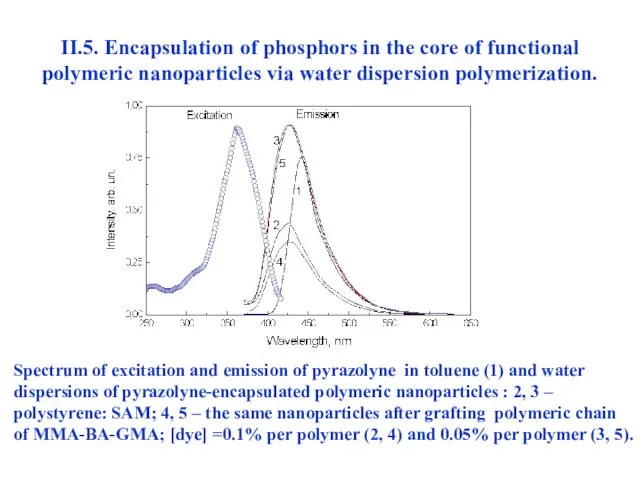
- 45. II.5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. Spectrum
- 46. II.5. Encapsulation of phosphors in the core of functional polymeric nanoparticles via water dispersion polymerization. Optical
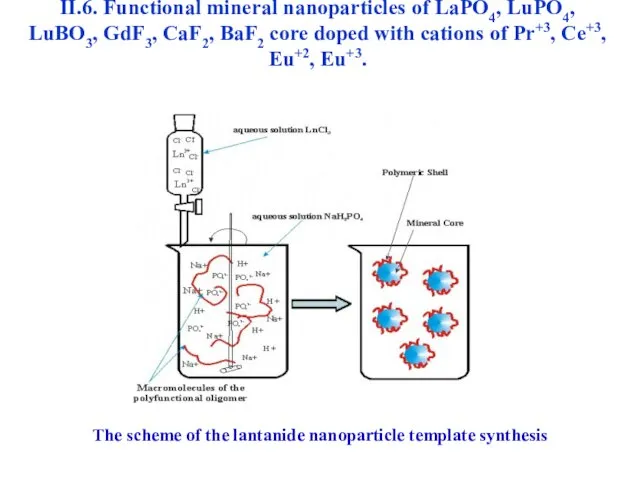
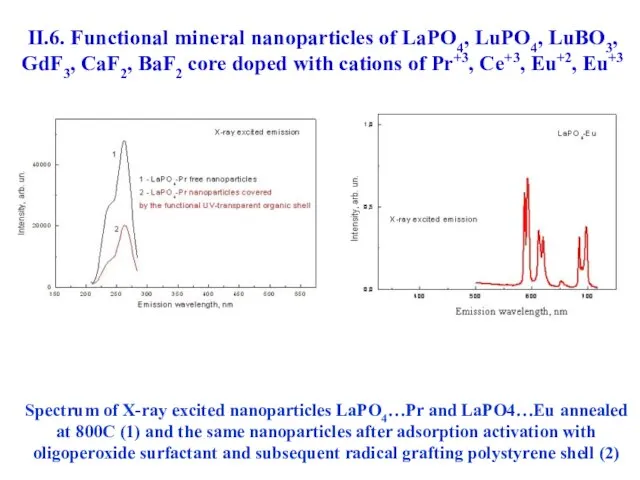
- 47. II.6. Functional mineral nanoparticles of LaPO4, LuPO4, LuBO3, GdF3, CaF2, BaF2 core doped with cations of
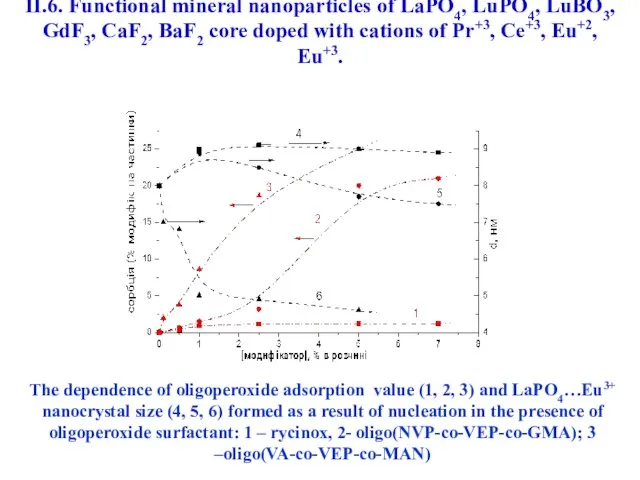
- 48. The dependence of oligoperoxide adsorption value (1, 2, 3) and LaPO4…Eu3+ nanocrystal size (4, 5, 6)
