Слайд 2Quantum Physics – introduction
Getting ‘into’ the atom
Late in the 19th century, physicists

explained properties of matter with particle model, light with wave model. Classical physics, which was continuous, had no theoretical limits on size of matter or radiation. Mechanics and electromagnetism together seemed to be enough.
However…
Слайд 3Black-body radiation remained unexplained
All hot bodies radiate.
The known spectrum did not coincide
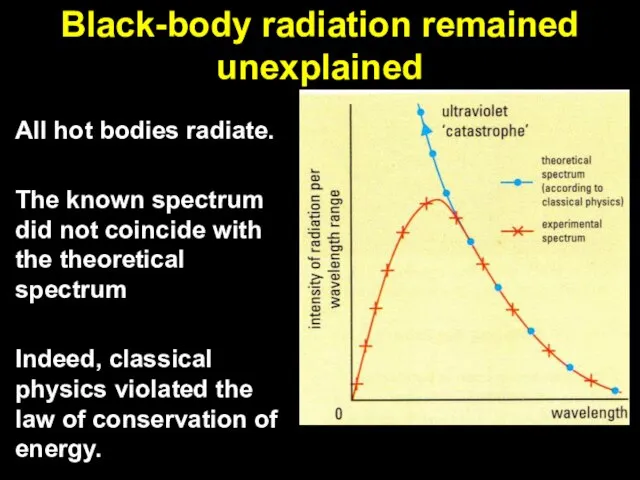
with the theoretical spectrum
Indeed, classical physics violated the law of conservation of energy.
Слайд 4In 1900, Planck postulated that
All oscillators that emit radiation can only emit

discrete energies. That is, radiation comes in packets or quanta (plural of quantum). These quanta are proportional to frequency:
E = hf
where h = 6.63 x 10-34 Js = 4.14 x 10-15 eV-s
h is known as “Planck’s constant”
Слайд 5Definition
What is one electronvolt?

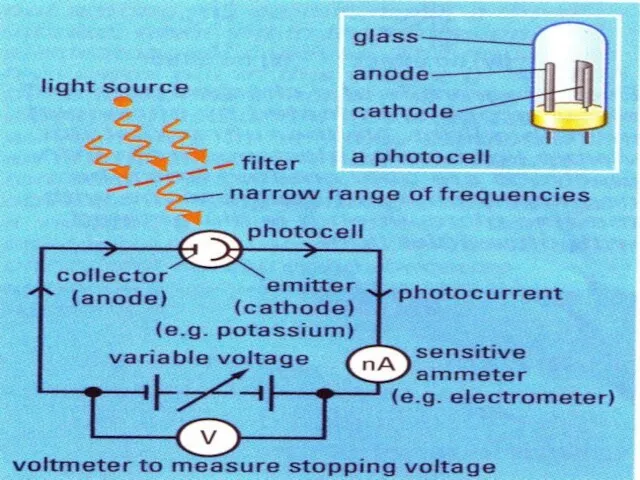
Слайд 6Along comes Albert…
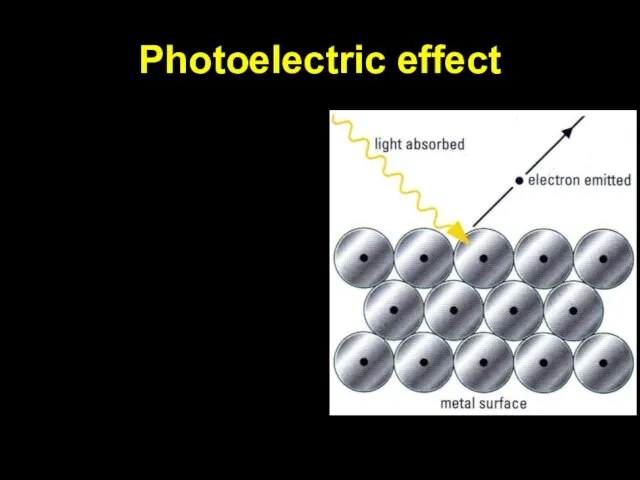
Photoelectric effect

Слайд 9This equation explained
1) For each metal, there is a threshold frequency below

which no electrons are emitted.
2) The number of electrons emitted is proportional to intensity of radiation.
3) Emitted electrons have KE up to a maximum value which depends on frequency of radiation*.
(* unexplained by classical physics)
Слайд 10
For his work on
the photoelectric
Effect, Einstein
was awarded
the Nobel
Prize in 1921