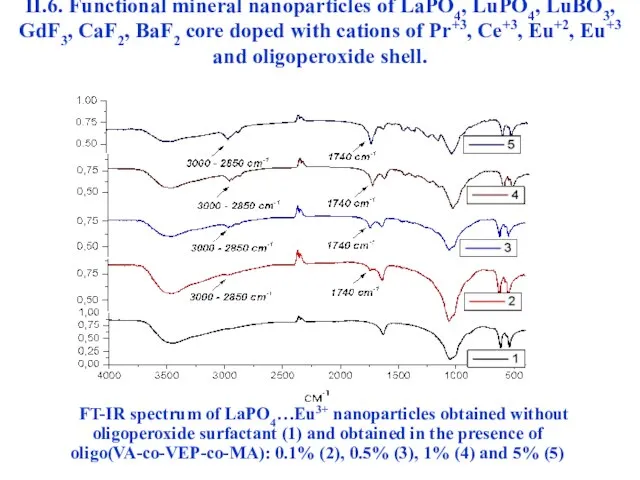
- 49. FT-IR spectrum of LaPO4…Eu3+ nanoparticles obtained without oligoperoxide surfactant (1) and obtained in the presence of
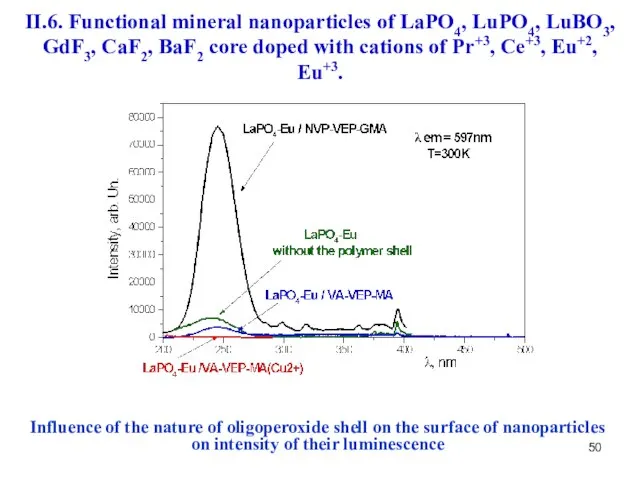
- 50. Influence of the nature of oligoperoxide shell on the surface of nanoparticles on intensity of their
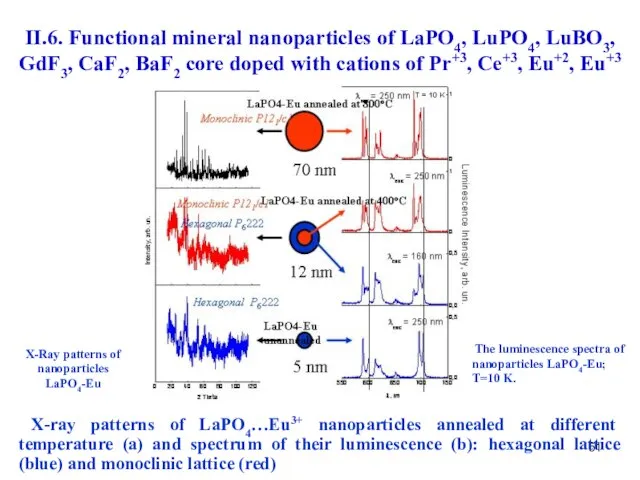
- 51. X-Ray patterns of nanoparticles LaPO4-Eu The luminescence spectra of nanoparticles LaPO4-Eu; T=10 K. X-ray patterns of
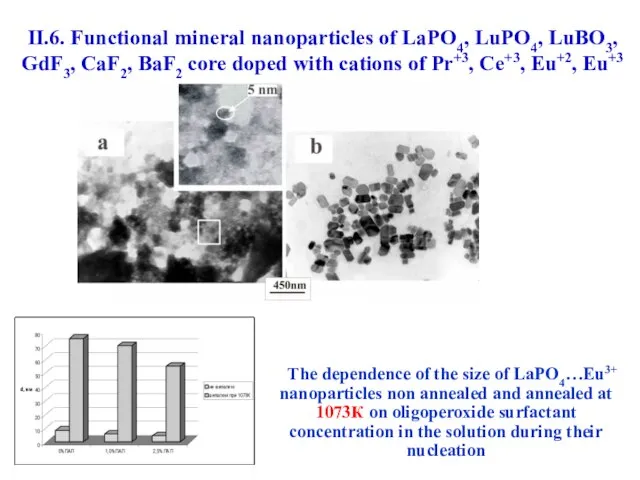
- 52. The dependence of the size of LaPO4…Eu3+ nanoparticles non annealed and annealed at 1073К on oligoperoxide
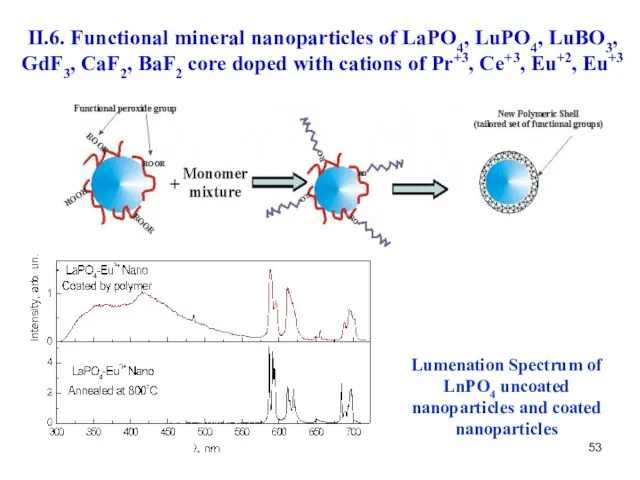
- 53. Lumenation Spectrum of LnPO4 uncoated nanoparticles and coated nanoparticles II.6. Functional mineral nanoparticles of LaPO4, LuPO4,
- 54. Spectrum of X-ray excited nanoparticles LaPO4…Pr and LaPO4…Eu annealed at 800С (1) and the same nanoparticles
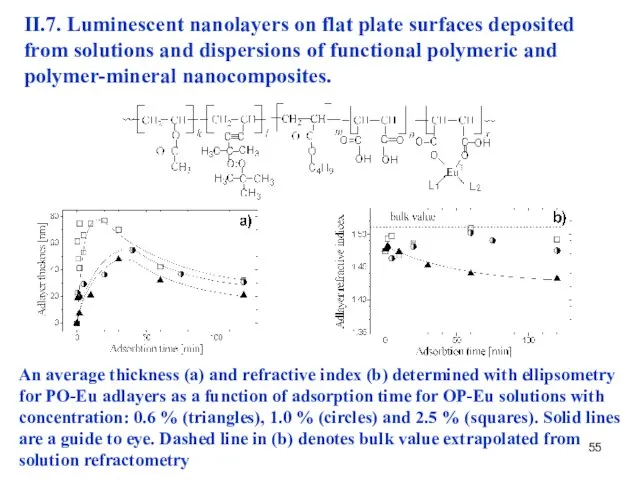
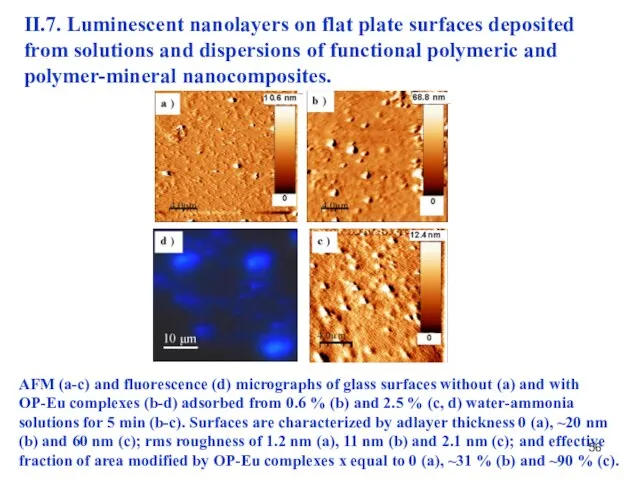
- 55. II.7. Luminescent nanolayers on flat plate surfaces deposited from solutions and dispersions of functional polymeric and
- 56. AFM (a-c) and fluorescence (d) micrographs of glass surfaces without (a) and with OP-Eu complexes (b-d)