Слайд 11Example 1
a) Find the longest wavelength beyond which no electrons are emitted

from a caesium surface if Φcaesium = 1.80 eV.
b) If the surface is illuminated with monochromatic light of wavelength λ = 450 nm, calculate KEmax of emitted photoelectrons.
c) What is the stopping potential which just prevents photoemission in this situation?
Слайд 12De Broglie equation
During his PhD work, de Broglie proposed that electrons might

behave as waves.
Слайд 13Electron diffraction
Using de Broglie equation for electrons accelerated through 1kV gives a

wavelength of about _0.0388 nm_.
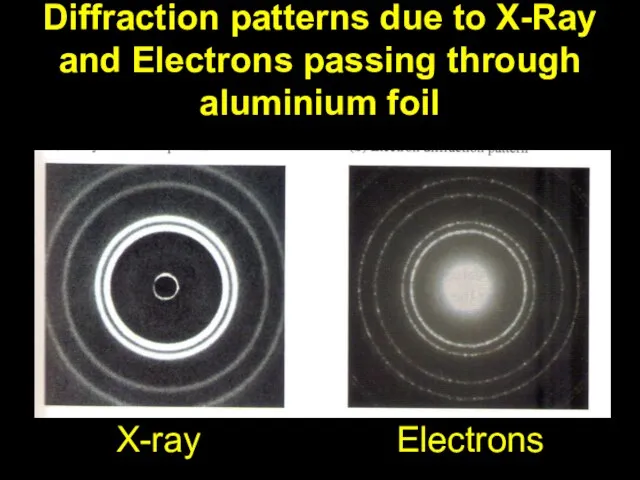
This is comparable to the wavelength of X-Rays.
Why are electrons easier to use than X-Rays when studying crystalline structures?
Слайд 14Diffraction patterns due to X-Ray and Electrons passing through aluminium foil
X-ray
Electrons
Слайд 15If all moving objects have an associated wave, why can’t we see

the waves associated with buses?
Слайд 16Wave-particle duality
Sometimes light behaves like a wave, sometimes light behaves like a

particle.
Wave-particle duality exists in the universe!
How accurately physical properties can be measured is limited.
Слайд 17Heisenberg’s uncertainty principle















 Формула цветка
Формула цветка Как составить резюме? Практические рекомендации
Как составить резюме? Практические рекомендации Классификация сварных швов и соединений
Классификация сварных швов и соединений Пути экономии энергии
Пути экономии энергии По волнам памяти
По волнам памяти Европейский Союз
Европейский Союз Презентация на тему Что такое туман
Презентация на тему Что такое туман Методика расследования карманных краж
Методика расследования карманных краж Презентация на тему Аутэкология. Факторная экология
Презентация на тему Аутэкология. Факторная экология EQUIVALENCE and TRANSLATION
EQUIVALENCE and TRANSLATION The draw
The draw AIRCRAFT ENGINES
AIRCRAFT ENGINES Рыба, запечённая с гречкой и тёртым сыром в сметанном соусе
Рыба, запечённая с гречкой и тёртым сыром в сметанном соусе I
I История национального костюма
История национального костюма Авторские таблицы к разделу Искусство народов разных стран. Греция
Авторские таблицы к разделу Искусство народов разных стран. Греция Театральная студия Солнышко
Театральная студия Солнышко Модель организации образовательного процесса до ФГТ Самостоятельная деятельность Совместная деятельность Занятия.
Модель организации образовательного процесса до ФГТ Самостоятельная деятельность Совместная деятельность Занятия. Интегрированное региональное исследование окружающей среды Сибири: первые шаги и уроки
Интегрированное региональное исследование окружающей среды Сибири: первые шаги и уроки Выпечка
Выпечка Овощи: характеристика и общие свойства
Овощи: характеристика и общие свойства Животные Сибири
Животные Сибири Детская Художественная Школа №1
Детская Художественная Школа №1 Презентация на тему Стекло история открытия и получение
Презентация на тему Стекло история открытия и получение  Конфликт. Его виды, примеры
Конфликт. Его виды, примеры Программа (концепция) развития муниципального образования Завьяловский район версия 1.0
Программа (концепция) развития муниципального образования Завьяловский район версия 1.0 Our impact
Our impact Novy_dokument_v_formate_RTF
Novy_dokument_v_formate_RTF