- 57. Controlled physically detectable characteristics of nanocomposites and nanoshells Presence of peroxide links on particle surface provides
- 58. III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment. * Cellular
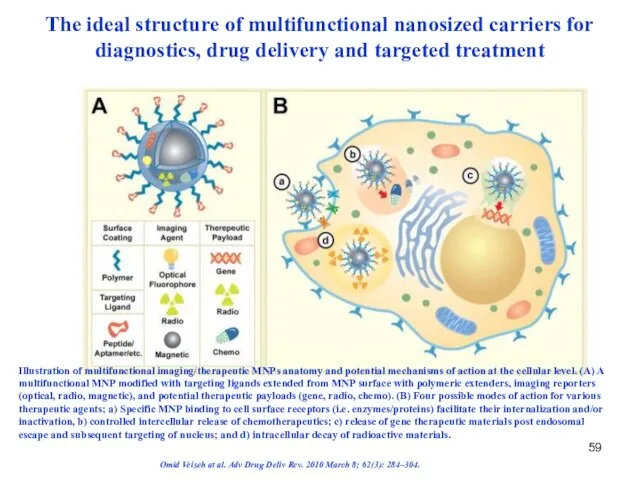
- 59. The ideal structure of multifunctional nanosized carriers for diagnostics, drug delivery and targeted treatment Illustration of
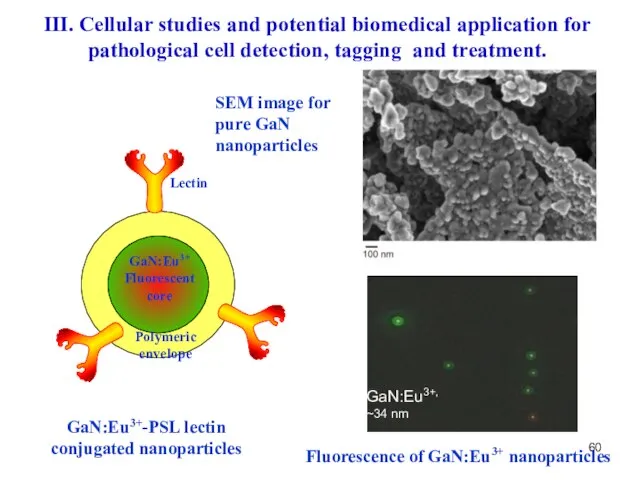
- 60. GaN:Eu3+-PSL lectin conjugated nanoparticles SEM image for pure GaN nanoparticles Fluorescence of GaN:Eu3+ nanoparticles III. Cellular
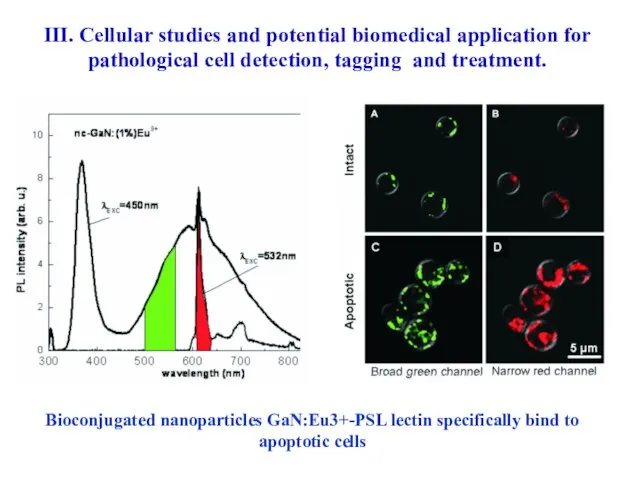
- 61. Bioconjugated nanoparticles GaN:Eu3+-PSL lectin specifically bind to apoptotic cells III. Cellular studies and potential biomedical application
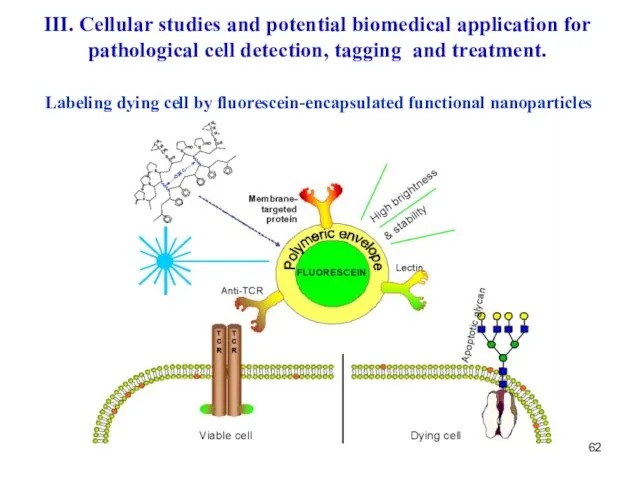
- 62. Labeling dying cell by fluorescein-encapsulated functional nanoparticles III. Cellular studies and potential biomedical application for pathological
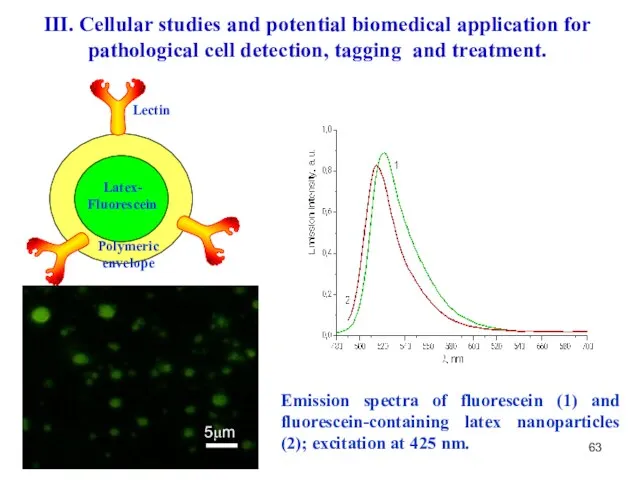
- 63. 5μm Emission spectra of fluorescein (1) and fluorescein-containing latex nanoparticles (2); excitation at 425 nm. B
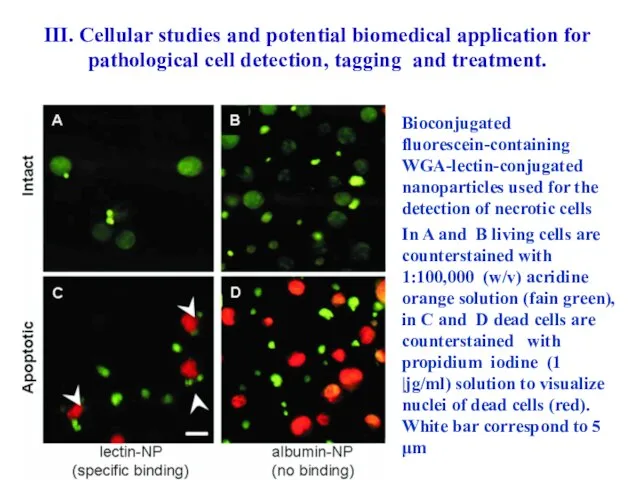
- 64. Bioconjugated fluorescein-containing WGA-lectin-conjugated nanoparticles used for the detection of necrotic cells In A and B living
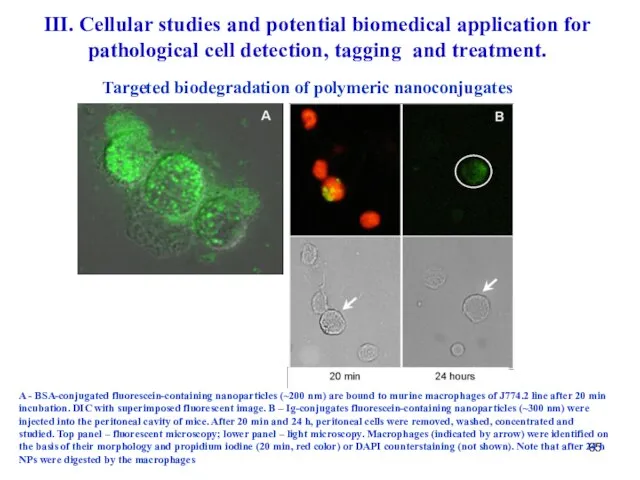
- 65. A - BSA-conjugated fluorescein-containing nanoparticles (~200 nm) are bound to murine macrophages of J774.2 line after
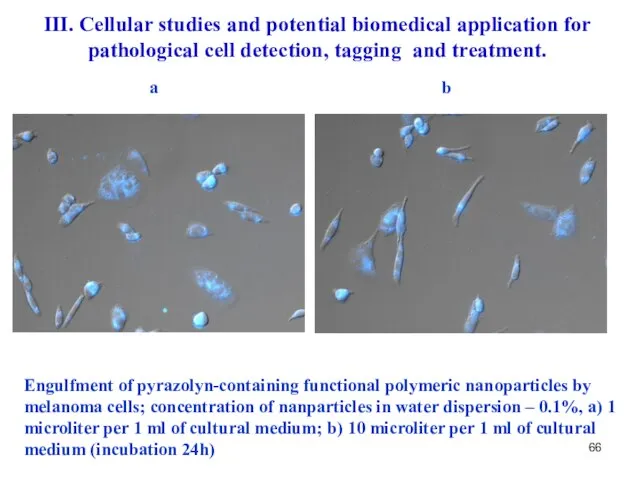
- 66. Engulfment of pyrazolyn-containing functional polymeric nanoparticles by melanoma cells; concentration of nanparticles in water dispersion –
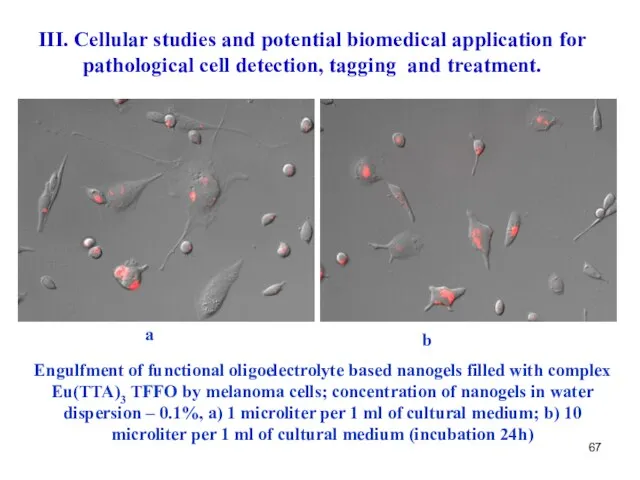
- 67. a b III. Cellular studies and potential biomedical application for pathological cell detection, tagging and treatment.
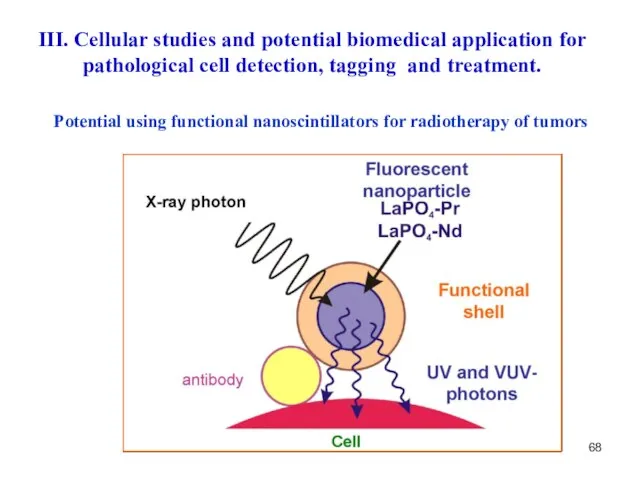
- 68. Potential using functional nanoscintillators for radiotherapy of tumors III. Cellular studies and potential biomedical application for
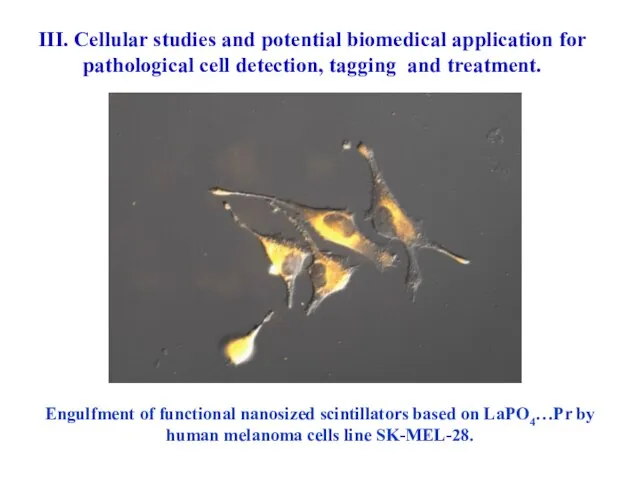
- 69. Engulfment of functional nanosized scintillators based on LaPO4…Pr by human melanoma cells line SK-MEL-28. III. Cellular
- 71. Скачать презентацию










 Русское искусство. Средневековая Русь
Русское искусство. Средневековая Русь «Знай правила дорожногодвижения – как таблицу умножения!»
«Знай правила дорожногодвижения – как таблицу умножения!» Презентация на тему Инструктаж по технике безопасности
Презентация на тему Инструктаж по технике безопасности  Формы финансовых отношений
Формы финансовых отношений История развития политических учений
История развития политических учений Фигура коммуникатора. Аудитория и сфера коммуникации
Фигура коммуникатора. Аудитория и сфера коммуникации Организационная культура учреждения
Организационная культура учреждения Оригами 1 класс
Оригами 1 класс Диагностика письменной речи
Диагностика письменной речи ПРОМЫШЛЕННЫЕ РОБОТЫ
ПРОМЫШЛЕННЫЕ РОБОТЫ Число и цифра 8 (1 класс)
Число и цифра 8 (1 класс) Bulienko_Siemion_Pavlovich
Bulienko_Siemion_Pavlovich Правила, которые следует помнить студенту
Правила, которые следует помнить студенту Партия народной свободы (Парнас)
Партия народной свободы (Парнас) Общение с детьми дошкольного возраста
Общение с детьми дошкольного возраста Презентация на тему Жанр портрета
Презентация на тему Жанр портрета  드라마와 함께 하는 한국어 수업 6강
드라마와 함께 하는 한국어 수업 6강 АУКЦИОННО ТЕНДЕРНЫЙ ДОМ
АУКЦИОННО ТЕНДЕРНЫЙ ДОМ Айсар
Айсар Интернет-отдел ББ
Интернет-отдел ББ Основы создания и работы команды. Распределение ролей в команде
Основы создания и работы команды. Распределение ролей в команде Движение крови по сосудам
Движение крови по сосудам Швейцария
Швейцария Teenage Risk Taking Behaviour
Teenage Risk Taking Behaviour Весёлый новогодний гном. Создание ёлочной игрушки
Весёлый новогодний гном. Создание ёлочной игрушки Домен TRAVEL: новые возможности для российской туристической индустрии Сергей Горбунов, Главный специалист департамента по связям
Домен TRAVEL: новые возможности для российской туристической индустрии Сергей Горбунов, Главный специалист департамента по связям  Поделка Кораблик Победы
Поделка Кораблик Победы Информация. Свойства и единицы измерения
Информация. Свойства и единицы измерения